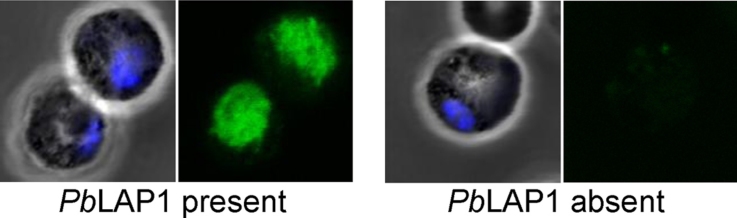
Graphical abstract
This paper identifies conformational codependence between Plasmodium berghei LCCL proteins by crossing PbLAP1-KO and PbLAP3/GFP parasite lines.
Highlight
► Co-dependent expression of LCCL proteins appears absent in Plasmodium berghei. ► However, P. berghei LCCL proteins are conformationally co-dependent. ► This mechanism promotes LCCL protein complex formation and stability. ► Conformational co-dependence could be the mechanism behind co-dependent expression.
Keywords: Plasmodium berghei, Crystalloid, GFP, Protein folding, LCCL domain
Abstract
Malaria parasites express a conserved family of LCCL-lectin adhesive-like domain proteins (LAPs) that have essential functions in sporozoite transmission. In Plasmodium falciparum all six family members are expressed in gametocytes and form a multi-protein complex. Intriguingly, knockout of P. falciparum LCCL proteins adversely affects expression of other family members at protein, but not at mRNA level, a phenomenon termed co-dependent expression. Here, we investigate this in Plasmodium berghei by crossing a PbLAP1 null mutant parasite with a parasite line expressing GFP-tagged PbLAP3 that displays strong fluorescence in gametocytes. Selected and validated double mutants show normal synthesis and subcellular localization of PbLAP3::GFP. However, GFP-based fluorescence is dramatically reduced without PbLAP1 present, indicating that PbLAP1 and PbLAP3 interact. Moreover, absence of PbLAP1 markedly reduces the half-life of PbLAP3, consistent with a scenario of misfolding. These findings unveil a potential mechanism of conformational interdependence that facilitates assembly and stability of the functional LCCL protein complex.
The LCCL protein family of malaria parasites is a group of six highly conserved and structurally related proteins that possess a modular architecture with multiple domains implicated in protein, lipid and carbohydrate binding [1–5]. The family is named after the Limulus clotting factor C, Coch-5b2, Lgl1 (LCCL) domain [6] which is found in all except one family member. LCCL proteins possess a consensus endoplasmic reticulum (ER) signal peptide at the amino-terminus, but other known organelle targeting sequences are absent [1,4]. The LCCL protein family members are referred to as Plasmodium LCCL domain-containing proteins (PCCp) [4] used mostly for Plasmodium falciparum, and Plasmodium LCCL-lectin adhesive-like proteins (PLAP) mostly used in the context of Plasmodium berghei [5]. In P. berghei, all of the family members except PbLAP3 have been studied by gene disruption, which has revealed that they have very similar loss-of-function phenotypes characterized by a loss of sporozoite development in the oocyst and concomitant loss of sporozoite transmission [1,7–9]. Conversely, knockout of PfCCp2 and PfCCp3 expression in P. falciparum does not appear to affect sporozoite development, but blocks the transition from midgut to salivary gland sporozoites [4]. Plasmodium LCCL proteins thus play vital roles in sporozoite transmission.
There is a clear consensus that all P. berghei LCCL protein family members are expressed in gametocytes based on a combination of GFP reporter studies and GFP-tagging experiments [7,10–12]. This fully agrees with the reported expression of the LCCL protein family members in P. falciparum as determined by immunofluorescence and immunoblot [4,13–16]. The similar expression patterns and the highly similar loss-of-function phenotypes of the PbLAPs strongly indicate that these molecules are functionally interdependent, are involved in the same molecular processes in the parasite, and operate as a protein complex. This notion is further supported by the observation that several PbLAPs are targeted to the crystalloid organelle in the downstream ookinete stage [7,11]. Indeed, molecular interactions between P. falciparum LCCL protein family members have been shown in co-immunoprecipitation experiments using gametocytes [16], providing evidence for protein complex formation.
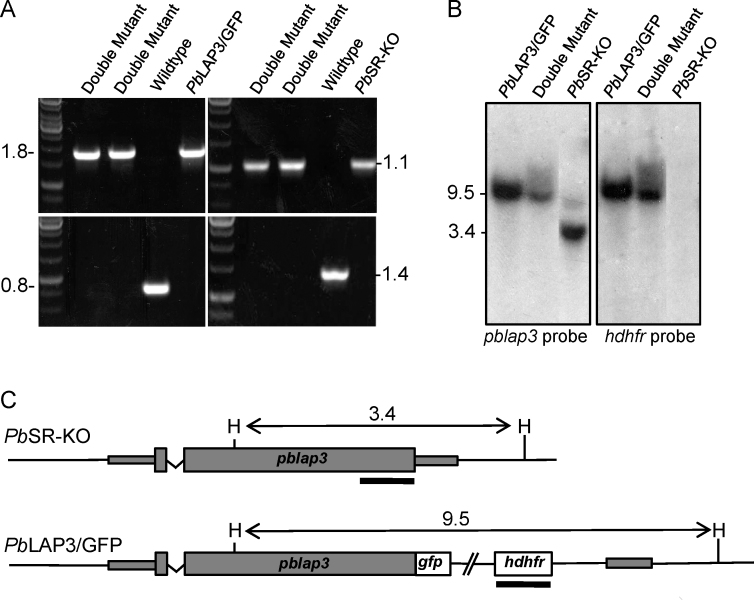
Another line of evidence that supports a functional relationship between the LCCL protein family members is provided by observations in P. falciparum that PfCCp proteins are co-dependently expressed [14,16]. These studies showed using immunofluorescence and immunoblotting that genetic disruption of a single pfccp gene not only abolishes expression of its cognate gene product as expected, but also reduces or abolishes the expression of other LCCL proteins. Moreover, this process occurs at the protein, but not at the transcript level. It remains unclear what the molecular mechanisms are that underlie this phenomenon. In this study we investigated whether co-dependent expression exists in P. berghei. To this purpose we generated a double mutant parasite line by crossing a structural gene knockout parasite line for PbLAP1 (also known as PbSR; PlasmoDB ID: PBANKA_103520), named PbSR-KO [7], with a mutant parasite line expressing full-length PbLAP3 (PlasmoDB ID: PBANKA_020450) tagged at the carboxy-terminus with GFP, named PbLAP3/GFP [11]. PbSR-KO parasites display the typical null mutant phenotype of all the PbLAP proteins, characterised by a loss of sporozoite development and transmission [7]. This parasite line does not exhibit GFP-based fluorescence. By contrast, parasite line PbLAP3/GFP exhibits strong GFP fluorescence in gametocytes and displays a wildtype phenotype throughout the life cycle [11]. The two parasite lines were crossed by culturing ookinetes from mixed gametocyte populations, followed by feeding the resulting ookinetes to Anopheles stephensi vector mosquitoes in membrane feeders. Because the pblap alleles are maternally inherited [9], cross-fertilization events between female gametes of PbLAP3/GFP and male gametes of PbSR-KO parasites should give rise to infective sporozoites. At three weeks post-infection, sporozoites were transmitted to naïve mice by infected mosquito bites, and double mutants containing both of the modified pblap alleles were selected from the resulting patent blood stage infection by drug selection followed by limiting dilution cloning, as described [17]. The presence of the two modified pblap alleles, as well as the absence of the equivalent unmodified alleles, was confirmed by diagnostic PCR. A ca. 1.1 kb fragment specific for the modified pblap1 allele was amplified from the double mutant and parental PbSR-KO parasite line (Fig. 1A). Similarly, a ca. 1.8 kb fragment specific for the modified pblap3 allele was amplified from both the double mutant and parental PbLAP3/GFP parasite line (Fig. 1A). In addition, fragments of ca. 1.4 kb and 0.8 kb diagnostic for the wildtype pblap1 and pblap3 alleles, respectively, were absent from the double mutant parasites (Fig. 1A). The presence of the pblap3::gfp allele in the double mutant was further verified by Southern analysis: a pblap3-specific probe detected a 3.4 kb fragment in HindIII-digested genomic DNA of the PbSR-KO parental line, but a 9.5 kb fragment in the PbLAP3/GFP parental line, as expected (Fig. 1B and C). A 9.5 kb fragment corresponding to the modified pblap3 allele was also detected in the double mutant, while the 3.4 kb fragment corresponding to the wild-type pblap3 allele was absent in this parasite (Fig. 1B). Similarly, a hdhfr-specific probe detected a 9.5 kb fragment in both the PbLAP3/GFP parent and double mutant, and no signal in the PbSR-KO parent, as expected (Fig. 1B and C).
Fig. 1.
Molecular analyses of the parental parasite lines PbSR-KO and PbLAP3/GFP, and double mutant parasites derived from a PbSR-KO × PbLAP3/GFP genetic cross. (A) PCR diagnostic for the disrupted pblap1 allele (top right panel; primers [CGCGATG ACCCCCAAGAGGGG] and [CGCCTTCACGCTGATGT]); the GFP-tagged pblap3 allele (top left panel; primers [ACAAAGAATTCATGGTTGGTTCGCTAAACT] and [CCTCAAGATAGTTACGAATTTAAC]); the wildtype pblap1 allele (bottom right panel; primers [CATAATATGCATCTAGAACCAACTTTTC] and [AACGGGATCTTCTAGAATTTAATATAAGCGTTTCAAAAAGGTAAATG]); and the wildtype pblap3 allele (bottom left panel; primers [ACGAAGTTATCAGT CGAGGTACCTAGCGGAAACAACAATGTTC] and [CCTCAAGATAGTTACGAATTTAAC]). B) Southern blot analysis of HindIII-digested genomic DNA using a pblap3-specific probe (left panel) and a hdhfr-specific probe (right panel). (C) Schematic diagram showing the structure of the pblap3 alleles in parasite lines PbSR-KO and PbLAP3/GFP. Indicated are the HindIII restriction sites (H), sizes of the predicted HindIII restriction fragments, and regions corresponding to probes used in B (thick lines).
Validated clones of the double mutant parasite line were assessed by western blot analysis for the expression of the PbLAP3::GFP fusion protein in gametocytes, in comparison with the parental lines. This showed normal expression of the protein in question (Fig. 2A), demonstrating that knockout of PbLAP1 had not adversely affected expression levels of PbLAP3 as is the case for the orthologous proteins in P. falciparum [16]. This result indicated that gametocyte stage co-dependent expression does not occur in P. berghei. Surprisingly, however, GFP fluorescence levels in gametocytes of the double mutant parasite line were dramatically reduced compared to the parental PbLAP3/GFP gametocytes (Fig. 2B), indicating that the absence of PbLAP1 has affected the ability of the PbLAP3::GFP chimera to generate fluorescence. To further investigate the expression and subcellular distribution of PbLAP3::GFP in gametocytes of the double mutant parasite line we performed immunofluorescence using commercially available anti-GFP antibody. Gametocytes were purified as described [18], fixed for 20 min in 4% paraformaldehyde, washed twice with PBS, blocked and permeabilized for 1 h in PBS supplemented with 0.1% Triton X-100 and 1% BSA. Then the gametocytes were labelled with anti-GFP antibodies conjugated to FITC (ab65180, Abcam, diluted 1 in 1000) for 1 h at room temperature. After a further two PBS washes the gametocyte suspension was examined by confocal microscopy. FITC signal in the double mutant gametocytes was clearly detectable compared to GFP-negative wildtype parasite controls (Fig. 2C), consistent with expression of the PbLAP3::GFP fusion protein in the double mutant. Moreover, the subcellular distribution of FITC signal (Fig. 2C) was comparable to that of GFP signal in the parental PbLAP3/GFP parasite line (Fig. 2B), demonstrating that the subcellular distribution of PbLAP3::GFP had not drastically changed in the absence of PbLAP1. It is therefore unlikely that a change in the protein's localization to a subcellular compartment less favourable for generating GFP fluorescence is responsible for the loss of GFP fluorescence in the double mutant. We postulate that a more likely explanation for the loss of GFP fluorescence is based on a PbLAP1–PbLAP3 molecular interaction: the imposed loss of this interaction in the double mutant parasite line causes a conformational change in PbLAP3 that is adverse to functionality of its GFP tag. Indeed, the folding of amino-terminal fusions with GFP has long been known to influence fluorescence levels of chimeric GFP [19].
Fig. 2.
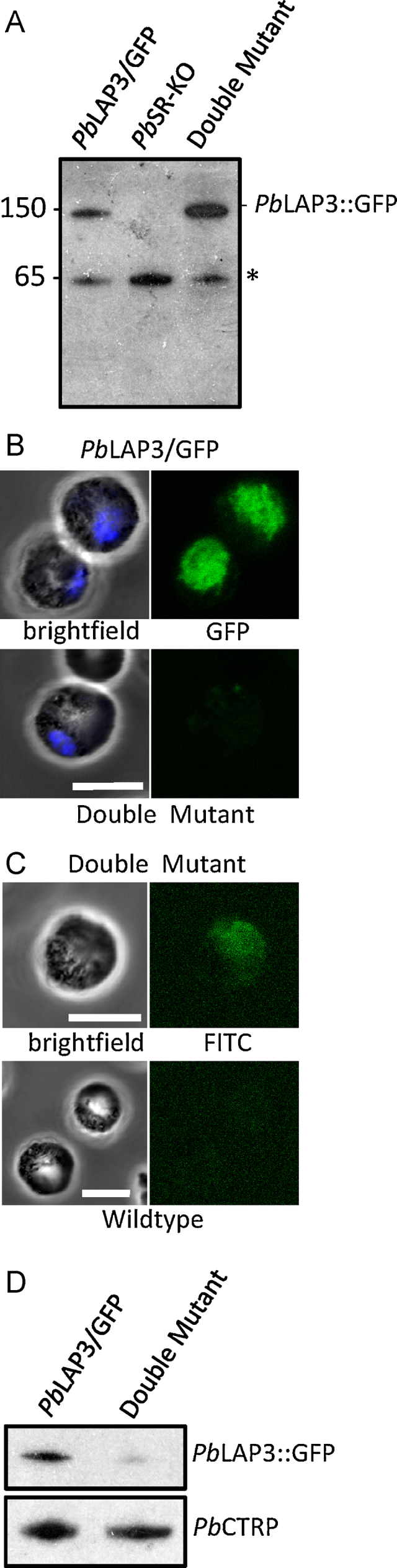
Expression and distribution of the PbLAP3::GFP fusion protein in the parental parasite lines PbSR-KO and PbLAP3/GFP, and double mutant parasites derived from a PbSR-KO × PbLAP3/GFP genetic cross. (A) Western blot of purified gametocyte samples of parasite lines PbSR-KO, PbLAP3/GFP, and the double mutant, using anti-GFP antibodies. The blot shows bands of ca. 150 kDa corresponding to the PbLAP3::GFP chimera, and of ca. 65 kDa (*) corresponding to a non-specific protein that cross reacts with the antibody. (B) Confocal GFP fluorescence images of macrogametocytes of parasite line PbLAP3/GFP and the double mutant parasite. GFP images were taken with the same gain settings. Nuclei were stained with Hoechst (blue). Bar = 5 μm. (C) Confocal FITC immunofluorescence images of macrogametocytes of double mutant and wildtype parasites. FITC images were taken with the same gain settings. Bar = 5 μm. (D) Western blot of purified ookinete samples of parasite lines PbLAP3/GFP and the double mutant showing PbLAP3::GFP fusion protein relative to the ookinete loading control PbCTRP. (For interpretation of the references to color in this figure caption, the reader is referred to the web version of the article.)
The notion that in the absence of PbLAP1 the PbLAP3::GFP fusion protein adopts a different conformation and hence is in a potential state of ‘misfolding’ led us to investigate the stability of the protein post-synthesis by western blot. For this purpose ookinete cultures were set up from gametocytemic blood as described [20] and after 24 h ookinetes were purified and analysed. Western blot revealed that considerably less PbLAP3::GFP was present in the double mutant than in the parental PbLAP3/GFP ookinete population, relative to the P. berghei ookinete-specific CS and TRAP related protein (PbCTRP) [21] (Fig. 2D). In fact, the double mutant ookinetes possessed only 16% of the PbLAP3::GFP level of the parental PbLAP3/GFP line as determined by ImageJ analysis as recommended [http://rsbweb.nih.gov/ij] and normalized against PbCTRP levels. This implies that PbLAP3 is markedly less stable in the absence of PbLAP1 than in its presence, a scenario that is consistent with a misfolded protein being actively removed from the cell.
Our combined findings unveil a mechanism of conformational interdependence between PbLAP1 and PbLAP3 that stabilizes the molecules when they interact and as such promotes the assembly and durability of the functional LCCL protein complex. It is highly plausible that such a mechanism extends to the other LCCL protein family members. The fact that all PbLAPs studied so far have exhibited very similar loss-of-function phenotypes strongly points to a functional interdependence of these molecules, which is obviously compatible with a scenario in which assembly of a functional LCCL protein complex is prevented when individual components are lacking, as is the case in PbLAP knockout parasites. A similar system of conformational interdependence, if it were present in P. falciparum, could provide the underlying mechanism for the observed co-dependent expression of its LCCL proteins in gametocytes. Gametocytogenesis in P. falciparum takes considerably longer than in P. berghei and, consequently, much more time is available for any misfolded LCCL proteins to be degraded and removed before the gametocytes reach maturity, especially as the PfCCp proteins are already expressed early during gametocytogenesis [15]. Moreover, the varying degrees of co-dependent expression observed in P. falciparum between different family members [16] could reflect quantitative differences in their dependence on each other to fold correctly.
Acknowledgements
This work was supported by grants from the Wellcome Trust (AZT), and a studentship from the Pakistan Higher Education Commission (SS).
References
- 1.Claudianos C., Dessens J.T., Trueman H.E., Arai M., Mendoza J., Butcher G.A. A malaria scavenger receptor-like protein essential for parasite development. Molecular Microbiology. 2002;45(6):1473–1484. doi: 10.1046/j.1365-2958.2002.03118.x. [DOI] [PubMed] [Google Scholar]
- 2.Dessens J.T., Sinden R.E., Claudianos C. LCCL proteins of apicomplexan parasites. Trends in Parasitology. 2004;20(3):102–108. doi: 10.1016/j.pt.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Lasonder E., Ishihama Y., Andersen J.S., Vermunt A.M., Pain A., Sauerwein R.W. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature. 2002;419(6906):537–542. doi: 10.1038/nature01111. [DOI] [PubMed] [Google Scholar]
- 4.Pradel G., Hayton K., Aravind L., Iyer L.M., Abrahamsen M.S., Bonawitz A. A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. Journal of Experimental Medicine. 2004;199(11):1533–1544. doi: 10.1084/jem.20031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trueman H.E., Raine J.D., Florens L., Dessens J.T., Mendoza J., Johnson J. Functional characterization of an LCCL-lectin domain containing protein family in Plasmodium berghei. Journal of Parasitology. 2004;90(5):1062–1071. doi: 10.1645/GE-3368. [DOI] [PubMed] [Google Scholar]
- 6.Trexler M., Banyai L., Patthy L. The LCCL module. European Journal of Biochemistry. 2000;267(18):5751–5757. doi: 10.1046/j.1432-1327.2000.01641.x. [DOI] [PubMed] [Google Scholar]
- 7.Carter V., Shimizu S., Arai M., Dessens J.T. PbSR is synthesized in macrogametocytes and involved in formation of the malaria crystalloids. Molecular Microbiology. 2008;68(6):1560–1569. doi: 10.1111/j.1365-2958.2008.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ecker A., Bushell E.S., Tewari R., Sinden R.E. Reverse genetics screen identifies six proteins important for malaria development in the mosquito. Molecular Microbiology. 2008;70(1):209–220. doi: 10.1111/j.1365-2958.2008.06407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raine J.D., Ecker A., Mendoza J., Tewari R., Stanway R.R., Sinden R.E. Female inheritance of malarial lap genes is essential for mosquito transmission. PLoS Pathogens. 2007;3(3):e30. doi: 10.1371/journal.ppat.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan S.M., Franke-Fayard B., Mair G.R., Lasonder E., Janse C.J., Mann M. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121(5):675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Saeed S., Carter V., Tremp A.Z., Dessens J.T. Plasmodium berghei crystalloids contain multiple LCCL proteins. Molecular and Biochemical Parasitology. 2010;170(1):49–53. doi: 10.1016/j.molbiopara.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavazec C., Moreira C.K., Mair G.R., Waters A.P., Janse C.J., Templeton T.J. Analysis of mutant Plasmodium berghei parasites lacking expression of multiple PbCCp genes. Molecular and Biochemical Parasitology. 2009;163(1):1–7. doi: 10.1016/j.molbiopara.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Delrieu I., Waller C.C., Mota M.M., Grainger M., Langhorne J., Holder A.A. PSLAP: a protein with multiple adhesive motifs, is expressed in Plasmodium falciparum gametocytes. Molecular and Biochemical Parasitology. 2002;121(1):11–20. doi: 10.1016/s0166-6851(02)00016-6. [DOI] [PubMed] [Google Scholar]
- 14.Pradel G., Wagner C., Mejia C., Templeton T.J. Plasmodium falciparum: co-dependent expression and co-localization of the PfCCp multi-adhesion domain proteins. Experimental Parasitology. 2006;112(4):263–268. doi: 10.1016/j.exppara.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Scholz S.M., Simon N., Lavazec C., Dude M.A., Templeton T.J., Pradel G. PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. International Journal for Parasitology. 2008;38(3–4):327–340. doi: 10.1016/j.ijpara.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Simon N., Scholz S.M., Moreira C.K., Templeton T.J., Kuehn A., Dude M.A. Sexual stage adhesion proteins form multi-protein complexes in the malaria parasite Plasmodium falciparum. Journal of Biological Chemistry. 2009;284(21):14537–14546. doi: 10.1074/jbc.M808472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tremp A.Z., Dessens J.T. Malaria IMC1 membrane skeleton proteins operate autonomously and participate in motility independently of cell shape. Journal of Biological Chemistry. 2011;286(7):5383–5391. doi: 10.1074/jbc.M110.187195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raabe A.C., Billker O., Vial H.J., Wengelnik K. Quantitative assessment of DNA replication to monitor microgametogenesis in Plasmodium berghei. Molecular and Biochemical Parasitology. 2009;168(2):172–176. doi: 10.1016/j.molbiopara.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waldo G.S., Standish B.M., Berendzen J., Terwilliger T.C. Rapid protein-folding assay using green fluorescent protein. Nature Biotechnology. 1999;17(7):691–695. doi: 10.1038/10904. [DOI] [PubMed] [Google Scholar]
- 20.Arai M., Billker O., Morris H.R., Panico M., Delcroix M., Dixon D. Both mosquito-derived xanthurenic acid and a host blood-derived factor regulate gametogenesis of Plasmodium in the midgut of the mosquito. Molecular and Biochemical Parasitology. 2001;116(1):17–24. doi: 10.1016/s0166-6851(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 21.Dessens J.T., Beetsma A.L., Dimopoulos G., Wengelnik K., Crisanti A., Kafatos F.C. CTRP is essential for mosquito infection by malaria ookinetes. EMBO Journal. 1999;18(22):6221–6227. doi: 10.1093/emboj/18.22.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]