Abstract
The objective of this study was to assess the radiation exposure levels in victims of a 60Co radiation accident using chromosome aberration analysis and the micronucleus assay. Peripheral blood samples were collected from three victims exposed to 60Co 10 days after the accident and were used for the chromosome aberration and micronucleus assays. After in vitro culture of the lymphocytes, the frequencies of dicentric chromosomes and rings (dic+r) and the numbers of cytokinesis blocking micronuclei (CBMN) in the first mitotic division were determined and used to estimate radiation dosimetry. The Poisson distribution of the frequency of dic+r in lymphocytes was used to assess the uniformity of the exposure to 60Co radiation. Based on the frequency of dic+r in lymphocytes, estimates of radiation exposure of the three victims were 5.61 Gy (A), 2.48 Gy (B) and 2.68 Gy (C). The values were estimated based on the frequencies of CBMN, which were 5.45 Gy (A), 2.78 Gy (B) and 2.84 Gy (C). The estimated radiation dosimetry demonstrated a critical role in estimating the radiation dose and facilitating an accurate clinical diagnosis. Furthermore, the frequencies of dir+r in victims A and B deviated significantly from a normal Poisson distribution. Chromosome aberration analysis offers a reliable means for estimating biological exposure to radiation. In the present study, the micronucleus assay demonstrated a high correlation with the chromosome aberration analysis in determining the radiation dosimetry 10 days after radiation exposure.
As early as the mid-1960s, ionising radiation was known to be capable of inducing chromosome aberrations in the metaphase of human peripheral lymphocytes [1, 2]. Since then, the chromosome aberration assay has been widely used as a sensitive biomarker for dose reconstruction following radiation exposure [3–6]. In particular, the analysis of dicentric chromosomes and rings (dic+r), two aberrations exemplifying inter- and intrachromosomal exchanges, respectively, has been generally considered to be the standard means for estimating biodosimetry based on its well-established dose–response relationship with radiation exposure and its low baseline levels in the general population [7, 8]. However, the utility of the chromosome aberration assay relies heavily on the expertise and experience of individual investigators. As an alternative method for determining radiation biodosimetry, the cytokinesis blocking micronucleus (CBMN) assay is easy to perform and less time-consuming, but its drawbacks include large individual variations in baseline values in the general population [7] and a markedly lower sensitivity when used for estimating low dose exposure compared with the chromosome aberration assay. Therefore, the micronucleus assay is widely used as a supplement to the chromosome aberration analysis [9]. In the present study, we made a comparative estimate of the radiation exposure in three victims of a 60Co radiation accident that occurred in 1999 using dose–response curves of γ-ray-induced chromosome aberration and micronuclei, which were previously established in our laboratory. The results provide evidence of the utility of both methods in estimating biological doses of radiation exposure in humans.
Materials and methods
Subjects
A 60Co radiation accident occurred on 26 April, 1999, in a village in the Henan Province, China. In short, a stainless steel stick with a 60Co radiation source was purchased in an illegal transaction without the buyer's knowledge of its radioactivity. The radiation source was kept in the buyer's bedroom. Three victims of this accident (A, B and C), who had been exposed to radiation for 1–4 h and experienced nausea and vomiting were enrolled in the present study. Subject A was a female aged 38 years who presented with a severe form of acute radiation sickness (bone marrow suppression); her 8-year-old son (Subject B) and 37-year-old husband (Subject C) both presented with a moderate level of acute radiation sickness (bone marrow suppression). A paper about the patients' clinical progress of these cases has been published in the Journal of Radiation Research [10]. Prior to the study, written informed consent was obtained from all subjects.
Reagents and instruments
Cytochalasin B, colchicines and Giemsa stain were purchased from Sigma-Aldrich (St Louis, MO). A Nikon 90i fluorescence microscope was purchased from Nikon (Tokyo, Japan). The chromosome image analysis system was purchased from United Scientific USA, Inc. (Cherry Hill, NJ); the SANYO MCO-20AIC CO2 incubator was purchased form SANY (Sakata, Japan).
Cell culture and sample preparation
Procedures for lymphocyte culture were essentially the same as described previously in an International Atomic Energy Agency (IAEA)-405 report [8]. A 0.3 ml sample of venous blood was collected from each subject 10 days after the accident in a heparinised syringe, then added to 4 ml of RPMI-1640 medium containing 20% of bovine calf serum and 50 μg ml−1 of phytohaemagglutinin; the mixture was incubated at 37°C in a 5% CO2 incubator for 24 h. The blood cultures were then divided into two aliquots for chromosome aberration analysis and micronucleus assay. For the former analysis, a final concentration of 0.06 mg ml−1 of colchicines was added to the culture, and, after being cultured for an additional 32 h, the lymphocytes were harvested and subjected to fixing and Giemsa staining in accordance with the method described previously [8]. Dicentric chromosomes (dic) and rings (r) were scored under a light microscope.
For the micronucleus assay, cells were cultured for 44 h before a final concentration of 6 μg ml−1 cytochalasin B was added to the culture. The lymphocytes were harvested and subjected to fixing and Giemsa staining 28 h later, according to the method described previously [8]. Micronuclei in 1000 bi-nucleated cells per subject were scored using the Giemsa-stained slides [8].
Biological dosimetry
The dose was estimated for each subject using the dose–response curves of 137Cs γ-ray-induced chromosome aberration (dic+r) and micronuclei. The dose–response data used in the present study curves were previously established in our laboratory as follows:
| (1) |
| (2) |
Y1 denotes the number of dic+r in each lymphocyte, D is the radiation dose (Gy) and Y2 denotes the frequency of micronuclei (‰). The dose range of the above two curves was 0.5–5.0 Gy. The mean radiation dose and its 95% confidence interval were calculated for each subject based on the two equations.
Statistical analysis
A u-test of the Poisson distribution of dic+r was used to determine whether the subjects received uniform radiation. The u and σ2 values were obtained using the following equations:
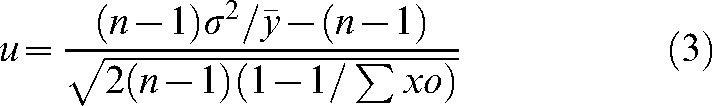 |
(3) |
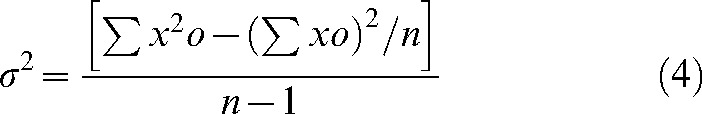 |
(4) |
where σ2 denotes variance, n is the number of lymphocytes, σxo is the total number of dic+r observed, x is the number of dicentric chromosomes in each lymphocyte, o is the number of lymphocytes observed and 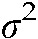 denotes the mean value (σxo/n).
denotes the mean value (σxo/n).
Results
Estimation of biological doses based on chromosome aberration analysis
The results of chromosome aberration analysis and estimates of biological dosimetry for each subject are presented in Table 1. The frequencies of dic+r in the three subjects were significantly higher than the reported 0.1–2.1‰ of spontaneous aberration frequency in general populations [11], demonstrating that the three subjects were exposed to a high dose of radiation. Because the number of peripheral lymphocytes in subject A was markedly reduced 10 days after the radiation, the number of harvested lymphocytes after culture was also low; only 150 lymphocytes in metaphase were analysed.
Table 1. Chromosome aberration analysis and estimates of biological dosimetry.
| Subject | Sex | Age (years) | Number of lymphocytes examined | Dicentric chromosomes and rings |
Estimated radiation dose (Gy) | 95% confidence interval | |
| Total count | Number per cell | ||||||
| A | Female | 38 | 150 | 357 | 2.38 | 5.61 | 2.29–5.90 |
| B | Male | 8 | 300 | 154 | 0.51 | 2.48 | 2.26–2.68 |
| C | Male | 37 | 300 | 178 | 0.59 | 2.68 | 2.46–2.89 |
Determination of the pattern of radiation exposure
Based on the results of the u-test of the Poisson distribution of dic+r, and the distribution of dic+r in the lymphocytes (Table 2) of the three victims exposed to 60Co radiation, subject C received uniform radiation, whereas subjects A and B were exposed to radiation in a non-uniform manner (Table 3).
Table 2. Distribution of dicentric chromosomes and rings in lymphocytes.
| Subject | Cell number | Number of dicentric chromosomes and rings per cell |
||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total | ||
| A | 150 | 33 | 26 | 29 | 22 | 16 | 11 | 7 | 3 | 2 | 1 | 357 |
| B | 300 | 195 | 68 | 27 | 8 | 2 | 0 | 0 | 0 | 0 | 0 | 154 |
| C | 300 | 170 | 97 | 21 | 9 | 3 | 0 | 0 | 0 | 0 | 0 | 178 |
Table 3. Results of the u-test of Poisson distribution of dicentric chromosomes and rings.
| Subject | 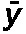 |
σ2/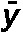
|
u | Uniformity coefficient |
| A | 2.38 | 1.77 | 6.70 | Non-uniform |
| B | 0.51 | 1.31 | 3.80 | Non-uniform |
| C | 0.59 | 1.15 | 1.87 | Uniform |
Dose estimation by another laboratory
4 days after the accident, the blood samples of the three affected individuals were obtained by the Institute of Occupational Disease Prevention of the Henan Province, China. Dose estimation was performed by chromosome aberration and the results are presented in Table 4. The estimated dose is similar to the results of our laboratory.
Table 4. Chromosome aberration analysis and dose estimation performed by another laboratory.
| Subject | Number of lymphocytes examined | Dicentric chromosomes and rings |
Estimated radiation dose (Gy) | 95% confidence interval | Poisson distribution | |
| Total count | Number per cell | |||||
| A | 40 | 79 | 1.98 | 5.09 | 4.46–5.64 | No |
| B | 217 | 113 | 0.52 | 2.49 | 2.23–2.74 | No |
| C | 334 | 188 | 0.56 | 2.61 | 2.40–2.80 | Yes |
Estimation of biological doses based on the micronucleus assay
The estimated mean radiation doses and 95% confidence intervals as obtained from Equation (2) are presented in Table 5. Because the number of peripheral lymphocytes in subject A was markedly reduced, the number of the harvested lymphocytes after culture was limited for the assay; only 140 binucleated lymphocytes were analysed.
Table 5. The frequencies of micronuclei in the three subjects and estimated biological doses.
| Subject | Number of binucleated lymphocytes | Frequency of micronuclei (p±sp ‰) | Estimated radiation dose (Gy) | 95% confidence interval |
| A | 140 | 500±42.3 | 5.45 | 4.28–6.62 |
| B | 1000 | 265±14.0 | 2.78 | 2.42–3.13 |
| C | 1000 | 271±14.1 | 2. 84 | 2.49–3.21 |
p, rate of sample (frequency of micronuclei); sp, standard error of the ratio.
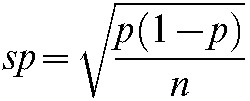 |
Distribution of micronucleated cells
As shown in Table 6, the lymphocytes with multiple micronuclei accounted for 37.8% of the total lymphocytes examined in subject A, whereas the proportions in subjects B and C were 23.0% and 19.1%, respectively. The above findings indicate that more lymphocytes with multiple micronuclei are observed in subjects who received higher doses of radiation. It should be noted that the number of lymphocytes with two or more micronuclei contributed greatly in the estimates of biological doses. The u-test of Poisson distribution of micronuclei was also performed and the results are shown in Table 7. As is shown in Table 7, all three victims received non-uniform exposure.
Table 6. Distribution of micronucleated cells in the three subjects.
| Subject | Lymphocytes with MN | 1 MN |
2 MN |
3 MN |
4 MN |
||||
| Counts | % | Counts | % | Counts | % | Counts | % | ||
| A | 45/140 | 28 | 62.2 | 10 | 22.2 | 6 | 13.3 | 1 | 2.3 |
| B | 196/1000 | 151 | 77.0 | 33 | 16.8 | 10 | 5.1 | 2 | 1.0 |
| C | 220/1000 | 178 | 80.9 | 35 | 15.9 | 5 | 2.3 | 2 | 0.9 |
MN, micronuclei.
Table 7. The results of the u-test of Poisson distribution of micronucleus.
| Subject | 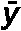 |
σ2/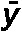
|
u | Uniformity coefficient |
| A | 0.32 | 2.76 | 14.87 | non-uniform |
| B | 0.20 | 1.87 | 19.49 | non-uniform |
| C | 0.22 | 1.57 | 12.74 | non-uniform |
Dose estimation using the CABAS-2 software [12]
Deperas et al [12] developed the CABAS software, which consists of the main curve-fitting and dose-estimating module, and modules for calculating the dose in cases of partial body exposure, for estimating the minimum number of cells necessary to detect a given dose of radiation and for calculating the dose in the case of a protracted exposure. Using the CABAS-2 software, we estimated the yield to exposed fraction, the partial body dose and other exposure measurements (Table 8). The results are similar to those shown in Tables 1 and 3.
Table 8. The results of dose estimation using CABAS-2.
| Subject | Yield to exposed fraction | Partial body dose (Gy) | Percentage of body exposed | Whole body dose (Gy) | u-value | 95% confidence interval (Gy) | Poisson distribution |
| A | 2.88 | 6.19 | 97.90 | 5.61 | 6.69 | No | |
| B | 0.82 | 3.19 | 84.43 | 2.48 | 3.79 | 2.26–2.70 | No |
| C | 2.68 | 1.86 | 2.47–2.90 | Yes |
Discussion
The dose was estimated for each subject using the dose–response curves of 137Cs γ-ray-induced chromosome aberration (dic+r) and micronuclei. In this accident, the dose rate of the 60Co source at a distance of 1 m away was 48.7 R min−1 [10] and the dose rate of our 137Cs source at the same distance was 49.5 R min−1 when the response curves were fitted. The dose rate of the two radiation sources is more important than the contribution of the radiation dose. Considering the different energy of the two sources, it might have little effect on the dose estimation. For the biological dose estimation, the error at this level is acceptable.
The application of chromosome aberration analysis to the estimation of biological dosimetry dates back more than 40 years, and has now been accepted as the most reliable means for estimating the radiation dose [8]. Its reliability and sensitivity in biodosimetry have been documented in investigations of several large radiation accidents [5, 9, 10, 13–17]. Furthermore, it may provide valuable dosimetry information to facilitate clinical diagnosis when individual dosimetry data are missing. In the present study, chromosome aberration analysis demonstrated a critical role in estimating the radiation dose and facilitating an accurate clinical diagnosis. Several previous studies have found that the distribution pattern of dic+r exhibits a Poisson distribution among radiated lymphocytes in subjects uniformly exposed to low linear energy transfer (LET) radiation [6, 8], whereas the pattern of dic+r distribution does not follow a Poisson distribution in subjects non-uniformly or locally exposed to LET radiation. Of the three subjects in the present study, C received uniform radiation, whereas A and B both received non-uniform radiation exposure. It should be noted that the wider 95% confidence interval for estimating the biodosimetry for subject A, using the dic+r aberrations, may be partly derived from the non-uniform radiation exposure and a smaller number of lymphocytes in metaphase available for the analysis.
The cytokinesis-blocked micronucleus assay demonstrated a good dose–response profile in the early stage of radiation exposure. Scoring of micronuclei in binucleated lymphocytes provides an easy yet rapid method for assessing the absorbed radiation dose. However, a large variation in background levels of micronuclei in the general population [7] has limited its use in estimating low dose radiation exposure where chromosome aberration analysis is more frequently employed. In the present study, the biodosimetry, as measured by dic+r and CBMN for each subject, was fully consistent with the clinical diagnoses, which included severe bone marrow forms of acute radiation sickness for subject A and more moderate forms for subjects B and C. In addition, the results obtained by the two methods showed a high level of correlation, suggesting that micronucleus assay and chromosome aberration analysis are both capable of providing reliable and accurate dosimetry data within a certain period post-irradiation [9]. However, the u-test of Poisson distribution on the micronucleus data was performed using Equations 3 and 4, and the results showed that all three victims received non-uniform exposure. The reason for this may be that there are so many influencing factors that can affect the results of a micronucleus test. For example, smoking is an important factor that can affect the results. Of all of the exposed individuals, case C had a smoking history of more than 10 years. This might have influenced the results. On the other hand, Equations 3 and 4 may not be fit for the micronucleus data.
In this accident, 10 days elapsed from the day of exposure to the day that the blood samples were taken. In the Istanbul accident [9], which had a similar delayed discovery and where the patients experienced marked leucopenia, the dicentric assay underestimated the dose. This also applies to the micronucleus assay, as both measure unstable damage in lymphocytes. In Istanbul, FISH (fluorescence in situ hybridisation) was also done and showed about 20% higher doses than dicentrics [9]. In IAEA report No 405, it was reported that the blood sample should be obtained before 4 weeks have elapsed, since after this time aberration yields begin to fall, causing greater uncertainty in any estimates of the radiation dose [8]. In the present study, there was a delay of 10 days from the exposure until the blood sample was obtained. Some uncertainty in the dose estimation may have occurred, but this did not influence the diagnosis of the victims.
In general, a reliable and accurate estimation of radiation dosimetry using dic+r can be achieved within the radiation dose range of 0.1–5.0 Gy, whereas the range is 0.25–5.0 Gy for the CBMN assay. When the radiation dose is higher than 5 Gy, there are fewer lymphocytes in metaphase, making it less likely that an accurate estimation of absorbed radiation can be made. Hayata et al [18] developed CHROSY (a culture and harvest robotic system) capable of recovering lymphocytes efficiently for performing chromosome aberration analysis. This system has demonstrated its use in collecting a large number of lymphocytes in metaphase even after exposure to large doses of radiation, thus enabling the estimation of radiation doses as high as 10 Gy. In addition, Kanda et el [19] proposed that premature condensed ring chromosome can be used for the assessment of biological radiation dose, achieving accurate estimates of radiation exposure for doses as high as 20 Gy. The latter method was found to be effective in determining radiation dosimetry in the critical nuclear accident that occurred in Japan in 1999 [20].
For estimating radiation dose, uniform whole-body equivalent and dose estimation in partial body exposures, excellent software are available. The results obtained with CABAS-2 are consistent with the results that were calculated manually. The yield to exposed fraction and partial body dose were more important to dose estimation for subjects A and B. Moreover, the data about the irradiated fraction and dose to the irradiated fraction has been published in another paper from our institute [21]. The use of this program is convenient, and we expect that its use will improve the precision of dose estimates by biological dosimetry in cases of radiation accidents [12].
Cytogenetic biodosimetry has been applied for a long time to obtain absorbed dose estimates in real or suspected overexposures. Biological monitoring of humans exposed to ionising radiation has relied heavily on cytogenetic indicators. In particular, the measurement of dicentric chromosome is considered to be the standard method for biodosimetry [22]. Scoring of micronuclei is easier and considerably faster than dicentric scoring and can be automated using computerised image analysis systems [7]. It is a valuable technique for rapid screening for triage purposes, especially in radiological accidents involving a large number of casualties.
Conclusions
In summary, the results from the present study indicate that both chromosome aberration analysis and the micronucleus assay provide a reliable estimate for biological exposure to radiation, which are shown to have a critical role in estimating the radiation dose and facilitating an accurate clinical diagnosis. This may enable faster and more reliable estimation of radiation exposure, leading to better treatment for patients.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 30800281), the Natural Science Foundation of Tianjin (No. 07JCYBJC09200) and the Development Foundation of Institute of Radiation Medicine, Chinese Academy of Medical Sciences and Peking Union Medical College (Nos. SF0818, SF0820). The authors are grateful to Prof. JX Wang and the language editing services of Medjaden Co.
Footnotes
Qiang Liu and Jia Cao contributed equally to this study and should be considered as co-first authors.
References
- 1.United Nations Report of the United Nations Scientific Committee on the Effect of Atomic Radiation. General Assembly Document, 24th Session, Annex C. Radiation-induced chromosome aberrations in human cells. Suppl. No. 13 New York, NY: United Nations, 1969: 98–155. [Google Scholar]
- 2.Lloyd DC, Purrott RJ, Dolphin GW. Chromosome aberration dosimetry using human lymphocytes in simulated partial body irradiation. Phys Med Biol 1973;18:421–31 [DOI] [PubMed] [Google Scholar]
- 3.Lloyd DC. An overview of radiation dosimetry by conventional cytogenetic methods. In: Eisert WG, and Mendelsohn ML, editors. Biological dosimetry. Berlin, Germany: Springer, 1984: 3–14 [Google Scholar]
- 4.Sasaki MS. Cytogenetic biomonitoring of human radiation exposures: possibilities, problems and pitfalls. J Radiat Res 1992;33:44–53 [DOI] [PubMed] [Google Scholar]
- 5.Lloyd DC, Edwards AA. Biological dosimetry after radiation accidents. In: Obe G, Natarajan AT, editors. Chromosome aberrations: basic and applied aspects. Berlin, Germany: Springer, 1989: 212–23 [Google Scholar]
- 6.Wojcik A, Gregoire E, Hayata I, Roy L, Sommer S, Stephane G, et al. Cytogenetic damage in lymphocytes for the purpose of dose reconstruction: a review of three recent radiation accidents. Cytogenet Genome Res 2004;104:200–05 [DOI] [PubMed] [Google Scholar]
- 7.Thierens H, De Ruyck K, Vral A, de Gelder V, Whitehouse CA, Tawn EJ, et al. Cytogenetic biodosimetry of an accidental exposure of a radiological worker using multiple assays. Radiat Prot Dosim 2005;113:408–414 [DOI] [PubMed] [Google Scholar]
- 8.International AtomicEnergyAgency Cytogenetic analysis for radiation dose assessment: a manual. Technical Report Series No. 405, Vienna, Austria: IAEA, 2001: 105–22. [Google Scholar]
- 9.International AtomicEnergyAgency The radiological accident in Istanbul. Vienna, Austria: IAEA, 2000: 42–3. [Google Scholar]
- 10.Liu Q, Jiang B, Jiang LP, Wu Y, Wang XG, Zhao FL, et al. Clinical report of three cases of acute radiation sickness from a 60Co radiation accident in Henan Province in China. J Radiat Res 2008;49:63–9 [DOI] [PubMed] [Google Scholar]
- 11.Wang JX, Jin CZ, Bai YS, Wang ZQ, editors Radiobiological dosimetry. Beijing, China: Atomic Energy Press, 1997: 19–26. [Google Scholar]
- 12.Deperas J, Szłuińska M, Deperas-Kaminska M, Edwards A, Lloyd D, Lindholm C, et al. CABAS – a freely available PC program for fitting calibration curves in chromosome aberration dosimetry. Radiat Prot Dosim 2007;124:115–23 [DOI] [PubMed] [Google Scholar]
- 13.International AtomicEnergyAgency The radiological accident in Gilan. Vienna, Austria: IAEA, 2002: 15–7. [Google Scholar]
- 14.International AtomicEnergyAgency The radiological accident in Cochabamba. Vienna, Austria: IAEA, 2004: 34–6. [Google Scholar]
- 15.International AtomicEnergyAgency The radiological accident at the irradiation facility in Nesvizh. Vienna, Austria: IAEA, 1996: 68. [Google Scholar]
- 16.Ramalho AT, Nascimento ACH, Natarajan AT. Dose assessments by cytogenetic analysis in the Goiania (Brazil) radiation accident. Radiat Prot Dosim 1988;25:97–100 [Google Scholar]
- 17.Littlefield LG, Joiner SP, Ricks RC, Lushbaugh CC, Hurtado-Monroy R. The 1989 San Salvador 60Co radiation accident: cytogenetic dosimetry and follow-up evaluation in three accident victims. Radiat Prot Dosim 1991;35:115–9 [Google Scholar]
- 18.Hayata I, Takuchi H, Furukawa A, Okabe N, Yamamoto M, Sato K. Robot system for preparing lymphocyte chromosome. J Radiat Res 1992;33:231–41 [DOI] [PubMed] [Google Scholar]
- 19.Kanda R, Hayata I, Lloyd DC. Easy biodosimetry for high-dose radiation exposures using drug-induced, prematurely condensed chromosomes. Int J Radiat Biol 1999;75:441–6 [DOI] [PubMed] [Google Scholar]
- 20.Hirohiko T, Makoto A. International symposium on the criticality accident in Tokaimura: medical aspects of radiation emergency proceedings. Chiba, Japan: National Institute of Radiological Sciences, 2000: 14–15, 71–101. [Google Scholar]
- 21.Xu ZY, Zhang LA, Dai GF. The estimation of absorbed doses received by a victim of a Chinese radiation accident. Radiat Prot Dosim 2003;103:163–7 [DOI] [PubMed] [Google Scholar]
- 22.Terzoudi GI, Pantelias GE. Cytogenetic methods for biodosimetry and risk individualisation after exposure to ionising radiation. Radiat Prot Dosim 2006;122:513–20 [DOI] [PubMed] [Google Scholar]


