Abstract
We studied the expression of three promoter 5′ deletion constructs (−218, −599, and −1312) of the LEA (late embryogenesis abundant)-class gene Dc3 fused to β-glucuronidase (GUS), where each construct value refers to the number of base pairs upstream of the transcription start site at which the deletion occurred. The Dc3 gene is noted for its induction by abscisic acid (ABA), but its response to other plant hormones and various environmental stresses has not been reported previously for vegetative cells. Fourteen-day-old transgenic tobacco (Nicotiana tabacum L.) seedlings were exposed to dehydration, hypoxia, salinity, exogenous ethylene, or exogenous methyl jasmonate (MeJa). GUS activity was quantified fluorimetrically and expression was observed by histochemical staining of the seedlings. An increase in GUS activity was observed in plants with constructs −599 and −1312 in response to dehydration and salinity within 6 h of stress, and at 12 h in response to hypoxia. No increase in endogenous ABA was found in any of the three lines, even after 72 h of hypoxia. An ABA-independent increase in GUS activity was observed when endogenous ABA biosynthesis was blocked by fluridone and plants were exposed to 5 μL L−1 ethylene in air or 100 μm MeJa. Virtually no expression was observed in construct −218 in response to dehydration, salinity, or MeJa, but there was a moderate response to ethylene and hypoxia. This suggests that the region between −218 and −599 is necessary for ABA (dehydration and salinity)- and MeJa-dependent expression, whereas ethylene-mediated expression does not require this region of the promoter.
LEA (late embryogenesis abundant) proteins accumulate in the seeds of many higher plants and are probably universal in occurrence in plant seeds. LEA proteins were first identified and characterized in cotton as a set of proteins that are highly accumulated in the embryos at the late stage of seed development (Dure et al., 1981). Subsequently, more than 100 LEA genes/cDNAs or their homologs have been identified from both monocots and dicots. In many cases, the timing of LEA mRNA and protein accumulation is correlated with the seed-desiccation process and associated with elevated in vivo ABA levels. The products of these genes are thought to function in protecting cells from dehydration (Baker et al., 1988; Dure et al., 1989), possibly by ion sequestration in dehydrated cells (Dure, 1993). The high abundance of LEA proteins in desiccation-tolerant seed embryos (Roberts et al., 1993), water-stress inducibility of specific LEA genes in vegetative tissues (Piatowski et al., 1990), and particular structural features (Baker et al., 1988; Dure, 1993) support this role. The Dc3 promoter fused to GUS can drive GUS expression in leaves of mature, transgenic plants in response to desiccation stress and/or treatment with ABA (Vivekananda et al., 1992).
ABA is involved in the signal transduction pathway regulating several genes that are expressed at specific developmental stages or as a result of an environmental stress. ABA accumulates in vegetative cells in response to water deficit, salinity, cold temperature, and light variation, and it is thought to act as a signal for the initiation of acclimation to these stresses (Marcotte et al., 1992; Chandler and Robertson, 1994; Weatherwax et al., 1996). Constitutive expression of the barley LEA gene HVA1 conferred increased tolerance to water deficit and salinity in second-generation transgenic rice plants (Xu et al., 1996). The extent of stress tolerance was correlated with the level of HVA1 protein accumulation. In rice more salt-tolerant varieties showed higher concentrations in their roots of dehydrins and group 3 LEA proteins compared with salt-sensitive varieties (Moons et al., 1995).
Dc3 is a carrot (Daucus carota) gene encoding an mRNA that is expressed at high levels in developing embryos (Seffens et al., 1990). Dc3 belongs to a small gene family (Wilde et al., 1988) encoding Dc3 and Dc3-like mRNAs, and is closely homologous to the D-7 LEA family of cotton (Dure et al., 1989). In transgenic tobacco (Nicotiana tabacum L.) containing a GUS reporter gene fused to the full-length 5′ promoter element of Dc3, there was expression in seedlings in response to desiccation or ABA (Seffens et al., 1990). Also, Dc3 is expressed in developing transgenic tobacco seeds, and detailed evidence has been obtained for the differential response of different promoter deletion constructs in response to desiccation and exogenous ABA during germination (Chung, 1996). The Dc3 promoter can be divided into two regulatory regions. The PPR from −117 to +27 is the minimal sequence for seed-specific expression and contains the cis-acting elements that are responsible for the regulation of Dc3 expression in seeds (Thomas, 1993; Kim et al., 1997). The second region, the DPR from −1457 to −118, contains several repeats of additional cis-regulatory regions, namely the TCGT motifs (Chung, 1996). The TCGT motif, interacting with the PPR, is required for ABA-induced expression in vegetative cells in transient expression assays. It is proposed that bZIP proteins bind to the PPRs and DPRs and regulate gene expression in response to developmental and environmental cues (Kim et al., 1997). Several studies have shown that bZIP proteins are induced by a variety of stresses, including: hypoxia (de Vetten and Ferl, 1995), low light (Feldbrugge et al., 1994; Mikami et al., 1995), low temperature (Kusano et al., 1995), and ABA (Lu et al., 1996). Thus, a bipartite structure for the Dc3 promoter is proposed for seed-specific expression, as well as for ABA-inducible expression in vegetative tissues. Deletion of the DPR eliminates all expression in vegetative tissues, allowing only seed-specific expression (Chung, 1996).
The aim of the present work was to study the response of the Dc3 promoter in vegetative cells to several environmental stresses that may involve mediation by ABA and other hormones. Although the sensitivity of the Dc3 promoter in transgenic plants to dehydration and/or ABA is well established, it is not known whether other environmental factors or hormonal signals are able to modulate the activity of the gene. Therefore, the sensitivity of Dc3-driven GUS expression was tested with ethylene and MeJa, and with the environmental stresses hypoxia and salinity. By examining the response of the Dc3 promoter to various 5′ upstream deletions, we hoped to identify regions that are essential for differential expression.
Plants encounter hypoxia or O2 shortage with excess soil water (Drew, 1997) and the ethylene-biosynthetic rate is then increased (Jackson et al., 1985; Atwell et al., 1988; He et al., 1996). Endogenous ABA levels may also increase under hypoxic conditions (Smit et al., 1990; Neuman and Smit, 1991). Ethylene biosynthesis is increased in response to stimuli such as wounding, pathogen attack, hypoxia, and water deficit (for review, see Abeles et al., 1992; Morgan and Drew, 1997). In leaves of plants growing under high-salt stress, ABA accumulation may assist in the acclimation to salinity (Zeevaart and Creelman, 1988).
JA and its methyl ester MeJa are plant growth substances that modulate plant development and defense mechanisms (Creelman and Mullet, 1997). JA and MeJa influence many physiological and developmental processes that are affected by ABA (Hildmann et al., 1992; Melan et al., 1993; Sembdner and Parthier, 1993; Lehmann et al., 1995). ABA and JA not only exert similar physiological effects, but they also share common actions at the level of gene expression by also inducing similar, or even the same, polypeptides, e.g. Pin II, the proteinase inhibitor of potato (Farmer and Ryan, 1992); VspB, the vegetative storage proteins of soybean (Mason et al., 1993); or the seed storage proteins of Brassica napus (Wilen et al., 1991). Jasmonates can accumulate in plants in response to water deficit and wounding (Creelman et al., 1992; Creelman and Mullet, 1995). Promoters of both the Pin II and VspB genes contain similar DNA domains called G-boxes, which are identical to the G-box in ABA-responsive promoters, including Dc3. One aim of our study was to determine whether exogenous MeJa can induce Dc3 when the synthesis of ABA is blocked.
Regulation of the Dc3 promoter in response to dehydration and exogenous ABA has been studied previously using GUS fusions in vegetative tissues of transgenic plants (Vivekananda, et al., 1992). Plants subjected to mild dehydration showed a slight decrease in leaf water potential, with a dramatic increase in the levels of GUS activity. However, this earlier study was with the full-length promoter construct. It focused only on leaves of mature plants and did not examine the first signs of expression in roots and shoots. The specific objectives of the present research were: (a) to study the tissue-specific expression of Dc3 in response to three major environmental stresses that might involve ABA-mediated responses, namely dehydration, salinity, and hypoxia; (b) to determine the responses of various promoter deletion lines to these stresses; and (c) to examine the possibility of ABA-independent expression of Dc3.
MATERIALS AND METHODS
Plant Material
Transgenic tobacco (Nicotiana tabacum L. cv Xanthi) seeds containing the promoter region of the gene for carrot (Daucus carota), Dc3, fused to a GUS reporter gene were used. Three different 5′ promoter deletion constructs were chosen, −218, −599, and −1312, where each construct value represents the number of base pairs upstream of the transcription start site at which the deletion occurred. The full-length Dc3 promoter is 1.5 kb long. Thus, the −218 construct is the shortest promoter sequence and the −1312 construct is the longest. For convenience, in the remainder of the text the promoter deletion constructs will be referred to as −218, −599, and −1312. Details concerning tobacco transformation and construction of these deletion lines are given by Chung (1996).
Agrobacterium tumefaciens strain LBA4404 was transformed with the pBI101.1 cassette containing the promoter deletions and chimeric constructs via electroporation. Leaf discs of tobacco were inoculated for 10 min with A. tumefaciens grown to saturation in M9 medium (Maniatis et al., 1982). The inoculated leaf discs were then incubated for 2 d on Murashige and Skoog medium (1× Murashige and Skoog salts with 3% Suc, 1 μg/mL pyridoxine-HCl, 1 μg/mL nicotinic acid, 100 μg/mL myo-inositol, and 0.25% Gel-Rite) at 25°C with 16 h of light and 8 h of darkness. The inoculated leaf discs were transferred to a shoot-inducing medium (Murashige and Skoog medium, 0.01 μg/mL NAA, and 2.0 μg/mL 6-BA) containing 100 μg/mL kanamycin and 200 μg/mL carbenicillin. Young shoots were transferred to a growth medium (Murashige and Skoog medium containing 100 μg/mL kanamycin and 200 μg/mL carbenicillin). Regenerated plants were transferred to soil and grown under optimal conditions. The seed used in this study was from plants that were representative of between 8 and 10 independent transformation events for each construct. Both histochemical and fluorimetric tests were made on embryos and on seedling leaves (Chung, 1996). Thus, the lines used in the present work gave GUS expression under a variety of environmental conditions that was close to the median for each group of independent lines. These particular constructs were chosen because in transgenic tobacco embryos the sequence and properties of −1312 were very close to those of the full-length promoter; −599 gave maximal response to ABA, whereas −218 was relatively insensitive to ABA.
Seeds were surface sterilized with 5% (v/v) bleach and washed with sterile distilled water several times. Seedlings were grown hydroponically on an inorganic nutrient solution containing (in mm): 0.1 KNO3, 0.4 Ca(NO3)2, 0.1 NH4H2PO4/(NH4)2PO4 (pH 5.0), 0.05 MgSO4, and 0.05 Fe-EDTA, together with micronutrients (Rodriguez et al., 1997). To reduce damage and stress to plants during transplanting, a hydroponic system was chosen for growing plants. In early experiments, in which plants were grown on Murashige and Skoog medium before transfer to hydroponics, they had significantly higher expression of GUS because of the physical damage to the plants (data not shown). Kanamycin (5 μg mL−1) was used for selection. This antibiotic concentration was chosen after a separate experiment in which plants were grown in the presence of kanamycin concentrations ranging from 5 to 100 μg mL−1. At 5 μg mL−1, there was clearly no visible damage to the kanamycin-resistant transgenic seedlings. A higher concentration resulted in late germination, restricted growth of roots, smaller cotyledons, delayed emergence of true leaves, and yellowing of leaves after 10 d (data not shown). Hydroponic containers were made from Magenta (Chicago, IL) boxes by mounting stainless-steel mesh over the top and laying a piece of cheesecloth to hold the seeds and provide a moist substratum for the germinating seeds. Plants were grown in a controlled-environment growth chamber at 25°C in 16 h of light and 8 h of darkness. Seeds were germinated in contact with nutrient solution containing kanamycin for 6 d and resistant seedlings were then transferred to kanamycin-free nutrient solution. Stress treatments started 14 d after germination.
Stress and Hormone Treatments
Fourteen-day-old plants were subjected to dehydration by removing water from the hydroponic container and then very gently blotting the plants with filter paper to remove excess water. Seedlings were then transferred to a Styrofoam box and covered with a lid to maintain a near-constant RH level inside of the box for uniform dehydration. The RH was monitored using an instant digital hygrometer (Fisher) at regular intervals during the stress period, and was maintained at 80% to 85% by adjusting the lid of the Styrofoam box. Control plants were grown in hydroponic containers during the stress period. Samples were taken every 6 h (starting at 0 h) for up to 24 h.
For salinity treatment, 14-d-old seedlings were transferred to nutrient solution containing 100 mm NaCl. Control plants continued to grow on regular nutrient solution with no NaCl. Samples were taken every 6 h for up to 24 h.
For hypoxia treatment, at 14 d after germination hydroponic containers were transferred to 4-L sealed plastic boxes. To induce hypoxia, air (20.6% [v/v] O2) and prepurified N2 gas, both from pressurized cylinders and regulated by electronic mass flow meters, were mixed to give an O2 concentration of 4% (v/v). This gas mixture was passed into the nutrient solution at a flow rate of 100 mL min−1 per container. The top of the plastic container was sealed except for a tube to vent the gas mixture to ensure that the tops of the plants were also hypoxic. Control plants were transferred to similar containers but received normal air.
For exogenous ethylene and MeJa treatments, plants were grown as described above and were then subjected to fluridone to block ABA biosynthesis. Fluridone solution was prepared by dissolving the solid in 100 μL of ethanol and then adding it to vigorously stirred water. The final concentration of ethanol in the nutrient solution was 100 μL L−1. Fluridone precipitates rapidly if it is added to unstirred water or if the solution is more than 3 to 4 d old. Fresh fluridone solution was prepared for each experiment. To determine the effect of fluridone exposure on growth, plants in a preliminary experiment were subjected to 100 μm fluridone for 1, 2, 3, and 4 d, and the morphological changes and GUS expression in seedlings were observed. No noticeable damage to seedlings was seen after 2 d of fluridone treatment, and no GUS activity was detected even after 24 h of dehydration. However, we were cautious about continuous exposure to fluridone because it can be lethal to plants if it is present for too long in the growing medium. These results suggested that 2 d of treatment might be enough to block endogenous ABA biosynthesis without any significant damage to the seedlings.
In all subsequent experiments involving fluridone, 14-d-old seedlings were transferred to nutrient solution containing 100 μm fluridone for 48 h before being transferred back to the regular nutrient solution. Hydroponic containers were transferred to sealed plastic boxes. Ethylene was continuously bubbled through the nutrient solution. Ethylene (10 mL min−1 of 100 μL L−1 [v/v]) and air (190 mL min−1), both from pressurized cylinders and regulated by electronic mass flow meters, were mixed to give a final ethylene concentration of 200 nmol L−1, equivalent to 5 μL L−1 (v/v). Control plants, initially grown on fluridone, were also transferred to the plastic containers but received no ethylene. Samples of plant tissue were taken at intervals for histochemical staining and GUS-activity analysis.
For exogenous MeJa treatment, 100 μm MeJa was prepared by dissolving 95% MeJa (Aldrich) in 200 μL of ethanol before adding it to the nutrient solution. The final concentration of ethanol in the nutrient solution was 100 μL L−1. A separate aliquot of MeJa (100 μm final concentration) was mixed in water instead of the nutrient solution. This solution was sprayed on the leaves at the start of the stress period. Control plants were grown on nutrient solution containing the same amount of ethanol used to dissolve MeJa and were also sprayed with the same concentration of ethanol mixed in water.
GUS Analysis
GUS activity was determined according to the method of Jefferson et al. (1987). For the fluorimetric assay, roots or shoots of stressed or control seedlings were homogenized in GUS extraction buffer (50 mm NaPO4 [pH 7.0], 10 mm EDTA, 0.1% sarkosyl, 0.1% Triton X-100, and 10 mm β-mercaptoethanol). The homogenate was centrifuged in a microcentrifuge for 5 min, and the supernatant was used for the GUS assay. For fluorimetric reactions, 100 μL of supernatant was used. Duplicate reactions were started by adding 100 μL of extraction buffer containing 2 mm 4-methylumbelliferyl β-d-glucuronide (Sigma) and incubating at 37°C for 60 min. One reaction was terminated at time 0 and the second at 60 min, with the addition of 800 μL of 0.2 m Na2CO3 to each reaction. The 4-methylumbelliferyl β-d-glucuronide fluorescence product was then measured on a fluorimeter (model no. TKO 100, Hoefer, San Francisco, CA) (excitation wavelength = 365 nm, photodetector wavelength = 460 nm). The protein content of the samples was determined by the procedure of Bradford (1976). For histochemical GUS analysis, whole seedlings were immersed in the GUS reaction buffer (2 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid, 1% dimethylformamide, 1× KFeCN, 1 mm EDTA, and 50 mm NaPO4 [pH 7.0]). Tissue was incubated in the dark at 37°C for 18 to 24 h. The reaction was stopped by washing the seedlings several times with 50 mm phosphate buffer without 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid. For better visualization of the stained tissue, seedlings were cleared with an ethanol series and stored in 80% glycerol. Mounted seedlings were photographed with Kodak Ektachrome 60 ASA tungsten film.
Endogenous ABA Measurements
Approximately 25 seedlings per sample were cut to separate roots and shoots and were immediately frozen in liquid N2. Three replicates of each sample were taken for a statistical comparison. Samples were lyophilized overnight before measuring dry weights. The extraction and determination (HPLC-GC) were done as described by Creelman et al. (1990). An internal standard of [3H](±)-ABA was added for the determination of ABA recovery.
RESULTS
Strong Expression of the −1312 and −599 Constructs in Response to Dehydration
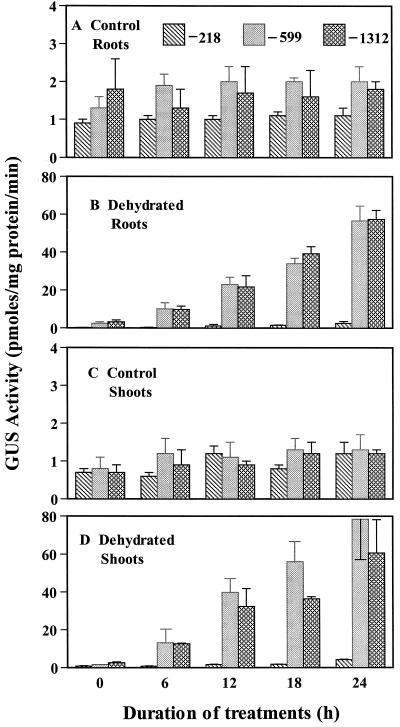
We studied the effects of dehydration on Dc3-driven GUS expression in roots and shoots of 14-d-old seedlings. The exact method used to bring about dehydration is critical in studying responses. The method frequently used to dehydrate plants or detached leaves is to expose them to laboratory air at an RH level of around 50% to 55%. This RH level brings about rapid desiccation, and damaged seedlings may be unable to acclimate or show expression of potentially responsive genes. To avoid this, we subjected seedlings to 80% to 85% RH, in which seedlings survived, even after 24 h of dehydration, in contrast to seedlings exposed to laboratory RH, which did not survive (data not shown). A 30-fold increase in GUS activity in roots and a 40-fold increase in GUS activity in shoots was observed after 24 h of dehydration in transgenic seedlings carrying the −1312 and −599 constructs (Fig. 1). The first sign of Dc3-GUS expression was observed at 6 h in root tips, vascular tissues, and hypocotyls. A low level of GUS expression was also observed in leaves of seedlings carrying the −1312 and −599 constructs at 6 h. A minimal increase in expression was observed in the tissue of the transgenic seedlings carrying the −218 construct. Figure 2, A to C, shows the lack of GUS expression in all tissues of the controls. By contrast, GUS expression in the −1312 and −599 constructs was uniformly increased throughout the seedlings at 24 h, with a concentration of activity in the densely packed cells of the root tip (Fig. 2, D–F). The relative responses of the different deletion constructs of the Dc3 promoter to dehydration, as determined by both fluorimetric and histochemical assays, are summarized in Table I.
Figure 1.
Effects of dehydration on GUS activity in roots and shoots of transgenic tobacco seedlings containing promoter deletion constructs of the carrot gene Dc3 fused to GUS. Stress started at 0 h. In this and subsequent figures, bars indicate se (n = 3).
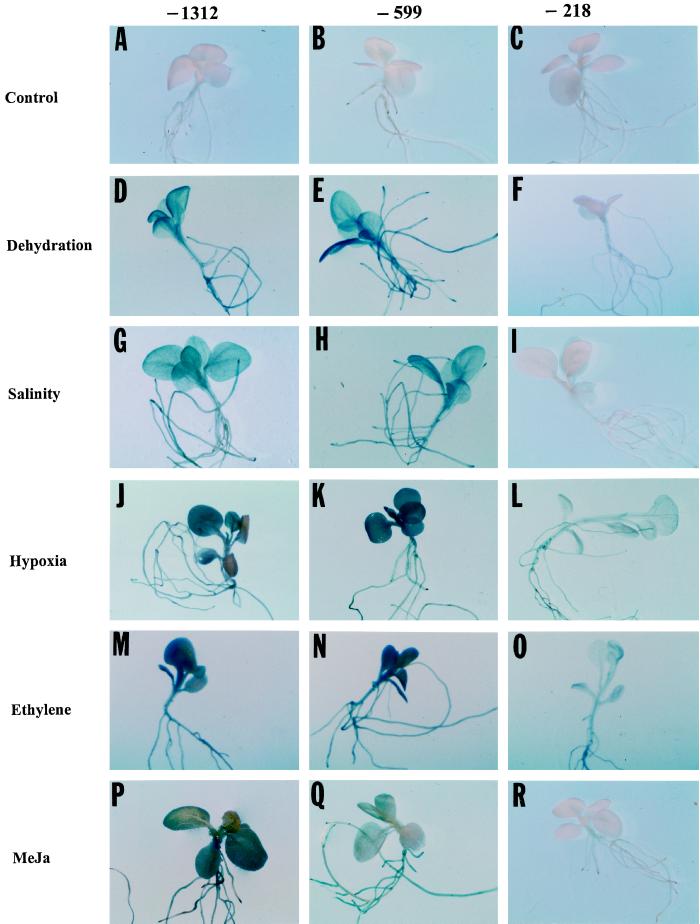
Figure 2.
Histochemically stained transgenic tobacco seedlings containing promoter deletion constructs of Dc3 fused to GUS. Line −1312 contained the longest promoter sequence and −218 contained the shortest. A to C, Control seedlings; D to F, 24 h of dehydration; G to I, 24 h of exposure to 100 mm NaCl; J to L, 24 h of hypoxia; M to O, 24 h of exposure to 5 μL L−1 exogenous ethylene; and P to R, 24 h of 100 μm exogenous MeJa treatment.
Table I.
Comparison of Dc3-driven GUS activity in roots and shoots of transgenic tobacco seedlings in response to environmental stresses and hormone treatments
| Construct | Stress and Hormone Treatment
(Roots/Shoots)
|
||||
|---|---|---|---|---|---|
| Dehydration | Salinity | Hypoxia | Ethylenea | MeJaa | |
| −1312 | +++ /+++ | ++ /+++ | + /++ | + /++ | ++ /++ |
| −599 | +++ /+++ | ++ /+++ | + /++ | + /++ | ++ /++ |
| −218 | − /− | − /− | + /+ | + /+ | − /− |
Activity is shown as strong (+++), medium (++), mild (+), or no activity (−).
Fluridone pretreated.
The possibility of ABA-independent expression during dehydration was tested with seedlings previously exposed to fluridone. Seedlings containing the −599 deletion construct showed no GUS expression when subjected to dehydration for 24 h, whereas control seedlings (those not treated with fluridone and subjected to similar dehydration) showed an increase in GUS activity (Table II). This test confirmed that fluridone effectively blocked ABA biosynthesis, and proved that fluridone itself did not induce Dc3 expression.
Table II.
Dc3-driven GUS expression in tobacco seedlings exposed to dehydration, in the presence (+) or absence (0) of fluridone
| Plant Part and Treatment | GUS Expression
|
|
|---|---|---|
| 0 h Dehydration | 24 h Dehydration | |
| pmol mg−1 protein min−1 | ||
| Roots | ||
| 0 Fluridone | 2.1 (0.56) | 53.7 (4.3) |
| + Fluridone | 1.4 (0.18) | 2.0 (0.30) |
| Shoots | ||
| 0 Fluridone | 3.0 (0.95) | 61.6 (6.4) |
| + Fluridone | 2.3 (0.46) | 1.9 (0.11) |
Values are means from three independent experiments with the −599 line; se values are in parentheses. Zero time corresponds to the end of the pretreatment with fluridone, immediately before the start of dehydration.
Dc3 Promoter Responds to Salt Stress
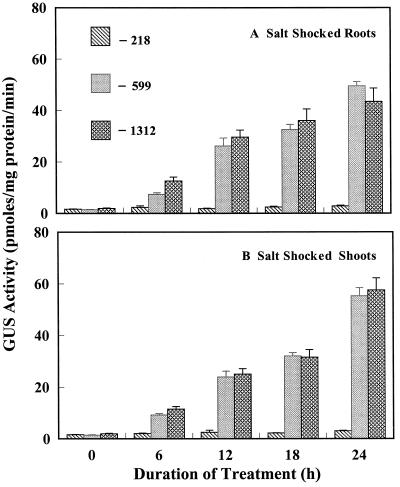
Expression of the three Dc3 promoter lines was studied with seedlings exposed to 100 mm NaCl. We decided to study the response of the Dc3 promoter to salt stress because endogenous ABA increases in plants exposed to salinity (for review, see Zeevaart and Creelman, 1988), and exogenous ABA or dehydration induces Dc3 expression. In a preliminary experiment, 50 mm NaCl caused very little change in GUS activity compared with the controls (data not shown). In later experiments, 14-d-old seedlings were exposed to 100 mm NaCl for 24 h. Salt was added to the nutrient solution, and plants continued to grow under ambient conditions of light, temperature, and photoperiod. NaCl (100 mm) caused the leaves to wilt within 2 h of exposure, a characteristic response of plants to salt-shock conditions, but the leaves regained turgor after 6 to 8 h. A 5-fold increase in GUS activity was observed in roots of the −599 and −1312 lines at 6 h, and the level of GUS activity increased to 20- to 25-fold over that of the controls at 24 h of salt stress (Fig. 3). No appreciable increase in GUS activity was observed in roots of the −218 line (Fig. 3). This response of the −218 line was similar to its response under dehydration stress, presumably because ABA mediates expression driven by either salt stress or dehydration. A 5-fold increase in GUS activity was also observed in shoots of the −599 and −1312 lines at 6 h, and up to a 30-fold increase was observed at 24 h of salt stress (Fig. 3). The −218 line showed only very small increases in GUS activity in shoots even after 24 h of salt stress (Fig. 3; Table I), which was similar to the response to dehydration. From histochemical tests (Fig. 2, G–I), GUS expression could be seen throughout roots and leaves, particularly in cotyledons and root tips of the −599 and −1312 lines, whereas there was no expression in any tissues in the −218 line.
Figure 3.
Effects of 100 mm NaCl on GUS activity in roots and shoots of transgenic tobacco seedlings containing promoter deletion constructs of Dc3. Stress started at 0 h. Control values in this experiment were <2 pmol mg−1 protein min−1.
ABA-Independent Expression under Hypoxia
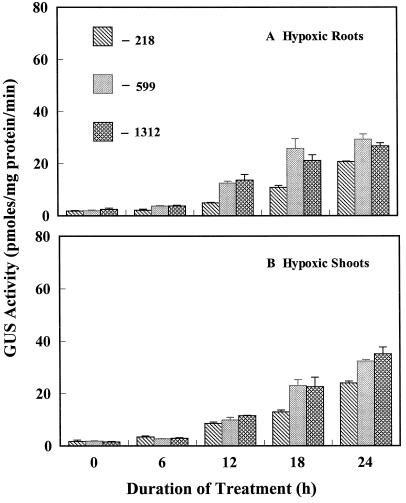
To investigate whether there was sufficient ABA released to induce Dc3 under hypoxic conditions and to study the tissue specificity and appearance of first expression, we subjected 14-d-old seedlings to hypoxia by supplying roots with 4% O2. ABA concentration did not change in hypoxic roots and shoots sampled at regular intervals over 72 h, with initial (prestress) values (nmol/g fresh weight) of 0.205 (roots) and 0.303 (shoots), and final values of 0.199 (roots) and 0.326 (shoots) at 72 h. Despite the absence of a change in ABA concentration, an increase in GUS activity was observed in all three lines, although −218 showed the least response (Fig. 4; Table I). Increases in GUS activity of 12-, 15-, and 10-fold were observed in roots of the −1312, −599, and −218 lines, respectively, at 24 h of hypoxia (Fig. 4). Increases of 12-, 15-, and 17-fold were observed in shoots of the −1312, −599, and −218 lines, respectively, at 24 h of hypoxia (Fig. 4). It is notable that there was a delay in GUS expression with hypoxia relative to the more rapid increase with dehydration or salt shock (Figs. 1 and 3). The weaker response of the −218 line is apparent in Figure 2, J–L, where the densely staining cotyledons and leaves that are apparent in lines −1312 and −599 are not seen.
Figure 4.
Effects of hypoxia on GUS activity in roots and shoots of transgenic tobacco seedlings containing promoter deletion mutant constructs of Dc3. Stress started at 0 h. Control values in this experiment were <1.5 pmol mg−1 protein min−1.
To test further whether Dc3 expression might be driven in an ABA-independent manner under hypoxia, seedlings were exposed to fluridone for 48 h and then transferred to nutrient solution lacking fluridone and subjected to 4% O2. The increase in GUS activity under hypoxia (after fluridone treatment) was similar to the GUS activity levels observed in the earlier experiment without fluridone treatment (data not presented). This suggests that Dc3 expression during hypoxia could be triggered by a signal other than ABA.
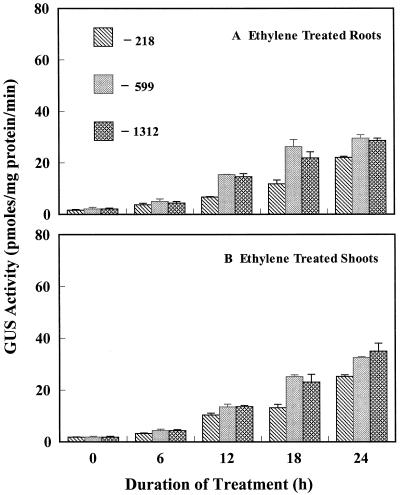
Expression of Dc3 Induced by Exogenous Ethylene
When 14-d-old seedlings were subjected to 5 μL L−1 ethylene in air after a 48-h fluridone treatment, GUS activity showed an increase in the roots and shoots of all three lines, but the induction of GUS was delayed, as it was under hypoxia. A slight increase in GUS activity was seen at 6 h. The GUS activity in roots at 12 h of stress increased 5-fold in the −218 line and approximately 15-fold in the −599 and −1312 lines, which increased to 22- and 30-fold, respectively, at 24 h (Fig. 5; Table I). A similar pattern was observed in shoots but the final expression at 24 h was slightly more than for the roots (Fig. 5). Histochemical staining showed an increase in GUS expression in all three lines (Fig. 2, M–O), although the weaker response of line −218 to ethylene, particularly the absence of intense expression in cotyledons and leaves, was apparent. Overall, the results with ethylene matched closely the results obtained under hypoxic conditions.
Figure 5.
Effects of exogenous ethylene on GUS activity in roots and shoots of transgenic tobacco seedlings containing promoter deletion constructs of Dc3. Stress started at 0 h, and seedlings had been exposed to fluridone. The concentration of ethylene in air was 5 μL L−1. Control values in this experiment were <1.5 pmol mg−1 protein min−1.
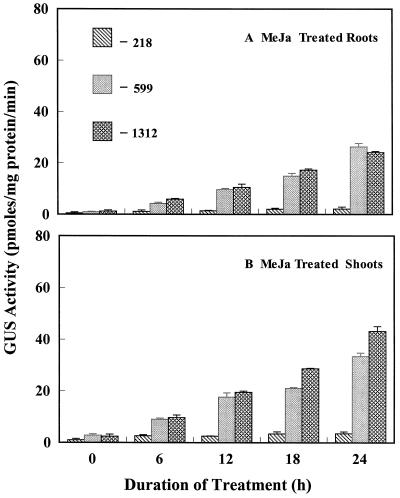
Expression of Dc3 by MeJa in the Absence of ABA
JA is primarily involved in wounding response, senescence, pathogen attack, and fruit removal (Creelman and Mullet, 1997), but it is also accumulated under water deficit. Several studies have shown an involvement of JA in ABA-mediated events. ABA and JA levels increase in response to water deficit, and some JA-inducible genes are also responsive to ABA, although it is still unknown whether they share common signal transduction pathways. To investigate whether MeJa can induce Dc3, we subjected 14-d-old seedlings to 100 μm MeJa. To isolate the effects of MeJa, seedlings were pretreated with 100 μm fluridone to block ABA synthesis. The −1312 and −599 lines showed a 3-fold increase in Dc3 expression in roots and a 5-fold increase in GUS activity in shoots after 6 h of exposure to MeJa. A 10-fold increase in GUS activity was observed in roots, and 20- and 15-fold increases were seen in shoots of −1312 and −599 mutants, respectively, after 24 h (Fig. 6). The increase in GUS activity was very small in roots or shoots of the −218 line compared with the controls even after 24 h of exposure to MeJa (Fig. 6; Table I). Histochemical staining of seedlings reflected the same pattern of expression (Fig. 2, P–R). Hypocotyls and cotyledons had a high expression of GUS. Irregular patches of GUS expression were also observed on the first true leaves. This pattern of expression among the three deletion lines resembles that found with dehydration. This suggests that jasmonates cause a similar but less-pronounced induction of Dc3, as does ABA.
Figure 6.
Effects of 100 μm MeJa on GUS activity in roots and shoots of transgenic tobacco seedlings containing promoter deletion constructs of Dc3. Stress started at 0 h, and seedlings had been exposed to fluridone. Control values in this experiment were <2.5 pmol mg−1 protein min−1.
DISCUSSION
This study shows the sensitivity of three promoter deletion lines of Dc3 to three major plant-stress hormones, ABA, ethylene, and JA, and the response of these Dc3 promoter deletion lines to three environmental stresses, dehydration, salinity, and hypoxia. Induction of GUS activity in vegetative cells by dehydration presumably was associated with water-deficit-induced increases in endogenous ABA in tobacco seedlings (Vivekananda et al., 1992). The Dc3 promoter has a bipartite structure, and interaction of upstream sequences (TCGT motifs) between −449 and −351 and the PPR from −117 and +27 is necessary for the ABA-driven expression in nonembryonic tissues (Chung, 1996). Only a very small increase in GUS activity was observed in the −218 line even after 24 h of dehydration, indicating that deletion of the upstream region prevented ABA-inducible expression in vegetative cells. GUS expression in the −1312 and −599 lines was observed in root tips and leaves within 6 h of water-deficit stress, followed by a more uniform expression in all vegetative tissues within 24 h. This indicates that the Dc3 promoter responds to ABA at the whole-plant level and expression is not localized to a specific tissue. GUS expression in leaves within 6 h of water deficit, before wilting was seen, could imply that the Dc3 promoter is sufficiently sensitive to ABA that it responds to presumed increases in endogenous ABA that occur before loss of cell turgor. Induction of Dc3 during dehydration is necessarily dependent on ABA signaling, as the experiments with fluridone showed. It is interesting that with the −218 construct, very small but possibly significant increases above the control levels of GUS activity occurred with dehydration, salinity, and exposure to MeJa (Figs. 1, 3, and 6). The possibility cannot be ruled out that within the 218-bp promoter sequence there are sequences that confer very weak but detectable levels of response to ABA or other signals.
Salinity stress is associated with increases in endogenous ABA (Zeevaart and Creelman, 1988; Moons et al., 1997). With 100 mm NaCl, there was a marked increase in Dc3-driven GUS activity that followed the same trend that was seen with dehydration, perhaps because the endogenous ABA levels increased similarly with dehydration stress. Moons et al. (1995) found expression of a group 3 LEA gene under salt stress in different cultivars of rice, with a simultaneous increase in endogenous levels of ABA and jasmonates. There was slightly greater GUS expression in tobacco leaves than in roots, as would be expected if there was a more rapid accumulation of ABA levels in leaves during salt shock.
In our study we also looked at Dc3 expression under root hypoxia. To explore the possibility of increased ABA levels, we measured the endogenous ABA levels in hypoxic roots and shoots. Although other studies have reported an increase in ABA level in hypoxic tissues (Zhang and Davies, 1987), we did not detect any increase even at 72 h of hypoxia. Hypoxia caused an increase in GUS expression within 12 h of stress imposition (Fig. 4). Transcription of LEA genes has been reported to be inducible by ABA-dependent and -independent signals (Chandler and Robertson, 1994; Shinozaki and Yamaguchi-Shinozaki, 1997). We observed expression in the −218 line, which did not respond to dehydration/ABA, salt shock, or exogenous MeJa. This raises the possibility of yet another signal involved in Dc3 expression. Ethylene may be working as the signal for hypoxia. Numerous studies have shown an increase in endogenous ethylene in hypoxic plants (Atwell et al., 1988; He et al., 1996; Drew, 1997). The endogenous ethylene levels in hypoxic tissues of tobacco seedlings were not determined, but we observed a very similar GUS expression in all three constructs in the presence of exogenous ethylene. This suggests that ethylene could be the signal for Dc3 expression in hypoxic tissues. ABA does not seem to be essential for the induction of Dc3 in hypoxic tissues because there was no increase in ABA concentration in roots or shoots, but there was GUS expression in hypoxic seedlings pretreated with fluridone. Fluridone effectively blocks synthesis of ABA in seedlings (Saab et al., 1990; Sharp et al., 1994) and inhibits expression of ABA-responsive genes (Lang and Palva, 1992). Although fluridone was removed from the root environment during the stress period in the present experiments, pretreatment with fluridone was clearly effective in inhibiting Dc3-driven GUS expression during dehydration. It could be argued that fluridone might be washed out of plants with their roots immersed in nutrient solution, as in the hypoxia or ethylene treatments, but that seems unlikely because the compound binds tightly to surfaces and could not be washed out of the shoots, which were exposed to the air.
Ethylene- and hypoxia-induced GUS expression in the −218 line suggests that the region between −599 and −218 is not absolutely necessary for ABA-independent expression, but is necessary for ABA-dependent expression. The transcription factors associated with ABA-independent expression in the Dc3 promoter are unknown. The dehydration-response element in a number of promoters is recognized as responding to some aspect of dehydration and not to ABA (Bray, 1997; Shinozaki and Yamaguchi-Shinozaki, 1997). However, the failure of dehydration to induce expression in fluridone-treated roots and shoots suggests that the dehydration-response element may not play a role in Dc3.
Induction of Dc3 expression by MeJa adds Dc3 to the growing list of ABA-inducible genes that are also sensitive to JA (Wilen et al., 1991; Hildmann et al., 1992; Lehmann et al., 1995). Several reports have shown that ABA and JA exert similar physiological effects and induce similar and/or related genes. However, it is not known whether MeJa-induced expression involves activation of transcription factors similar to those involved in ABA-dependent expression, or activation of a new set of transcription factors specific to MeJa. It is interesting that MeJa, like ABA, could not induce expression in the −218 line. This implies that the region between −599 and −218 is also necessary for MeJa-induced expression. Therefore, our results point to a similar mode of action of ABA and MeJa at the level of the Dc3 gene promoter. This contrasts with rice, in which a group 3 LEA protein that is induced by ABA was not induced by JA (Moons et al., 1997). Furthermore, ABA and JA exerted antagonistic effects with respect to induction of some salinity-inducible genes in rice seedlings (Moons et al., 1997). Clearly, ABA and jasmonates cannot be regarded as exerting overlapping influences on all genes.
In summary, GUS expression driven by the Dc3 promoter in transgenic tobacco seedlings is inducible not only by ABA, but also by salt shock, hypoxia, ethylene, and MeJa. Induction is virtually arrested in the −218 deletion mutants in response to dehydration, salt shock, and exogenous MeJa treatments, implying that the promoter region between −599 and −218 is absolutely necessary for ABA- and MeJa-dependent expression, but not for ethylene-mediated expression. This work shows that Dc3, an ABA-responsive, embryogenesis-specific gene, can be modulated by many different stress signals. The potential significance of these observations is that subtle differences in the pattern of response to different environmental/hormonal factors may result from various combinatorial interactions between cis- and trans-acting factors in the Dc3 promoter.
ACKNOWLEDGMENTS
We thank Dr. Robert Creelman for his assistance in HPLC-GC work on ABA. We also thank Dr. Keerti Rathore for his help with photography.
Abbreviations:
- DPR
distal promoter region
- JA
jasmonic acid
- MeJa
methyl jasmonate
- PPR
proximal promoter region
Footnotes
This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (no. 9437304 to T.L.T.).
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME., Jr . Ethylene in Plant Biology. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Atwell BJ, Drew MC, Jackson MB. The influence of oxygen deficiency on ethylene synthesis, 1-aminocyclopropane-1-carboxylic acid levels and aerenchyma formation in roots of Zea mays L. Physiol Plant. 1988;72:15–22. [Google Scholar]
- Baker J, Steele C, Dure L., III Sequence and characterization of 6 LEA proteins and their genes from cotton. Plant Mol Biol. 1988;11:277–291. doi: 10.1007/BF00027385. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray EA. Plant responses to water deficit. Trends Plant Sci. 1997;2:48–54. [Google Scholar]
- Chandler PM, Robertson M. Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:113–141. [Google Scholar]
- Chung H-J (1996) Analysis of the 5′ upstream region of the carrot Dc3 gene: bipartite structure of the Dc3 promoter for embryo-specific expression and ABA-inducible expression. PhD thesis. Texas A&M University, College Station
- Creelman RA, Mason HS, Bensen RJ, Boyer JS, Mullet JE. Water deficit and abscisic acid cause differential inhibition of shoot versus root growth in soybean seedlings. Analysis of growth, sugar accumulation, and gene expression. Plant Physiol. 1990;92:205–214. doi: 10.1104/pp.92.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 1995;92:4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JE. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA. 1992;89:4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vetten NC, Ferl RJ. Characterization of a maize G-box binding factor that is induced by hypoxia. Plant J. 1995;6:133–140. doi: 10.1046/j.1365-313x.1995.7040589.x. [DOI] [PubMed] [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Dure L., III A repeating 11-mer amino acid motif and plant desiccation. Plant J. 1993;3:363–369. doi: 10.1046/j.1365-313x.1993.t01-19-00999.x. [DOI] [PubMed] [Google Scholar]
- Dure L, III, Crouch M, Harada J, Ho THD, Mundy J, Quatrano R, Thomas TL, Sung ZR. Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol. 1989;12:475–486. doi: 10.1007/BF00036962. [DOI] [PubMed] [Google Scholar]
- Dure L, III, Greenway SC, Galau GA. Developmental biochemistry of cotton seed embryogenesis and germination. XIV. Changing mRNA populations as shown by in vitro and in vivo protein synthesis. Biochemistry. 1981;20:4162–4168. doi: 10.1021/bi00517a033. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldbrugge M, Sprenger M, Dinkelbach M, Yazaki K, Harter K, Weisshaar B. Functional analysis of a light-responsive plant bZIP transcriptional regulator. Plant Cell. 1994;6:1607–1621. doi: 10.1105/tpc.6.11.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Finlayson SA, Drew MC, Jordan WR, Morgan PW. Ethylene biosynthesis during aerenchyma formation in roots of Zea mays subjected to mechanical impedance and hypoxia. Plant Physiol. 1996;112:1679–1685. doi: 10.1104/pp.112.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildmann T, Ebneth M, Pena-Cortes H, Sanchez-Serrano JJ, Willmitzer L, Prat S. General roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell. 1992;4:1157–1170. doi: 10.1105/tpc.4.9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Fenning TM, Drew MC, Saker LR. Stimulation of ethylene production and gas-space (aerenchyma) formation in adventitious roots of Zea mays L. by small partial pressures of oxygen. Planta. 1985;165:486–492. doi: 10.1007/BF00398093. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Chung H-J, Thomas TL. Isolation of a novel class of bZIP transcription factors that interact with ABA-responsive and embryo-specification elements in the Dc3 promoter using a modified yeast one-hybrid system. Plant J. 1997;11:1237–1251. doi: 10.1046/j.1365-313x.1997.11061237.x. [DOI] [PubMed] [Google Scholar]
- Kusano T, Berberich T, Harada M, Suzuki N, Sugawara D. A maize DNA-binding factor with a bZIP motif is induced by low temperature. Mol Gen Genet. 1995;248:507–517. doi: 10.1007/BF02423445. [DOI] [PubMed] [Google Scholar]
- Lang V, Palva ET. The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol. 1992;20:951–962. doi: 10.1007/BF00027165. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Atzorn R, Bruckner C, Reinbothe S, Leopold J, Wasternack C, Parthier B. Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed barley leaf segments. Planta. 1995;197:156–162. [Google Scholar]
- Lu G, Paul AL, McCarty DR, Ferl RJ. Transcription factor veracity: is GBF3 responsible for ABA-regulated expression of Arabidopsis Adh? Plant Cell. 1996;8:847–857. doi: 10.1105/tpc.8.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J (1982) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, p 440
- Marcotte WRJ, Guiltinan MJ, Quatrano RS. ABA-regulated gene expression: cis-acting sequences and trans-acting factors. Biochem Soc Trans. 1992;20:93–97. doi: 10.1042/bst0200093. [DOI] [PubMed] [Google Scholar]
- Mason HS, DeWald DB, Mullet JE. Identification of a methyl jasmonate-responsive domain in the soybean vspB promoter. Plant Cell. 1993;5:241–251. doi: 10.1105/tpc.5.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melan MA, Dong X, Endara ME, Davis KR, Ausubel FM, Peterman TK. An Arabidopsis thaliana lipoxygenase gene is induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol. 1993;101:441–450. doi: 10.1104/pp.101.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami K, Katsura M, Ito T, Okada K, Shimura Y, Iwabuchi M. Developmental and tissue-specific regulation of the gene for the wheat basic/leucine zipper protein HBP-1a(17) in transgenic Arabidopsis plants. Mol Gen Genet. 1995;248:573–582. doi: 10.1007/BF02423453. [DOI] [PubMed] [Google Scholar]
- Moons A, Bauw G, Prinsen E, Van Montagu M, Van Der Straeten D. Molecular and physiological responses to abscisic acid and salts in roots of salt-sensitive and salt-tolerant Indica rice varieties. Plant Physiol. 1995;107:177–186. doi: 10.1104/pp.107.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons A, Prinsen E, Bauw G, Van Montagu M. Antagonistic effects of abscisic acid and jasmonates on salt-stress-inducible transcripts in rice roots. Plant Cell. 1997;9:2243–2259. doi: 10.1105/tpc.9.12.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PW, Drew MC. Ethylene and plant response to stress. Physiol Plant. 1997;100:620–630. [Google Scholar]
- Neuman DS, Smit BA. The influence of leaf water status and ABA on leaf growth and stomata of Phaseolus seedlings with hypoxic roots. J Exp Bot. 1991;42:1499–1506. [Google Scholar]
- Piatowski D, Schneider K, Salamini F, Bartels D. Characterization of five abscisic acid responsive cDNA clones isolated from the desiccation tolerant plant Craterostigma plantagineum, and their relationship to other water stress genes. Plant Physiol. 1990;94:1682–1688. doi: 10.1104/pp.94.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JK, DeSimone NA, Lingle WL, Dure L., III Cellular concentrations and uniformity of cell type accumulation of two LEA proteins in cotton embryos. Plant Cell. 1993;5:769–780. doi: 10.1105/tpc.5.7.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez HG, Roberts JKM, Jordan WR, Drew MC. Growth, water relations, and accumulation of organic and inorganic solutes in roots of maize seedlings during salt stress. Plant Physiol. 1997;113:881–893. doi: 10.1104/pp.113.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab IN, Sharp RE, Pritchard J. Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol. 1990;93:1329–1336. doi: 10.1104/pp.93.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seffens WS, Almoguera C, Wilde HD, Vonder Harr RA, Thomas TL. Molecular analysis of a phylogenetically conserved carrot gene: developmental and environmental regulation. Dev Genet. 1990;11:65–76. doi: 10.1002/dvg.1020110108. [DOI] [PubMed] [Google Scholar]
- Sembdner G, Parthier B. The biochemistry and the physiological and molecular actions of jasmonates. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:569–589. [Google Scholar]
- Sharp RE, Wu Y, Voetberg GS. Confirmation that abscisic acid accumulation is required for maize primary root elongation at low water potentials. J Exp Bot. 1994;45:1743–1751. [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit BA, Neuman DS, Stachowiak ML. Root hypoxia reduces leaf growth. Role of factors in the transpiration stream. Plant Physiol. 1990;92:1021–1028. doi: 10.1104/pp.92.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas TL. Gene expression during plant embryogenesis and germination: an overview. Plant Cell. 1993;5:1401–1410. doi: 10.1105/tpc.5.10.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekananda J, Drew MC, Thomas TL. Hormonal and environmental regulation of the carrot lea-class gene Dc3. Plant Physiol. 1992;100:576–581. doi: 10.1104/pp.100.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherwax SC, Ong MS, Degenhardt J, Bray EA, Tobin EM. The interaction of light and abscisic acid in the regulation of plant gene expression. Plant Physiol. 1996;111:363–370. doi: 10.1104/pp.111.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde HD, Nelson WS, Booij H, de Vries SC, Thomas TL. Gene expression programs in embryogenic carrot cultures. Planta. 1988;176:205–211. doi: 10.1007/BF00392446. [DOI] [PubMed] [Google Scholar]
- Wilen RW, Van-Rooijen GJH, Pearce DW, Pharis RP, Holbrook LA, Moloney MM. Effects of jasmonic acid on embryo-specific processes in Brassica and Linum oilseeds. Plant Physiol. 1991;95:399–405. doi: 10.1104/pp.95.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Duan X, Wang B, Hong B, Ho T-HD, Wu R. Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol. 1996;110:249–257. doi: 10.1104/pp.110.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol. 1988;39:439–473. [Google Scholar]
- Zhang JH, Davies WJ. ABA in roots and leaves of flooded pea plants. J Exp Bot. 1987;38:649–659. [Google Scholar]