Abstract
We report the possibility to modify the wetting properties of the surfaces of a diversity of seeds including: lentils (Lens culinaris), beans (Phaseolus vulgaris) and wheat (Triticum, species C9) by cold radiofrequency air plasma treatment. Air plasma treatment leads to the dramatic decrease in the apparent contact angle. Moreover, the speed of germination and yield (germination rate) of seeds can be modified by preliminary plasma treatment. The change in the wetting properties of seeds is at least partially due to oxidation of their surface under plasma treatment. Significant growth of the peaks corresponding to the nitrogen containing groups in the mass spectra of air plasma treated seeds was registered by TOF-SIMS spectroscopy.
The plasma treatment of polymer surfaces is a widely used method to modify the physical and chemical properties of the surface1,2,3,4,5,6,7,8,9,10,11. The plasma treatment creates a complex mixture of surface functionalities which influence physical and chemical properties of the surface. In particular, it results in a dramatic change of wetting behavior of the surface3,4,5. Not only the chemical structure but also the roughness of the surface is affected by the plasma treatment, this also can change the wettability of the surface12. It has also been demonstrated that wetting of biological tissue (keratin) can be modified by low-temperature radiofrequency plasma13,14,15.
Cold (non-equilibrium) radiofrequency plasma treatment of biological objects becomes an important tool for modification of their chemical and physical properties13,14,15,16,17. Cold plasma is capable of bacterial and fungi inactivation and noniflammatory tissue modification16,17. Wound healing and tissue regeneration can be achieved following various types of plasma treatment in a multitude of wound pathologies18. There are still many open issues with regard to the mechanisms of plasma action on cells and tissues16. For example, the chemistry of the plasma/tissue interaction and the exact roles of various plasma constituents in tissue treatment remain obscure. In our paper, we concentrate on the modification of wetting properties of the surfaces of various seeds by plasma leading to a significant change in their germination speed and eventual yield.
Results
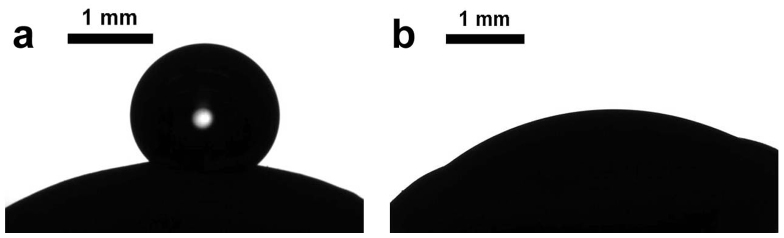
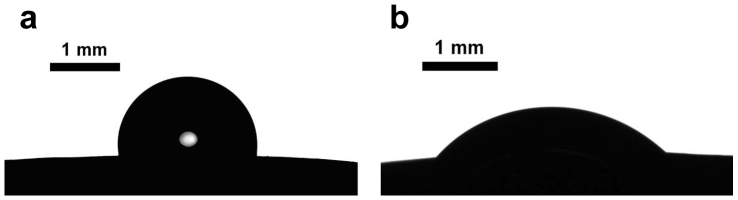
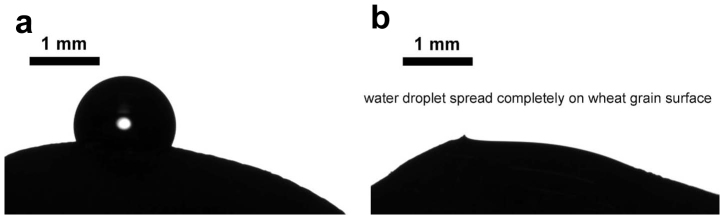
Let us start from the examination of the surface of seeds. SEM images of the seeds and grains used in our investigation are depicted in Figure 1. It can be recognized that the surface of lentils and wheat grains is much rougher when compared with that of beans. The surface of lentils comprises granules with a characteristic size varying from 0.2 to 30 µm. It was supposed that these irregularly shaped, randomly distributed granules are built from proteins19. However, the surface of beans is rough also on a microscopic scale. Wetting of such microscopically-scaled rough surfaces is characterized by the so-called apparent contact angle defined as the angle between the tangent to the liquid-air interface and the apparent solid surface as macroscopically observed (the detailed topography of a rough surface cannot be viewed with regular optical means)20,21. The wetting of miscroscopically rough random surfaces is an extremely complicated phenomenon. The apparent contact angle results from a complex interplay of chemical composition and roughness of the surfaces20,21. It is agreed that the analysis of the wetting of rough inhomogeneous surfaces can be reduced to the Cassie and Wenzel models22,23,24. We will not enter into details of the wetting regimes occurring on surfaces of lentil and bean seeds, but rather focus on the changes in these regimes caused by cold plasma treatment. The apparent contact angles were established as 127±2° and 98±2° for untreated lentils and beans correspondingly, as shown in Figures 2a, 3a, 4. The 15s cold air plasma irradiation of seeds decreased these angles to 20±1° and 53±1.5° for lentils and beans correspondingly (see Figures 2b, 3b). It should be stressed that this 15 s plasma treatment did not change the topography of the seed surfaces, a fact established with high resolution SEM and ESEM monitoring. The most pronounced change in the wettability was observed with plasma-treated wheat C9, when the apparent contact angle changed from 115±2° to zero, as shown in Figure 5. Thus, plasma treatment in this case caused a transition from partial to complete wetting. The variation of the time span of plasma treatment in the range of 15s – 2min did not influence the wetting of irradiated seeds.
Figure 1. SEM images of (a) lentil seeds, scale bar is 1µm; (b) bean's surface, scale bar is 20 µm; (c) wheat C9 grains, scale bar is 1 µm.
Figure 2. Water droplet deposited on untreated (a) and cold plasma treated (b) lentil seed.
Figure 3. Water droplet deposited on untreated (a) and cold plasma treated (b) bean.
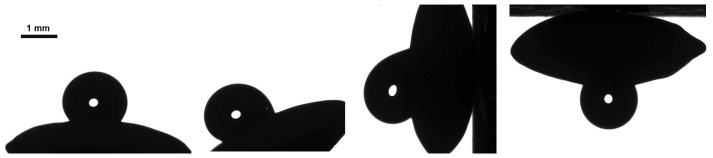
Figure 4. The “rose petal effect” observed on lentil seeds.
High apparent contact angles are attended by a high adhesive wetting state. The droplet is attached to the surface even in the pending position. The same effect was observed with wheat grains.
Figure 5. Water droplet deposited on untreated (a) and cold plasma treated (b) wheat grains.
The transition from partial to total wetting is seen.
Formation of cold radiofrequency plasma is accompanied by strong emission of UV radiation16. It is well-known that UV radiation modifies wetting properties of synthetic polymers25,26. In order to check the influence of UV emission on the wettability of seeds we exposed them solely to UV radiation at the wavelength of 254 nm during two minutes (corresponding to the maximal time of plasma treatment). We established that UV radiation did not modify the apparent contact angle of all kinds of seeds used in our study.
The high initial apparent contact angles observed on lentil seeds and wheat grains catch the eye. It is reasonable to suggest that the rough surface of these seeds gives rise to the so-called “lotus” effect27,28. However, both lentils and wheat grains do not demonstrate true superhydrophobicity. Actually, the high apparent contact angles observed on lentil seeds and wheat grains are accompanied by high contact angle hysteresis, as illustrated in Figure 4. Such behavior, when a superhydrophobic state is simultaneously sticky (high-adhesive), is typical for the “rose petal effect” inherent to a variety of biological objects which have been subjected recently to the intensive experimental and theoretical research29,30,31.
Increased hydrophilicity, resulting in decreasing the apparent contact angle, caused by cold plasma treatment is well-known for synthetic polymers1,2,3,4,5,6,7,8,9,10. However, the initial hydrophobicity of synthetic polymers is restored with time (this effect is called the “hydrophobic recovery”)10. It should be emphasized that we did not observe restored hydrophobicity while studying the seeds exposed to cold plasma. An absence of hydrophobic recovery was observed recently by our group for other biological objects i.e. keratin-built pigeon feathers and lycopodium15,32. The mechanism of hydrophobic recovery remains mysterious, and various physical and chemical hypotheses have been proposed for its explanation10,33.
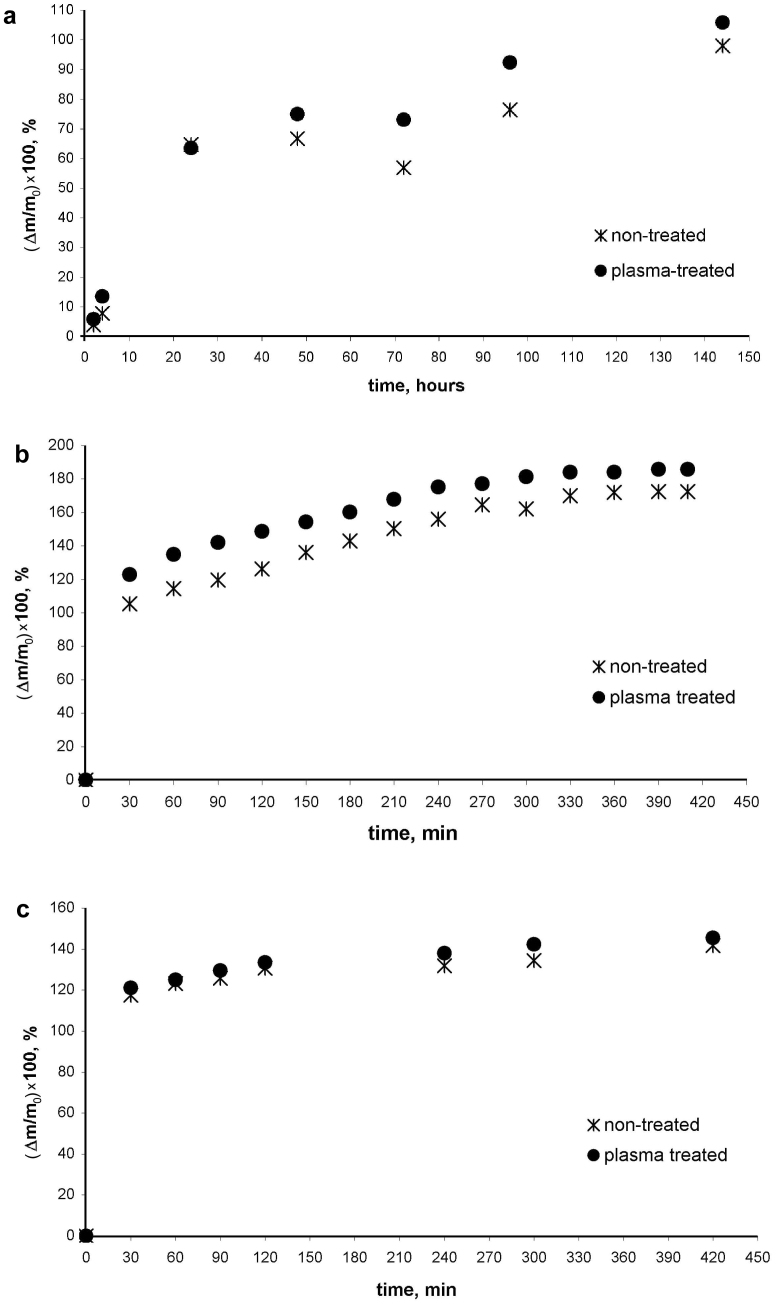
The change in the wettability of beans and lentils gave rise to a change in the water absorption (imbibition) of these seeds, presented by Figure 6a–b. These graphs demonstrate the time dependence of the average water absorption normalized by the initial masses of seeds. This change is very pronounced for lentils during the first two hours of the experiment; however, the saturation line for both the irradiated and the non-irradiated lentils is the same. For beans, the difference between the irradiated and non-irradiated seeds is noticeable throughout entire time span of the experiment. For wheat grains, only a slight (but resolvable within the accuracy of the experiment) increase of water imbibition was observed during 240–300 min of the experiment, as shown in Figure 6c.
Figure 6. The time dependence of water absorption (imbibition) by irradiated and non-irradiated (a) beans, (b) lentil seeds, (c) wheat grains.
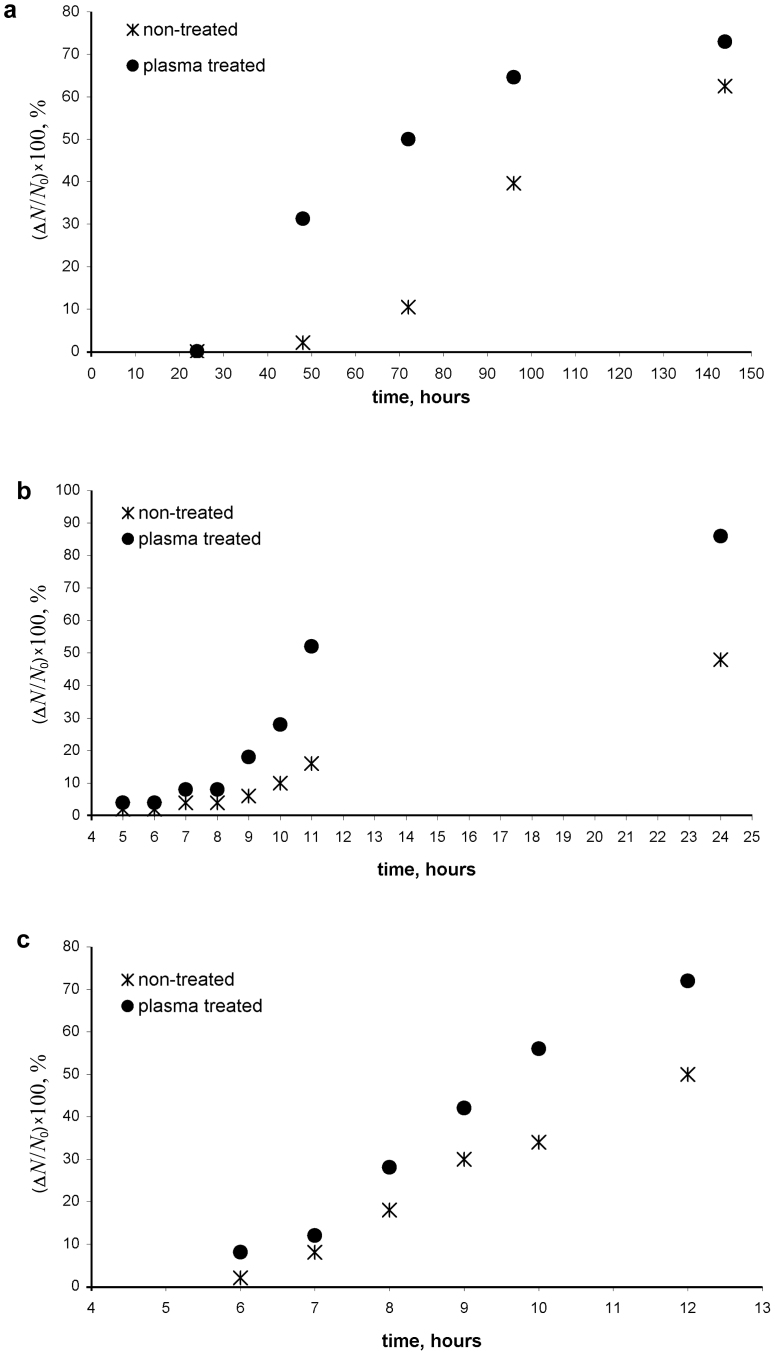
The change in water imbibition caused by plasma irradiation was noticeable, but the increase in the germination speed was dramatic, as shown in Figure 7a–c. And it was more pronounced for lentils and wheat grains. The processes induced by plasma treatment of biological objects are extremely complicated (and include amongst other effects of decontamination of seeds amongst other effects16,17); but it is reasonable to relate the observed drastic change in the germination speed at least partially to the plasma modification of the wetting properties of seeds, as discussed above. It should be stressed that the eventual germination rates corresponding to the saturation part of the curves presented in Figure 7a–c increased for all the kinds of seeds included in the study.
Figure 7. Time dependence of the germination speed of irradiated and non-irradiated (a) beans, (b) lentils, (c) wheat grains.
Discussion
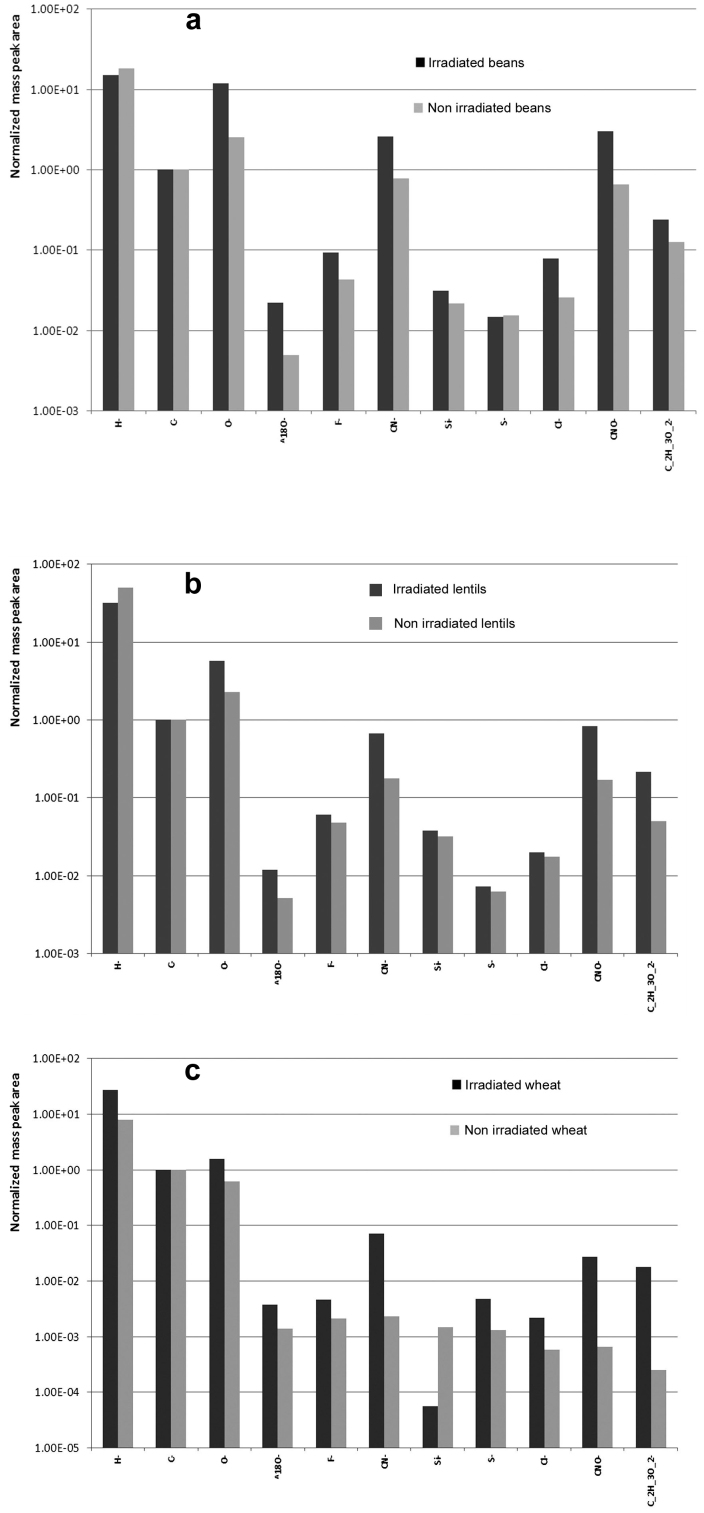
Modification of the seed germination performance through cold plasma treatment was investigated by Volin et al.34 They reported a significant delay in the germination speed of seeds treated by Fluorocarbon plasmas34. The results reported by Volin et al. are opposite to our findings34. This discrepancy can be understood if we consider that in our experiments, the air plasma was exploited. It is agreed that the wetting properties of organic surfaces are correlated with the amount of oxygen-containing functional groups at the sample surface3,4,35. Indeed, the negative ion spectra of the seed surfaces treated with plasma that were obtained with TOF-SIMS spectroscopy, demonstrated 2.5–3 times more intense mass peaks of oxygen than in the spectra of non-treated seeds of all kinds used in our study, as shown in Figure 8a–c (note, that the ordinate axis is represented in the logarithmic scale). Cold air plasma treatment in our experiments enriched the surface of seeds with oxygen containing functional groups. This resulted in the essential improvement in the wettability of seeds, and eventually influenced their germination speed. The delay of germination performance observed by Volin et al. in Ref. 34 obtains a natural explanation, if the increase in hydrophobicity of seeds exposed to Fluorocarbon plasmas is suggested. The significant growth of peaks corresponding to the CN− groups in the spectra of air plasma treated seeds is noteworthy. Volin et al. noted that incorporation of nitrogen onto the surface of seeds (see Figure 8a–c) has a positive effect on germination34.
Figure 8. TOF-SIMS mass spectrometry data comparing surface compositions of non-treated (grey columns) and plasma-treated (black columns) (a) beans, (b) lentils, (c) wheat seeds.
We also conclude that the modification of the wettability of seeds is not related to UV irradiation co-produced under plasma treatment. It looks reasonable to relate the phenomenon to the interaction of electrons and ions of plasma with the outer layer of a biological tissue.
A lot of questions remain open, and perhaps the most important of them is the influence of cold plasma treatment on the genetics of seeds. The increased eventual yield of plasma-treated seeds can be at least partially related to anti-microbial and antifungal activity of the cold plasma17. The precise role of the surface chemistry and biological factors in enhancing the yield has to be cleared up36. In particular, the O2 and CO2 permeability essential for germination could be modified by plasma treatment36,37,38. However, the reported results demonstrate that cold plasma treatment has a potential as a method of pre-treatment of seeds, increasing yield and controlling the germination speed. The increase in hydrophilicity of the treated seeds may save a significant amount of water necessary for irrigation. This makes the reported result even more actual. The absence of hydrophobic recovery makes possible a time span between plasma treatment and planting of seeds. It is reasonable to suggest that the existence of such a time span makes the proposed method of treatment of seeds flexible and convenient for agriculture.
We conclude that cold radiofrequency air plasma treatment of seeds supplied the effective method of modification of their surface properties including wettability. Plasma treatment leads to the dramatic decrease in the apparent contact angle of seeds. As a result, water imbibition of treated seeds increased. Perhaps, the most important result is the increase in the eventual germination rate (yield) of all kinds of seeds used in the investigation. TOF-SIMS spectroscopy has shown the significant increase in the concentration of oxygen- and nitrogen-containing groups at the surface of the plasma treated seeds. It is reasonable to relate the change of wettability of seeds to oxidation of their surfaces under plasma treatment. The absence of hydrophobic recovery was registered for the studied seeds.
Methods
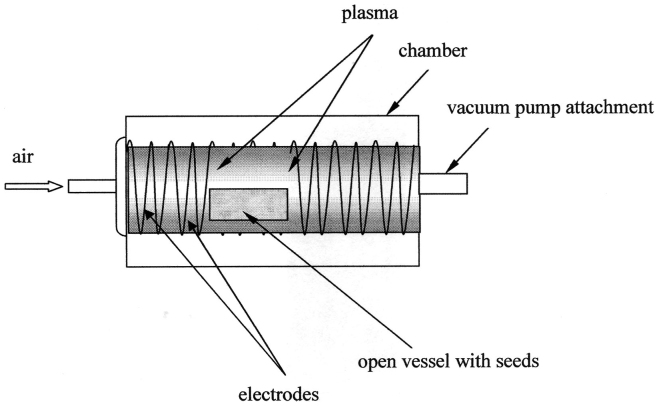
Lentils (Lens culinaris), beans (Phaseolus vulgaris) and wheat (Triticum species C9) grains were exposed to inductive air plasma discharge under the following parameters: the plasma frequency was of the order of 10 MHz, the power was 20 W, the pressure was 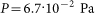 , the volume of the discharge chamber was 45 cm3. The time span of irradiation was varied from 15s to 2 min. The scheme of the experimental unit used for plasma treatment of seeds is depicted in Figure 9. Series of 10 experiments were carried out for all kinds of seeds.
, the volume of the discharge chamber was 45 cm3. The time span of irradiation was varied from 15s to 2 min. The scheme of the experimental unit used for plasma treatment of seeds is depicted in Figure 9. Series of 10 experiments were carried out for all kinds of seeds.
Figure 9. Scheme of the plasma treatment of seeds.
After exposure to plasma the seeds were imaged by high resolution SEM (JSM-6510 LV) once more. Irradiated and non-irradiated seeds were also imaged by environmental scanning electron microscopy (ESEM), carried out with a Quanta 200 FEG (field emission gun) ESEM microscope.
The wetting properties of lentils and beans were established using a Ramé-Hart goniometer (model 500). Ten measurements were taken to calculate the mean apparent contact angles for both kinds of seeds. The plasma treatment was carried out under low vacuum conditions. Thus, it was necessary to study the influence of air evacuation on the wettability of seeds and grains. Seeds and grains were evacuated under the aforementioned pressure, corresponding to the conditions of plasma treatment, and the apparent contact angles were measured as described above. We established that evacuation did not influence the wetting properties of seeds and grains. For the study of hydrophobic recovery apparent contact angles were measured every day during one month after exposure of seeds to the plasma treatment.
For the study of the impact of UV radiation on the wetting properties of seeds they were exposed to UV radiation produced by Camag UV lamp during 2 min. at the wavelength of 254 nm at the ambient conditions, the temperature was 24°C, the relative humidity was 30–40%. Apparent contact angles of UV-irradiated seeds were established as described above.
For the study of the time dependence of water absorption (imbibition) by irradiated and non-irradiated beans, lentils and wheat grains (48 seeds of every kind) were placed on humid cotton batting at ambient conditions; the temperature was 24°C. Beans were weighed every two hours with a MRC ASB-220-C2 analytical balance. The relative water imbibition (absorption) was defined as: 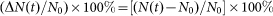 , where N0 is the total initial number of seeds, and N(t) is the running total number of germinated seeds.
, where N0 is the total initial number of seeds, and N(t) is the running total number of germinated seeds.
For the study of the time dependence of the germination speed of irradiated and non-irradiated beans, lentils and wheat grains (48 seeds of every kind) were placed on humid cotton batting at ambient conditions; the temperature was 24°C, the relative humidity was 30–40%. Germination of seeds was determined when a distinct (visible to the eye) sprout appeared (for grounding this point as the “germination time” see Ref. 38). The relative portion of germinated seeds was plotted as a function of time.
TOF-SIMS mass spectrometry of non-treated and treated seeds was carried out with the TOF SIMS5 Instrument (ION-TOF GmbH, Germany). The analysis beam was Bi1+; the sputter beam was 500 eV Cs+ beam. Electron flooding for sample charging neutralization was applied. The secondary negative ions spectra have been recorded in the static mode from 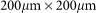 sample areas. All peak areas were normalized to C- mass peak area. Seeds were supplied by Sugat Co (Israel).
sample areas. All peak areas were normalized to C- mass peak area. Seeds were supplied by Sugat Co (Israel).
Author Contributions
EB analyzed data and proposed experimental concepts. RG carried out plasma treatment of seeds and measured the water imbibition and germination rate. YB studied wetting properties of the seeds. ED analyzed data from the biological point of view. All authors reviewed the manuscript.
Acknowledgments
We are thankful to Mrs. N. Litvak for the high resolution SEM and to Dr. Z. Barkay for the ESEM imaging of seeds. We are indebted to Dr. A. Gladkikh and Dr. C. Cytermann for TOF-SIMS study of the seeds. We are thankful to Dr. G. Whyman and Mrs. A. Musin for their help in preparing this manuscript.
References
- Yasuda H. Plasma for modification of polymers. Journal of Macromolecular Science: A 10, 383–420 (1976). [Google Scholar]
- Plasma surface modification of polymers: Relevance to adhesion, ed. by M. Strobel, C. S. Lyons, K. L. Mittal, VSP, Utrecht, 1994.
- France R. M. & Short R. D. Plasma treatment of polymers: the effects of energy transfer from an argon plasma on the surface chemistry of polystyrene, and polypropylene. A high-energy resolution X-ray photoelectron study. Langmuir 14, 4827–4835 (1998). [Google Scholar]
- France R. M. & Short R. D. Effects of energy transfer from an argon plasma on the surface chemistry of poly (styrene), low density poly(ethylene), poly(propylene) and poly(ethylene terephthalate. J. Chem. Soc., Faraday Transactions 93, 3173–3178 (1997). [Google Scholar]
- Wild S. & Kesmodel L. L. High resolution electron energy loss spectroscopy investigation of plasma-modified polystyrene surfaces. J. Vac. Sci. Technology 19, 856–860 (2001). [Google Scholar]
- Kondoh E., Asano T., Nakashima A. & Komatu M. Effect of oxygen plasma exposure of porous spin-on-glass films. J. Vac. Sci. Technology 18, 1276–1280 (2000). [Google Scholar]
- Fernández-Blázquez J. P., Fell D., Bonaccurso E. l. & del Campo A. Superhydrophilic and superhydrophobic nanostructured surfaces via plasma treatment. J. Colloid and Interface Science 357, 234–238 (2011). [DOI] [PubMed] [Google Scholar]
- Hegemann D., Brunner H. & Oehr,. Ch. Plasma treatment of polymers for surface and adhesion improvement. Nuclear Instruments and Methods in Physics Research B 208, 281–286 (2003) [Google Scholar]
- Balu B., Breedveld V. & Hess D. W. Fabrication of “Roll-off” and “Sticky” superhydrophobic cellulose surfaces via plasma processing. Langmuir 24, 4785–4790 (2008). [DOI] [PubMed] [Google Scholar]
- Kaminska A., Kaczmarek H. & Kowalonek J. The influence of side groups and polarity of polymers on the kind and effectiveness of their surface modification by air plasma action. European Polymer Journal 38, 1915–1919 (2002). [Google Scholar]
- Major S., Kumar S., Bhatnagar M. & Chopra K. L. Effect of hydrogen plasma treatment on transparent conducting oxides. Applied Physics Letters 49, 394–396 (1986). [Google Scholar]
- Lommatzsch U., Noeske M., Degenhardt J., Wubben T., Strudthoff S. G., Ellinghorst G. & Hennemann O.-D. Pretreatment and surface modification of polymers via atmospheric-pressure plasma jet treatment, in Polymer Surface Modification: Relevance to Adhesion, v. 4, ed. by K. L. Mittal, VSP, Leiden, 2007, pp. 25–32. [Google Scholar]
- Canal C., Molina R., Bertran E. & Erra P. Study on the influence of scouring on the wettability of keratin fibers before plasma treatment. Fibers and Polymers 9, 444–449 (2008). [Google Scholar]
- Molina R., Jovancic P., Jocic D., Bertran E. & Erra P. Surface characterization of keratin fibres treated by water vapour plasma. Surf. Interface Anal. 35, 128–135 (2003). [Google Scholar]
- Bormashenko, Ed. & Grynyov, R. Plasma treatment induced wetting transitions on biological tissue (pigeon feathers). Colloids and Surfaces B 92, 367– 371 (2012). [DOI] [PubMed] [Google Scholar]
- Stoffels E., Sakiyama Y. & Graves D. B. Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Transactions on Plasma Science 36, 1441–1451 (2008). [Google Scholar]
- Selcuk M., Oksuz L. & Basaran P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresource Technology 99, 5104–5109 (2008). [DOI] [PubMed] [Google Scholar]
- Friedman G., Gutsol A., Shekhter A., Vasilets V. N. & Fridman A. Applied plasma medicine. Plasma Process. Polym. 5, 503–533 (2008). [Google Scholar]
- Sotomayor,. Cr.,. Frias J., Fornal J., Sadowska J., Urbano G. & Vidal-Valverde C. Lentil starch content and its microscopical structure as influenced by natural fermentation. Starch 5, 152–156 (1999). [Google Scholar]
- de Gennes P. G., Brochard-Wyart F. & Quéré D. Capillarity and Wetting Phenomena; Springer, Berlin, 2003. [Google Scholar]
- Marmur A.. A guide to the equilibrium contact angles maze, in Contact Angle Wettability and Adhesion, V. 6, pp.3-18, ed. by K. L. Mittal, Brill/VSP, Leiden, 2009. [Google Scholar]
- Wenzel R. N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 28, 988–994 (1936). [Google Scholar]
- Cassie A. B. D. Contact angles. Discuss. Faraday Soc. 3, 11–16 (1948). [Google Scholar]
- Bormashenko E. Wetting transitions on biomimetic surfaces. Phil. Trans. Royal Society A 368, 4695–4711 (2010). [DOI] [PubMed] [Google Scholar]
- Kessler F., Kuhn S., Radtke,. Cl. & Weibel D. E. Controlling the surface wettability of poly(sulfone) films by UV-assisted treatment: benefits in relation to plasma treatment. Polymer International (2012) DOI 10.1002/pi.4302. [Google Scholar]
- Bormashenko,. Ed.,. Pogreb R., Whyman G., Bormashenko,. Ye.,. Jager R., Stein T., Schechter A. & Aurbach D. The Reversible giant change in the contact angle on the polysulfone and polyethersulfone films exposed to UV irradiation. Langmuir 24, 5977–5980 (2012). [DOI] [PubMed] [Google Scholar]
- Barthlott W. & Neinhuis C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202, 1–8 (1997). [Google Scholar]
- Quéré D. & Reyssat M. Non-adhesive lotus and other hydrophobic materials. Phil. Trans. R. Soc A 366, 1539–1556 (2008). [DOI] [PubMed] [Google Scholar]
- Feng L., Zhang,. Ya.,. Xi J., Zhu Y., Wang N., Xia F. & Jiang L. Petal Effect: A superhydrophobic state with high adhesive force. Langmuir 24, 4114–4119 (2008). [DOI] [PubMed] [Google Scholar]
- Bhushan B. & Nosonovsky M. The rose petal effect and the modes of superhydrophobicity. Phil. Trans. Royal Society A 368, 4713–4728 (2010). [DOI] [PubMed] [Google Scholar]
- Bormashenko E., Stein T., Pogreb R. & Aurbach D. “Petal Effect” on surfaces based on lycopodium: High-stick surfaces demonstrating high apparent contact angles. J. Phys. Chem. C 113, 5568–5572 (2009). [Google Scholar]
- Bormashenko, Ed. & Grynyov, R. Plasma treatment allows water suspending of the natural hydrophobic powder (lycopodium). Colloids & Surfaces B 97, 171–174 (2012). [DOI] [PubMed] [Google Scholar]
- Mortazavi M. & Nosonovsky M. A model for diffusion-driven hydrophobic recovery in plasma treated polymers. Applied Surface Science 258, 6876–6883 (2012). [Google Scholar]
- Volin J. C., Denes F. S., Young R. A. & Park S. M. T. Modification of seed germination performance through cold plasma chemistry technology. Crop. Sci 40, 1706–1718 (2000). [Google Scholar]
- Occhiello E., Morra M. & Garbassi F. SSIMS studies of hydrophobic recovery: oxygen plasma treated PS. Applied Surface Science 47, 235–242 (1991). [Google Scholar]
- Toole E. H., Hendricks S. B., Borthwick H. A. & Toole V. K. Physiology of Seed Germination. Annual Review of Plant Physiology 7, 299–324(1956). [Google Scholar]
- Kawakami M., Yamashita,. Yu., Iwamoto M. & Kagawa S. Modification of gas permeabilities of polymer membranes by plasma coating. Journal of Membrane Sci. 19, 249–258 (1984). [Google Scholar]
- Bewley D. & Black M. Seeds. Physiology of Development and Germination, Plenum Press, New York, 1994.