Abstract
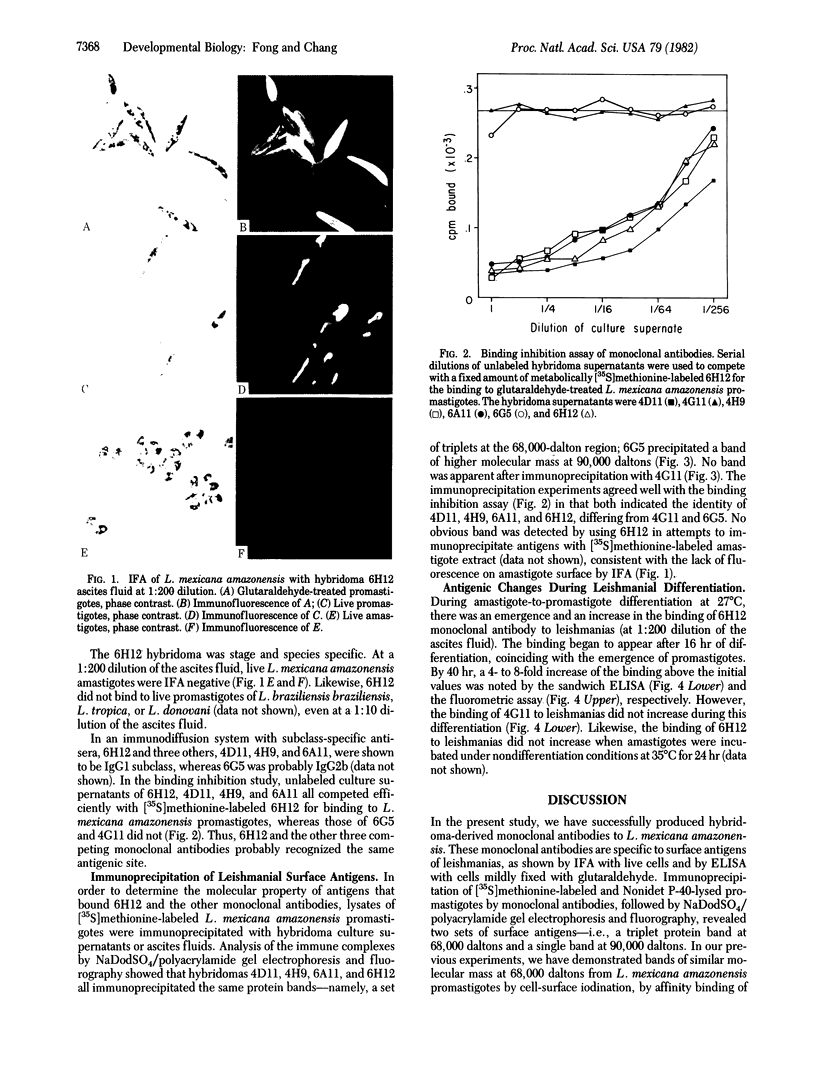
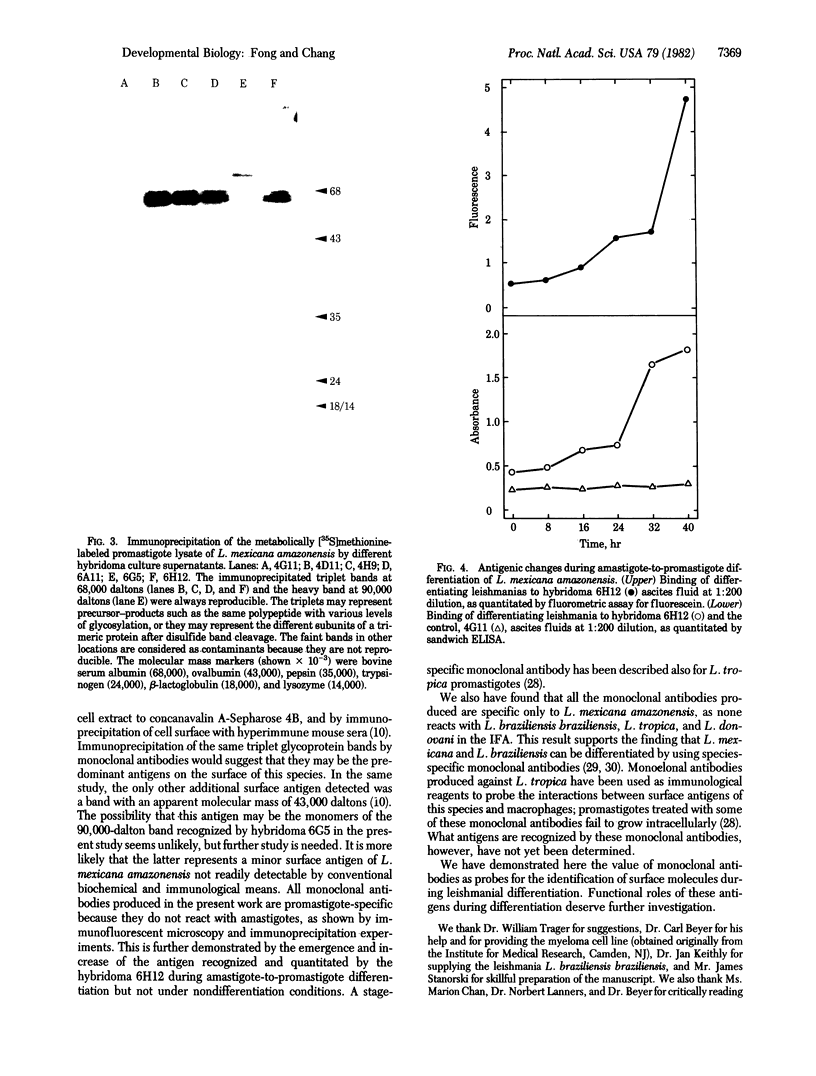
The fusion of SP2/0 myeloma cells with spleen cells from mice immunized with Leishmania mexicana amazonensis promastigotes produced hybridoma clones. Indirect immunofluorescent antibody assay with live leishmanias showed that the monoclonal antibody 6H12 recognized only the antigens bound to the surface of L. mexicana amazonensis promastigotes. It also showed that the antibody bound to neither amastigotes of this species nor to other Leishmania species--i.e., L. braziliensis braziliensis, L. tropica, and L. donovani. Monoclonal antibodies from three other clones (4D11, 4H9, and 6A11) were found to compete with 6H12 for binding to L. mexicana promastigotes. With lysates of [35S]methionine-labeled promastigotes, all four monoclonal antibodies precipitated the same triplet set of protein bands at the approximately equal to 68,000-dalton region, whereas another monoclonal antibody (6G5) precipitated a different band at approximately equal to 90,000 daltons. During differentiation of L. mexicana amazonensis from amastigotes to promastigotes, there was a 4- to 8-fold increase above the initial level in the binding of 6H12 monoclonal antibody to leishmanias, as detected by enzyme-linked immunosorbent assay and quantitative fluorometric assay, respectively. Thus, we have demonstrated the use of monoclonal antibodies as probes for antigens that change during leishmanial differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang K. P., Fong D. Antigenic changes during intracellular differentiation of Leishmania mexicana in cultured macrophages. Infect Immun. 1982 Apr;36(1):430–431. doi: 10.1128/iai.36.1.430-431.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- Coombs G. H., Craft J. A., Hart D. T. A comparative study of Leishmania mexicana amastigotes and promastigotes. Enzyme activities and subcellular locations. Mol Biochem Parasitol. 1982 Mar;5(3):199–211. doi: 10.1016/0166-6851(82)90021-4. [DOI] [PubMed] [Google Scholar]
- Coombs G. H. Proteinases of Leishmania mexicana and other flagellate protozoa. Parasitology. 1982 Feb;84(1):149–155. doi: 10.1017/s003118200005174x. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Tubulin biosynthesis in the developmental cycle of a parasitic protozoan, Leishmania mexicana: changes during differentiation of motile and nonmotile stages. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7624–7628. doi: 10.1073/pnas.78.12.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C., Wright B. Rat x rat hybrid myelomas and a monoclonal anti-Fd portion of mouse IgG. Nature. 1979 Jan 11;277(5692):131–133. doi: 10.1038/277131a0. [DOI] [PubMed] [Google Scholar]
- Handman E., Hocking R. E. Stage-specific, strain-specific, and cross-reactive antigens of Leishmania species identified by monoclonal antibodies. Infect Immun. 1982 Jul;37(1):28–33. doi: 10.1128/iai.37.1.28-33.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. T., Coombs G. H. The effects of carbon dioxide and oxygen upon the growth and in vitro transformation of Leishmania mexicana mexicana. Mol Biochem Parasitol. 1981 Nov;4(1-2):117–127. doi: 10.1016/0166-6851(81)90034-7. [DOI] [PubMed] [Google Scholar]
- Hart D. T., Vickerman K., Coombs G. H. A quick, simple method for purifying Leishmania mexicana amastigotes in large numbers. Parasitology. 1981 Jun;82(Pt 3):345–355. doi: 10.1017/s0031182000066889. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- North M. J., Coombs G. H. Proteinases of Leishmania mexicana amastigotes and promastigotes: analysis by gel electrophoresis. Mol Biochem Parasitol. 1981 Sep;3(5):293–300. doi: 10.1016/0166-6851(81)90003-7. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies that distinguish between New World species of Leishmania. Nature. 1981 Jun 18;291(5816):581–583. doi: 10.1038/291581a0. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Steiger R. F., Black C. D. Simplified defined media for cultivating Leishmania donovani promastigotes. Acta Trop. 1980 Jun;37(2):195–198. [PubMed] [Google Scholar]
- Stocker J. W., Heusser C. H. Methods for binding cells to plastic: application to a solid-phase radioimmunoassay for cell-surface antigens. J Immunol Methods. 1979;26(1):87–95. doi: 10.1016/0022-1759(79)90044-9. [DOI] [PubMed] [Google Scholar]