Abstract
We report a case of a mucinous cystadenoma of the pancreas communicating with the main pancreatic duct. To our knowledge, this is the first case in which a communication between the mucinous cystadenoma and the main pancreatic duct could be demonstrated by MRI.
Mucinous cystic neoplasms (MCNs) of the pancreas are low-grade tumours and represent approximately 10% of pancreatic cysts and 1% of pancreatic neoplasms. MCN is a clinical and pathologic entity, distinctly different from intraductal papillary mucinous neoplasms (IPMNs). The absence of communication of the cyst with the pancreatic duct has been used by some as a criterion for diagnosing MCNs and differentiating these neoplasms from IPMNs [1].
We report a case of a mucinous cystadenoma communicating with the main pancreatic duct. To our knowledge, this is the first case in which a communication has been demonstrated on MRI.
Case presentation
A 56-year-old woman with no significant medical history presented for evaluation of left lumbar pain. She denied alcohol consumption, trauma and previous pancreatitis. She also denied significant nausea, vomiting, dyspepsia, diarrhoea and weight loss. Initial laboratory analyses, which included a complete blood cell count, liver function tests and measurement of electrolyte and pancreatic enzyme levels, were normal. Physical examination did not reveal a palpable mass.
Abdominal sonography demonstrated a large cystic mass in the left pancreas, with back wall enhancement. This mass was multilocular with bright foci within the septation. Sonography showed a markedly dilated main pancreatic duct (Figure 1).
Figure 1.
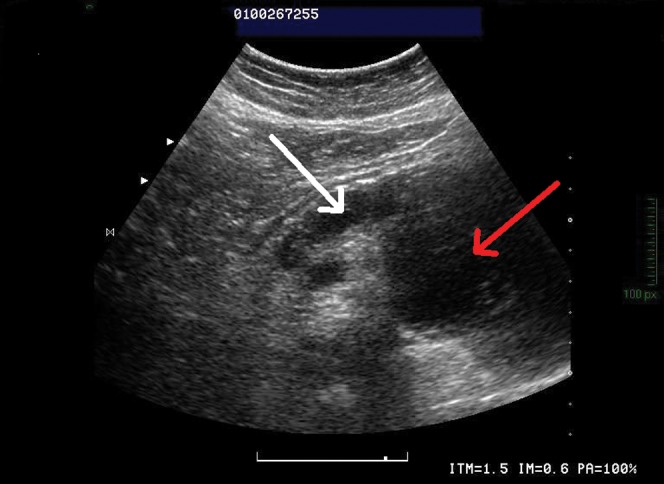
Abdominal sonography demonstrates a large cystic multilocular mass (red arrow) in the left pancreas and a markedly dilated main pancreatic duct (white arrow).
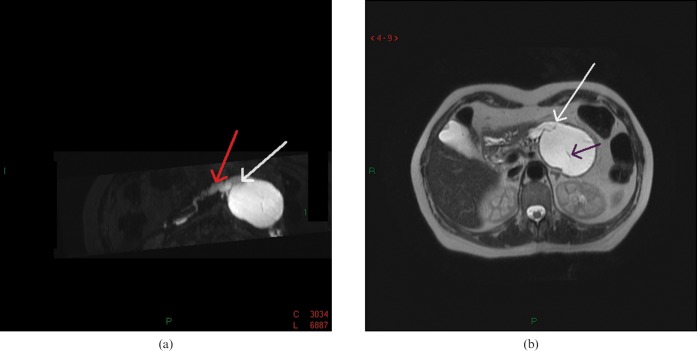
T2 weighted MRI revealed an overall high signal intensity of the lesion and low signal intensity of the internal septations. Two-dimensional (2D) and three-dimensional (3D) MR cholangiopancreatographies showed a communication between the cystic lesion and the dilated main pancreatic duct (Figure 2). The diameter of the main pancreatic duct gradually decreased up to the papilla. There was no dilatation of the side branch system in the head of the pancreas, or of the common bile duct. A 3D T1 weighted gradient echo sequence, performed after gadolinium contrast injection, showed a low-intensity mass with contrast enhancement of the capsule and the internal septae.
Figure 2.
(a) Two-dimensional and three-dimensional MR cholangiopancreatography demonstrates a communication between the cystic lesion (white arrow) and the dilated main pancreatic duct (red arrow). (b) T2 weighted MRI reveals the overall high signal intensity of the lesion and low signal intensity of the internal septations (black arrow). It also shows the communication between the cystic lesion and the dilated main pancreatic duct (white arrow).
From the MRI findings, and largely because of the communication with the main pancreatic duct, a diagnosis of IPMN was proposed.
The lesion was removed by means of laparoscopic partial distal pancreatectomy with spleen and splenic vessel conservation. The gross specimen showed an approximately 8 × 7 cm cystic tumour. Drainage of the multiple cysts produced fluid. The walls were composed of homogeneous greyish white and glistening tan tissue. No papillary or solid areas were present. The communication between the main pancreatic duct and the cystic tumour was confirmed.
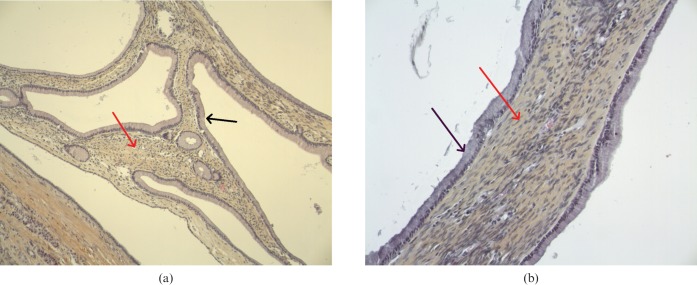
Histological examination of the cyst wall showed mucin-producing columnar epithelium overlying ovarian-type dense stroma and fibrous tissue (Figure 3). Extensive sampling revealed no area of malignancy. The histopathological diagnosis was MCN of the pancreas.
Figure 3.
(a,b) Histological examination of the cyst wall showed mucin-producing columnar epithelium (black arrow) overlying ovarian-type dense stroma and fibrous tissue (red arrow).
Discussion
The term “cystadenoma” was used to describe cystic neoplasms until 1978. At that time, the tumours were first classified and divided into serous cystic neoplasms and MCNs by Compagno and Oertel [2]. More recently, the mucinous tumours have been further subclassified into two separate entities, i.e. MCNs and IPMNs, by the World Health Organization (WHO) in 1996 [3] and the Armed Forces Institute of Pathology (AFIP) in 1997 [4].
MCNs are low-grade tumours that occur predominantly in middle-aged women, arise within the tail of the pancreas, and appear as large thick-walled septated cysts, with no communication with the ductal system. All MCNs are considered to be malignant neoplasms of low-grade malignant potential.
Histologically, these cysts are characterised by the presence of ovarian-type stroma (OS). Understandably, this maturation has led to confusion over their categorisation, management and prognosis. Since the landmark paper by Compagno and Oertel [2] in 1978, there have been many studies of MCNs in the literature; however, the diagnostic criteria adopted by these different studies were inconsistent, and so there was confusion between MCNs and IPMNs. A high rate of communication between the main pancreatic duct and MCNs was (wrongly) reported [5].
In his review of literature, Goh et al [6] demonstrated that when the definition of MCNs, as proposed by the WHO and AFIP, is adopted (the presence of OS is essential for this diagnosis), MCNs have unique clinical and pathological features. In a first group of 344 patients with MCNs defined by OS, MCNs occurred almost exclusively in females (99.7%), were almost always located in the body or tail of the pancreas (94.6%), and rarely communicated with the pancreatic duct (6.8 %). It confirmed, however, that a small proportion of MCNs may communicate with the pancreatic duct. In a study by Grogan et al [7], such a communication was due to the development of a fistula between the cyst and the pancreatic duct.
A review of the literature shows that the few cases of communication between MCN and the main pancreatic duct have been demonstrated only by endoscopic retrograde cholangiopancreatography, and thus reported in the surgical literature [5]. To our knowledge, there is no study in which MRI shows the communication between MCNs and the main pancreatic duct.
In this case, the diagnosis of IPMN was proposed at MRI because of the communication between the lesion and the main pancreatic duct. However, the MRI features of the cystic lesion (location in the body of the pancreas, macro cyst type, peripheral enhancement, internal septation) were strongly indicative of the diagnosis of MCN.
Furthermore, the absence of dilatation of the main pancreatic duct all the way to the ampulla was another factor counting against the diagnosis of IPMN.
Conclusions
In conclusion, the presence of a communication between the cystic lesion and the main pancreatic duct cannot be used as a definite criterion for diagnosing IPMN, as this can be observed in rare cases of MCN. MCNs are defined by the presence of OS in pathological examinations, as this is the necessary characteristic that differentiates MCNs from IPMNs.
References
- 1.De Lima J, Javitt M, Mathur S. Residents' teaching files: mucinous cystic neoplasm of the pancreas. RadioGraphics 1999;19:807–11 [DOI] [PubMed] [Google Scholar]
- 2.Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol 1978;69:573–80 [DOI] [PubMed] [Google Scholar]
- 3.Kloppel G, Solcia E, Longnecker DS. World Health Organization international classification of tumours. Histological typing of tumors of the exocrine pancreas, 2nd edn. Berlin, Germany: Springer, 1978:1–61. [Google Scholar]
- 4.Buetow PC, Rao P, Thompson LD. From the Archives of the AFIP. Mucinous cystic neoplasms of the pancreas: radiologic-pathologic correlation. RadioGraphics 1998;18:433–49 [DOI] [PubMed] [Google Scholar]
- 5.Le Borgne J, De Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg 1999;230:152–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goh BK, Tan YM, Chung YF, Chow PK, Cheow PC, Wong WK, et al. Intraductal papillary-mucinous neoplasms and mucinous cystic neoplasms of the pancreas differentiated by ovarian-type stroma. World J Surg Dec 2006;30:2236–45 [DOI] [PubMed] [Google Scholar]
- 7.Grogan JR, Saeian K, Taylor AJ, Quiroz F, Demeure MJ, Komorowski RA. Making sense of mucin-producing pancreatic tumors. AJR Am J Roentgenol 2001;176:921–9 [DOI] [PubMed] [Google Scholar]