Abstract
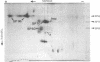
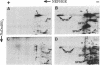
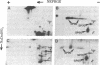
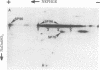
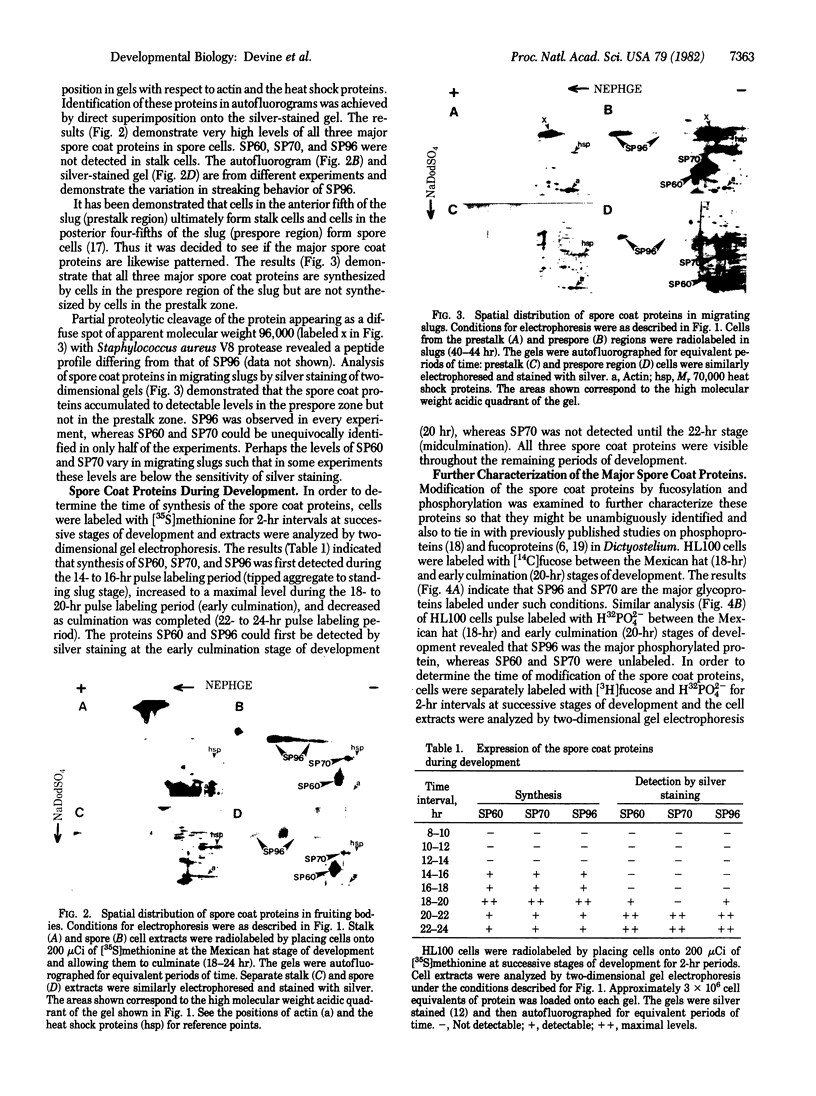
The major spore coat proteins (SP60, SP70, and SP96) of Dictyostelium discoideum have been analyzed by using two-dimensional nonequilibrium pH gradient electrophoresis. These proteins have been characterized with respect to electrophoretic behavior, metachromatic staining with silver, and posttranslational modifications; these techniques allow unambiguous identification of these proteins in silver-stained gels and autofluorograms of total cell extracts. They are synthesized by cells in the prespore region of migrating slugs, ultimately becoming major components of mature spores, but are not detectable either in cells in the prestalk region of slugs or in mature stalk cells. The ease with which these relatively abundant proteins can be recognized on two-dimensional gels makes them very useful markers for spatial and temporal differentiation in this organism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Brenner M. Comparison of proteins synthesized by anterior and posterior regions of Dictyostelium discoideum pseudoplasmodia. Dev Biol. 1979 Jul;71(1):1–7. doi: 10.1016/0012-1606(79)90077-0. [DOI] [PubMed] [Google Scholar]
- Alton T. H., Lodish H. F. Developmental changes in messenger RNAs and protein synthesis in Dictyostelium discoideum. Dev Biol. 1977 Oct 1;60(1):180–206. doi: 10.1016/0012-1606(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Coffman D. S., Leichtling B. H., Rickenberg H. V. Phosphoproteins in Dictyostelium discoideum. J Supramol Struct Cell Biochem. 1981;15(4):369–385. doi: 10.1002/jsscb.1981.380150407. [DOI] [PubMed] [Google Scholar]
- Coloma A., Lodish H. F. Synthesis of spore- and stalk-specific proteins during differentiation of Dictyostelium discoideum. Dev Biol. 1981 Jan 30;81(2):238–244. doi: 10.1016/0012-1606(81)90287-6. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Gregg J. H., Karp G. C. Patterns of cell differentiation revealed by L-[3H]fucose incorporation in Dictyostelium. Exp Cell Res. 1978 Mar 1;112(1):31–46. doi: 10.1016/0014-4827(78)90522-0. [DOI] [PubMed] [Google Scholar]
- Hemmes D. E., Kojima-Buddenhagen E. S., Hohl H. R. Structural and enzymatic analysis of the spore wall layers in Dictyostelium discoideum. J Ultrastruct Res. 1972 Dec;41(5):406–417. doi: 10.1016/s0022-5320(72)90047-0. [DOI] [PubMed] [Google Scholar]
- Hohl H. R., Hamamoto S. T. Ultrastructure of spore differentiation in Dictyostelium: the prespore vacuole. J Ultrastruct Res. 1969 Mar;26(5):442–453. doi: 10.1016/s0022-5320(69)90050-1. [DOI] [PubMed] [Google Scholar]
- Lam T. Y., Siu C. H. Synthesis of stage-specific glycoproteins in Dictyostelium discoideum during development. Dev Biol. 1981 Apr 15;83(1):127–137. doi: 10.1016/s0012-1606(81)80015-2. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- MacWilliams H. K., Bonner J. T. The prestalk-prespore pattern in cellular slime molds. Differentiation. 1979;14(1-2):1–22. doi: 10.1111/j.1432-0436.1979.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H., Farnsworth P. A., Loomis W. F. Pattern formation in Dictyostelium discoideum: an analysis of mutants altered in cell proportioning. Dev Biol. 1981 Apr 15;83(1):1–8. doi: 10.1016/s0012-1606(81)80002-4. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H., Wheeler S., Loomis W. F. New Loci in DICTYOSTELIUM DISCOIDEUM Determining Pigment Formation and Growth on BACILLUS SUBTILIS. Genetics. 1980 Sep;96(1):115–123. doi: 10.1093/genetics/96.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Loomis W. F. Plasma membrane proteins of Dictyostelium: the spore coat proteins. Dev Biol. 1979 Aug;71(2):297–307. doi: 10.1016/0012-1606(79)90171-4. [DOI] [PubMed] [Google Scholar]