Abstract
Calcifying fibrous pseudotumour (CFPT) is a rare lesion that has only recently been reported in the literature. Usually, the lesion develops in subcutaneous tissue, deep soft tissues or viscera. It appears as a uniform, hypocellular and well-circumscribed mass without a capsule. Only nine cases of gastric CFPT have been reported in the literature so far. Here, we report a new case of gastric CFPT, which was, surprisingly, associated with an ulcer. To our knowledge, a gastric CFPT with an ulcer has not been previously reported in the literature. The patient (a healthy 49-year-old man) had vomited approximately 300 g brown liquid and developed syncope once. CT scan and gastroscopy revealed a polypoid mass at the great curvature of the gastric body with a larger ulcer on its top. The mass was removed by surgery. During a follow-up of 5 months, the patient was asymptomatic with no recurrence. We discuss the imaging findings, as well as the clinicopathological features of this unusual case and review the related literature.
Calcifying fibrous pseudotumour (CFPT) has characteristic pathological features, but its aetiology and course are still uncertain. Pathologically, CFPT is characterised by abundant psammomatous and dystrophic calcications [1–3] on a background of rich collagen and lymphoplasmacytic infiltration. Until now, there have been only nine cases reported in the literature of the lesion being located in the stomach. There have been no previous reports of a CFPT with an ulcer. In addition, there are few reports concerning the imaging findings of CFPT in the literature. Here, we report a case of CFPT in which the lesion developed in the stomach and was associated with a large ulcer. We believe that this is the first case report of a CFPT developing in the stomach that was associated with a larger ulcer. We also review the relevant literature in an attempt to summarise the features of CFPTs, and discuss their pathology, clinical manifestations and imaging findings.
Case report
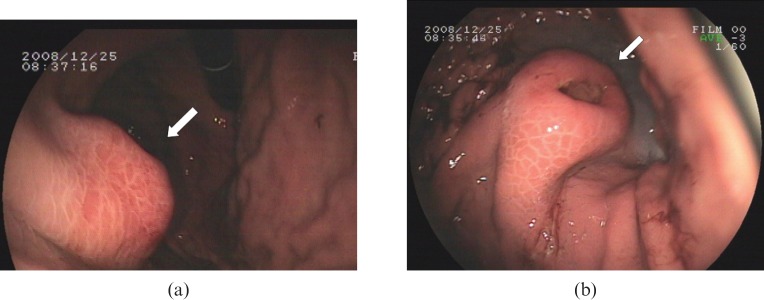
A 49-year-old man was admitted to our hospital because of sudden vomiting of brown liquid (approximately about 300 ml) followed by syncope. The patient had no history of trauma or surgery. Physical examination detected no positive signs except for mild pains in the upper abdomen upon deep palpation. Gastroscopic examination revealed a 1.5 × 2.0 cm polypoid lesion at the greater gastric curvature. The lesion was covered by normal mucosa with an ulcer of approximately 1 cm in diameter in the centre (Figure 1a,b). Further investigations included plain chest radiography, ultrasonography, cardiography, carcinoembryonic antigen (CEA), routine blood examination and routine urinalysis, and all results were normal except that routine stool examination revealed occult blood (+++).
Figure 1.
Gastroscopic findings. (a) A polypoid lesion of approximately 1.5 × 2 cm, covered by normal mucosa and situated along the great gastric curvature (arrow).( b) There is an ulcer of approximately 1 cm in diameter on the top of the lesion (arrow).
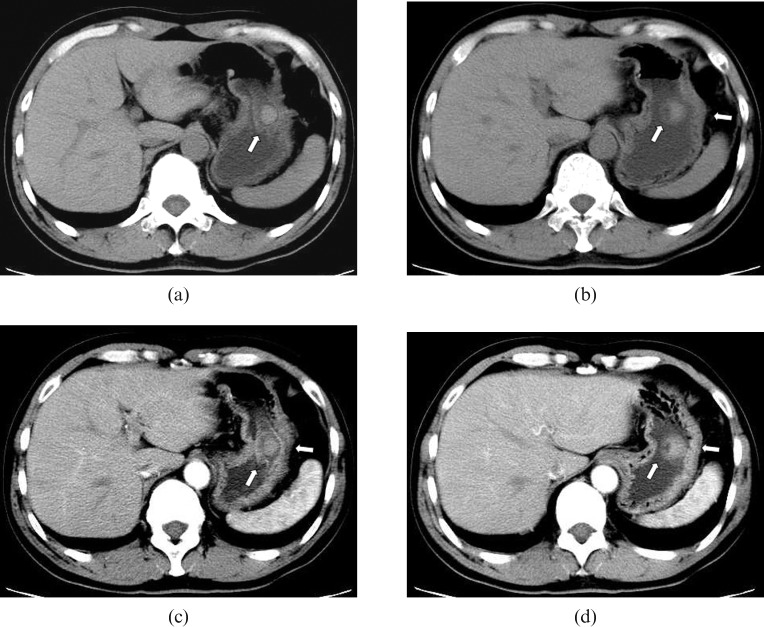
Plain CT scan of the upper abdomen showed a round, well-circumscribed and slightly hyperdense mass with a maximum cross-sectional area of 1.4 × 1.5 cm and with an ulcer on its surface (Figure 2a,b). Contrast-enhanced CT scanning with a bolus injection of 80 ml of iohexol (300 mg ml–1 of iodine) via an antecubital vein at a velocity of 2.5 ml s–1 was conducted; 60 s delayed scans demonstrated that the mass showed slight homogeneous enhancement, with an increasing CT value from 51.1–56.3 HU to 62.1–69.8 HU, and the ulcer was displayed more clearly. No sign of serous membrane involvement was found. CT diagnosis of a benign tumour originating from gastric stromal tissue was thus made (Figure 2c,d).
Figure 2.
CT findings. (a, b) Pre-enhanced CT scan shows a round mass of slight higher density with a depression on its cupula (arrows); the mass is 1.4 × 1.5 cm in size and has a CT value of 51.1–56.3 HU. (c, d) 60 s after intravenous contrast injection, the lesion shows slight homogeneous enhancement with an increase in CT value to 62.1–69.8 HU, and the depression in the lesion is clearer. The serous membrane of the stomach has not been involved (arrows).
The lesion was successfully excised with a Billroth I procedure and the patient was discharged from hospital 7 days after surgery. During a follow-up of 5 months, the patient was asymptomatic with no recurrence.
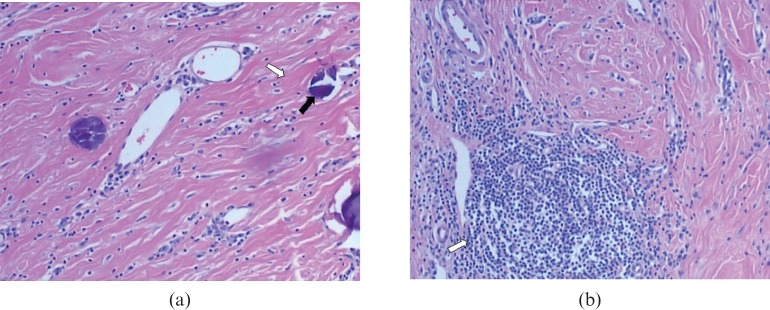
Macropathologically, the mass was solid, cylinder-like, non-encapsulated and measured 2 × 2.5 cm, with a 1 cm diameter ulcer on its top. The cross-section was greyish white and semitransparent. The lesion's texture was rather hard. Microscopically, there were abundant collagenous fibrils and numerous psammomatous calcifications in the lesion (Figure 3a) with lymphocytic infiltration and scattered lymph follicles (Figure 3b). Immunohistochemical examinations of CD117, CD34, SMA, Des and S-100 were all negative. Pathologically, a CFPT was diagnosed.
Figure 3.
Histological findings (haematoxylin and eosin staining (×200). (a) Hyalinised collagenous tissue (white arrow) with psammomatous calcifications (black arrow) is clearly revealed. (b) Dense hyalinised collagenous tissue and focal lymphocytic infiltration with lymphoid follicle formation (arrow) are demonstrated.
Discussion
CFPT was first described by Fetsch and associates in 1993 and was renamed calcifying fibrous tumour (CFT) by the World Health Organization in 2002. CFPT is an uncommon and phymatoid lesion [1–3]. Until now, only 100 cases (40 males and 60 females) of CFPT have been reported in the literature [3–16], with a mean age of 28.37 ± 17.17 years (range 5 weeks to 67 years). The lesions have a wide anatomical distribution, including the head, neck, limbs, trunk, mediastinum, heart, lung, axillary fossa, groin, scrotum, retroperitoneum, pleura, peritoneum, omentum, mesentery, and subserosa or submucosa. A solitary lesion was seen in 78 patients and multiple lesions in 22 patients. When CFPT occurs in the subcutaneous tissue, deep soft tissue, viscera or at subserosal or submucosal sites, the lesion is usually a well-circumscribed mass, and the patient may be asymptomatic for years. When the lesion is large, it can cause pressure symptoms from the neighbouring structures, such as local discomfort, numbness and pain [3–14]. Our patient had upper gastrointestinal bleeding, which may have been caused by the ulcer associated with the lesion.
The treatment for CFPT is surgical resection. Long-term follow-up is required for incompletely excised or disseminated lesions, although recurrence or metastasis is extremely rare [13–14].
The pathological features of CFPT are now well established [2–7]. On gross inspection, the mass is well circumscribed, sometimes lobulated, non-encapsulated and homogeneously solid. The cross-section of the lesion is uniformly greyish white, as it is composed of abundant collagenous fibres and degenerated hyaline fibres. Microscopically, there are spindle cells with scattered lymphoid follicles and chronic inflammatory infiltration within the lesion (Figure 3). The presence of calcifications, either psammomatous or dystrophic, is characteristic of the lesion. Immunohistochemically, the tumour shows only focal or, sometimes, negative expression of CD34 and non-specific expression of α smooth muscle actin and desmin. Anaplastic lymphoma kinase-1, which is a distinctive feature of inflammatory myofibroblastic tumours, is not a common finding in CFPT.
To the best of our knowledge, only nine cases of gastric CFPT have been reported to date that presented as a submucosal nodule or polypoid lesion less than 2.5 cm in diameter [3, 7–12]. A case of intestinal submucosal CFPT with a superficial ulcer was reported in the medical literature. However, no case of gastric CFPT associated with an ulcer has ever been reported before [14]. In our patient, the size of the lesion was up to 2 × 2.5 cm and it was associated with an ulcer of 1.0 cm diameter. The cause of the ulcer remains unclear; however, we suggest that it may have been formed by compressed erosion and/or digestion of the mucosa.
CFPT of the gastric wall can be identified by barium gastroenterography and/or gastroscopy, but the findings are not specific. Typical imaging findings of CFPT are thick, band-like, irregular or punctate calcifications, which are more easily demonstrated on CT and ultrasound (especially in echoendoscopy) than on conventional radiograph. On MRI, the lesion is usually manifested as a hypointense signal mass on T1 weighted and T2 weighted imaging, and as an iso-intense signal mass on gadolinium-enhanced T1 weighted imaging [3, 7–12]. The appearance of the calcifications may be changeable during the growth of the tumour. Small calcified lesions may not be detected on imaging examinations.
In our patient, the lesion density was slightly higher than that of gastric wall on the CT scan. On the contrast-enhanced CT scan, the lesion showed slight homogeneous enhancement 60 s after contrast injection. Unfortunately, we did not perform a delayed scan. Cho et al [15] have reported a CFPT which was located in the retroperitoneum, the mass manifested as iso- to lower signal intensity mass when compared with the signal intensity of back muscle on MRI. Dynamic gadolinium-enhanced coronal magnetic resonance (MR) images (60 s and 5 min after contrast injection) showed as progressive contrast enhancement from the periphery to the centre of the mass. The inhomogeneous and strong enhancement remained for 7 min after contrast injection. Eftekhari et al [16] described a CFPT in the left adrenal gland, which showed delayed enhancement of moderate intensity on CT scan. The delayed enhancement may be one of the imaging features of CFPT, which might correlate with its abundant hyalinised collagen [17].
Gastric CFPT should be differentiated from submucosal gastric tumours, including gastrointestinal stromal tumours, leiomyoma, leiomyosarcoma, schwannoma, neurofibroma, plexosarcoma, lipoma, liposarcoma, glomus tumour, haemangioma and lymphangioma, which can also present as a well-circumscribed submucosal mass with or without ulceration. Misdiagnosis may result in unnecessary surgery or inappropriate follow-up treatment. Fortunately, these gastric tumours usually have some imaging characteristics that indicate a specific diagnosis. The morphological features of gastric CFPT are usually pathognomonic. Correct diagnosis can often be obtained when a combination of endoscopic findings with imaging manifestations are carried out.
References
- 1.Fetsch JF, Montgomery EA, Meis JM. Calcifying fibrous pseudotumor. Am J Surg Pathol 1993;17:502–8 [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CD, Unni KK, Mertens F, eds World Health Organization classification of tumours: pathology and genetics of tumours of soft tissue and bone.Lyon, France:IARC Press,2002:77–8 [Google Scholar]
- 3.Elpek GO, Kupesiz GY, Ogus M. Incidental calcifying fibrous tumor of the stomach presenting as a polyp. Pathol Int 2006;56:227–31 [DOI] [PubMed] [Google Scholar]
- 4.Chon SH, Lee CB, Oh YH. Calcifying fibrous pseudotumor causing thoracic outlet syndrome. Eur J Cardiothorac Surg 2005;27:353–5 [DOI] [PubMed] [Google Scholar]
- 5.Bell DM, Dekmezian RH, Husain SA, Luna MA. Oral calcifying fibrous pseudotumor: case analysis and review. Head Neck Pathol 2008;2:343–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyano Y, Kanzaki M, Obara T, Ohnuki T. Multiple calcifying fibrous pseudotumor of the pleura. Kyobu Geka 2008;61:857–60 [PubMed] [Google Scholar]
- 7.Sato S, Ooike N, Yamamoto T, Wada M, Miyamoto A, Matsukawa M, et al. Rare gastric calcifying fibrous pseudotumor removed by endoscopic submucosal dissection. Dig Endosc 2008;20:84–6 [Google Scholar]
- 8.Chatelain D, Lauzanne P, Yzet T, Guernou M, Delcenserie R, Regimbeau JM, et al. Gastric calcifying fibrous pseudotumor: a rare mesenchymal tumor of the stomach. Gastroenterol Clin Biol 2008;32:441–4 [DOI] [PubMed] [Google Scholar]
- 9.Delbecque K, Legand M, Boniver J, Lauwers GY, Leval L. Calcifying brous tumour of the gastric wall. Histopathology 2004;44:399–400 [DOI] [PubMed] [Google Scholar]
- 10.Puccio F, Solazzo M, Marciano P, Benzi F. Laparoscopic resection of calcifying fibrous pseudotumor of gastric wall. A unique case report. Surg, Endosc 2001;5:1227. [DOI] [PubMed] [Google Scholar]
- 11.Emanuel P, Qin L, Harpaz N. Calcifying fibrous tumor of small intestine. Ann Diagn Pathol 2008;12:138–41 [DOI] [PubMed] [Google Scholar]
- 12.Attila T, Chen D, Gardiner GW, Ptak TW, Marcon NE. Gastric calcifying fibrous tumor. Can J Gastroenterol 2006;20:487–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata K, Yuki D, Sakata K. Multiple calcifying fibrous pseudotumors disseminated in the pleura. Ann Thorac Surg 2008;85:e3–5 [DOI] [PubMed] [Google Scholar]
- 14.Liang HH, Chai CY, Lin YH, Lee CH, Wu CH, Chang CC. Jejunal and multiple mesenteric calcifying fibrous pseudotumor induced jejunojejunal intussusception. J Formos Med Assoc 2007;106:485–9 [DOI] [PubMed] [Google Scholar]
- 15.Cho JH, Hwang MS, Chang JC, Park BH, Kim DS. Calcifying fibrous pseudotumor of the retroperitoneum: a case report. J Korean Radiol Soc 1999;40:953–6 [Google Scholar]
- 16.Eftekhari F, Ater JL, Ayala AG, Czerniak BA. Calcifying fibrous pseudotumour of the adrenal gland. Br J Radiol 2001;74:452–4 [DOI] [PubMed] [Google Scholar]
- 17.Helm PA, Caravan P, French BA, Jacques V, Shen L, Xu Y, et al. Postinfarction myocardial scarring in mice: molecular MR imaging with use of a collagen-targeting contrast agent. Radiology 2008;247:788–96 [DOI] [PMC free article] [PubMed] [Google Scholar]