Abstract
The aim of this study was to determine the frequency of subdural haematomas (SDHs) occurring in infants presenting following atraumatic cardiorespiratory collapse. This study was a review of retrospective case notes, brain imaging and post-mortem examinations carried out in the paediatric intensive care unit (PICU) and emergency department (ED) in a tertiary paediatric centre in the UK. The study included infants and children less than 4 years old dying in the ED or admitted to the PICU after atraumatic cardiorespiratory arrest. We identified macroscopic SDHs on brain imaging or post-mortem examination. Of those children who experienced a cardiorespiratory arrest from a non-traumatic cause and met inclusion criteria, 33 presented and died in the ED and 17 were admitted to the PICU. These children had a post-mortem examination, brain imaging or both. None of these infants had a significant SDH. One child had a small clot adherent to the dura found on post-mortem and two had microscopic intradural haemorrhage, but it is unclear in each case whether this was artefact, as each had otherwise normal brains. Subdural haematoma arising in infants or young children in the context of catastrophic cardiorespiratory compromise from a non-traumatic cause was not observed.
There is wide consensus that the triad of coincidental injuries consisting of encephalopathy, subdural haematoma (SDH) and retinal haemorrhages is strongly associated with non-accidental head injury (NAHI). However, in the absence of other somatic signs or symptoms of trauma, such as unusual bruises or fractures, the diagnosis of NAHI based solely upon the triad of findings remains contentious. The possibility of a non-accidental cause may be suggested by an inconsistent or changing history. In such cases the interpretation of radiological and pathological findings is often central to the determination of a diagnosis.
Recent publications have reviewed the anatomy of the dura, with particular reference to that of the infant [1], and the putative role of hypoxia–ischaemia in the causation of SDHs in infancy [2]. If a causal relationship between hypoxia–ischaemia associated with cardiorespiratory arrest and SDH is confirmed, the significance of SDHs seen in infants admitted with unexplained encephalopathy and who are found to have SDHs on admission brain imaging may have potentially profound medicolegal implications. So far, this association has been generated by research emanating from neuropathological sources and has not been supported by widespread clinical experience, the general literature of hypoxic–ischaemic encephalopathy in early life [3, 4], radiological literature [5–10] and a recent multicentre multinational post-mortem (PM) examination study [11]. It is particularly pertinent that the extensive imaging literature relating to radiological manifestations of hypoxic–ischaemic injury in early life does not refer to or identify SDH as a component of the injury spectrum or as a sequela.
In this retrospective study, we investigate a cohort of infants and young children who have experienced cardiorespiratory arrest, with the aim of determining whether severe hypoxic–ischaemic episodes result in macroscopic SDH.
Methods and materials
The clinical coding department, for the period January 2001 to May 2007, identified all children less than 4 years of age admitted to our regional paediatric intensive care unit (PICU). In addition, all children aged less than 4 years admitted to, and dying in, the emergency department (ED) were identified from the bereavement services database.
All children were included in the study if they were in the eligible age group and had suffered a cardiorespiratory arrest, defined as requiring chest compressions or a collapse that led to the child's death. Children who had suffered a cardiorespiratory arrest that accompanied cranial trauma (including occult trauma identified subsequently at PM examination) were excluded. Other exclusion criteria included an isolated respiratory arrest, coagulopathy, haemolytic uraemic syndrome and children with neurological conditions (meningitis, encephalitis, cerebral infection, metabolic and neurodegenerative disorders).
The clinical coding database with the ICD-10 (International Classification of Diseases, World Health Organization) diagnosis code and the PICU discharge database were examined. Those cases identified as having a cardiorespiratory arrest or where assessment could not be made from the database had a full review. All case notes of those children who had died in the ED were examined.
Demographic details of each case were recorded, as were details of the resuscitation, where available. In the event of the child's death, details of the PM examination and/or PM brain imaging were documented. In the event of the child's survival, if the child had post-arrest brain imaging, the results of these examinations were documented. All brain imaging examinations were independently reported by two experienced neuroradiologists (TJ and RAD) blinded to the clinical information. In cases where there were discrepancies in opinion (three cases, all regarding the extent of hypoxic–ischaemic changes on CT scans), consensus was reached following review of the imaging by a third experienced neuroradiologist (NSM). The presence or absence of intracranial haemorrhage located over the surface of the brain (classified as subarachnoid haemorrhage, SDH or uncertain compartment) and the extent, if present, of hypoxic–ischaemic brain injury were documented.
The study was part of a larger ongoing project investigating NAHI in infancy and had ethical approval from the Local Research Ethics Committee.
Results
Demographic analysis
The final cohort of 50 cases included 31 males and 19 females. The age range of these children was 0–39 months. 48 children were less than 24 months old, with 40 being less than 6 months old. The median age of the cohort was 3.1 months (mean 5.7 months). The median duration of resuscitation that was retrievable from documentation in 26 cases was 21 min (range 15–55 min; mean 17.34 min). Periresuscitative blood gas analysis was available in 18 cases, with a median pH of 6.6 (range 6.3–7.2; mean 6.6).
All 50 children were investigated by either PM examination or post-arrest brain imaging, as summarised in Table 1. 40 children underwent a PM examination, with full histological detail available in 31 cases. 14 children had both PM examination and brain imaging.
Table 1. Summary of investigations to identify the presence subdural haematoma.
| Number included |
Number who died |
Post-insult, pre-mortem imaging only |
Both pre-mortem imaging and PM examinationa | Post-mortem investigation only |
|||||
| Cranial CT only | Cranial MRI only | Cranial CT and MRI | Cranial CT only | PM examination onlya | Both CT and PM examinationa | ||||
| PICU | 17 | 10 | 6 | 1 | 2 | 2b | 1 | 4 | 1 |
| ED | 33 | 33 | 0 | 0 | 0 | 0 | 0 | 22 | 11 |
| Total | 50 | 43 | 6 | 1 | 2 | 2b | 1 | 26 | 12 |
ED, emergency department; PICU, paediatric intensive care unit; PM, post-mortem.
aWhere post-mortem examination report was available.
bIn both cases imaging was by CT only.
Of the 50 cases included in the cohort, 24 (48% of the cohort) children underwent brain imaging. The imaging was judged to be of good quality in all cases. 13 of these examinations were undertaken after death. 22 children were initially examined by CT (GE Lightspeed Plus, Milwaukee, WI, or Philips MX 8000, Erlangen, the Netherlands) and two by MRI (Philips Intera 1.5 T). Three of those who had an initial CT went on to have an MRI, one child who had an initial MRI had a repeat MRI and another child had an initial CT and MRI followed by a repeat CT a day later.
29 children underwent a radiographic skeletal survey, which was normal in all cases; a formal ophthalmological examination was undertaken in 5 children, which was normal in all cases.
Case identification
PICU cases
The clinical coding database identified 581 children less than 4 years of age who had been admitted to the PICU over the period January 2001 to May 2007 (Figure 1). Each case was examined by reference either to the ICD-10 coding database or to the PICU discharge summary database or through hand searching the case notes. 125 cases were excluded as identified from the ICD-10 coding database owing to meeting pre-defined exclusion criteria. A further 312 were excluded after examination of the PICU discharge summary database determined that these cases did not suffer a cardiorespiratory arrest. The details on the database for the remaining 144 cases demonstrated either that the case met the inclusion criteria or that further information was needed. The case notes for these children were selected for review.
Figure 1.
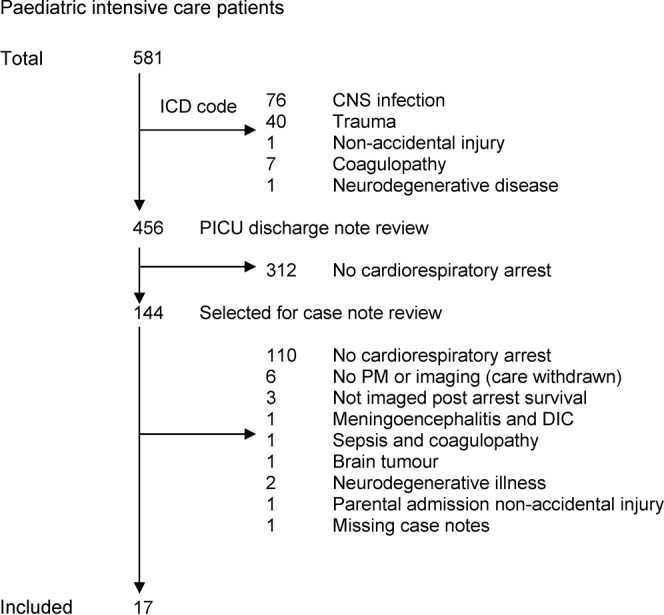
Case identification of children admitted to the paediatric intensive care unit. ICD, International Classification of Diseases; CNS, central nervous system; PM, post-mortem; DIC, disseminated intravascular coagulation.
From this group, 17 children are included in the final analysis. Those not included were excluded because the case note review determined that no cardiorespiratory arrest had occurred (n = 110), the child had a pre-defined exclusion criterion (n = 16) or the case notes were missing with no scan or PM records (n = 1).
Of the 17 children included, 10 had died. Of these, seven PM examinations were available for review. Of those who had died in PICU, the average time from admission to death was 2.9 days (median 2.5 days; range 0–7 days). We do not have PM results for three children. In one case, care was withdrawn and a PM examination was not deemed necessary (although post-arrest brain imaging had occurred prior to withdrawal of care). For the remaining two children we do not have access to the PM result as in one case it was unavailable and the other was performed out of the area.
ED cases
From the bereavement services database, 38 children were identified as having died in the emergency department (Figure 2). The final analysis included 33 cases; five were excluded because they either met pre-defined exclusion criteria (n = 2) or the PM examination results were unavailable and no PM imaging occurred (n = 3). Of the former two excluded cases, one had meningococcaemia and the other was excluded because of the likelihood of a traumatic cause: the child had diffuse retinal haemorrhages, petechial haemorrhages within the brain parenchyma accompanied by subarachnoid and intraventricular blood. No SDH was identified in either of these cases.
Figure 2.
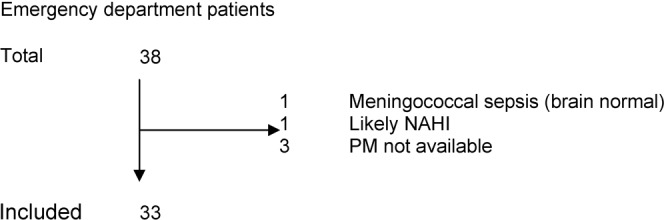
Case identification of children dying in the emergency department. NAHI, non-accidental head injury; PM, post-mortem.
Radiology
The imaging was classified as having features of diffuse hypoxic–ischaemic injury (global cerebral hypodensity) in 18 cases (75% of those imaged) (Figures 3 and 4). This includes 10 of the children who in which the brain imaging was performed PM (Figure 5). Four cases (17.4%) were classified as patchy hypoxic–ischaemic injury, characterised by discrete hypodense lesions with normal intervening brain (Figures 6 and 7). In two cases (8.7%), the brain appeared normal. No extra-axial haemorrhage (subarachnoid, subdural or indeterminate compartment) or parenchymal brain haemorrhage was identified in any of the 24 children who underwent brain imaging (Figure 6).
Figure 3.
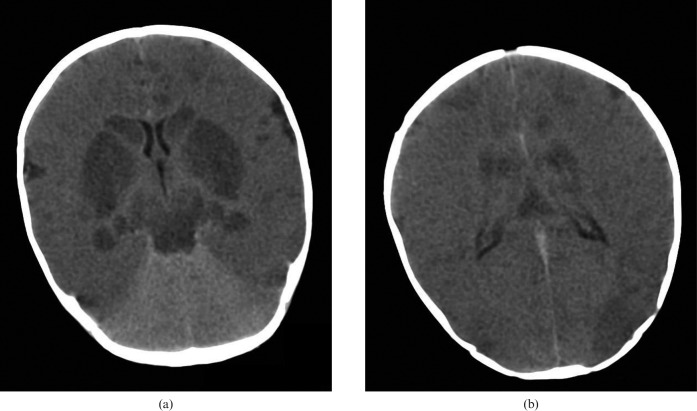
Diffuse hypoxic brain injury. 3-month-old infant scanned post mortem after presenting following a history of overlying. Unenhanced cranial CT at (a) the basal ganglia and (b) the bodies of the lateral ventricles showing a global reduction in cerebral density with loss of cortical visualisation and a “bright cerebellum” sign, with more pronounced hypodensity of the deep grey matter nuclei, the hippocampi, the midbrain and parts of the cortex.
Figure 4.
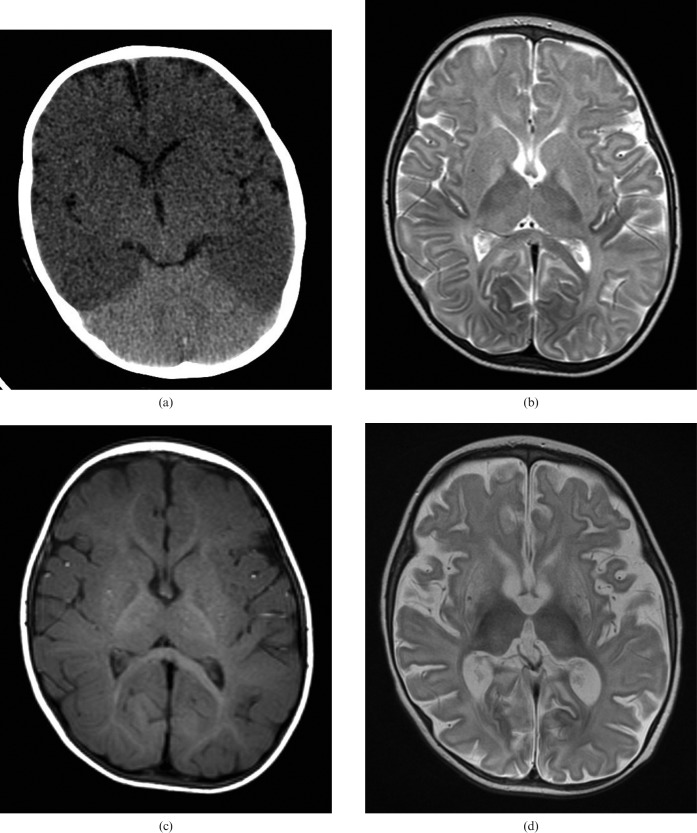
Diffuse hypoxic brain injury in a 7-month-old infant admitted following a successful resuscitation of cardiac arrest with associated dilated cardiomyopathy. (a) Unenhanced cranial CT scan on the day of admission shows global reduction in cerebral density with reduced grey/white matter differentiation and a “bright cerebellum sign”. (b) Unenhanced axial T2 and (c) T1 weighted MRI scans 4 days following initial CT showing T2 hyperintensity in the globi pallidi, with subtle T2 hyperintensity in the putamen and thalami bilaterally. Note the absence of subdural haematoma on the T1 weighted image. (d) Axial T2 weighted MRI at 3 weeks following the acute presentation, demonstrating generalised atrophy, particularly affecting the basal ganglia.
Figure 5.
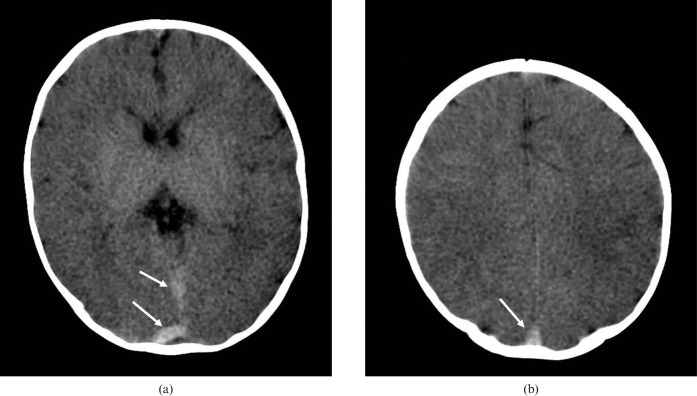
Post-mortem cranial CT in a 3-month-old infant who presented to the emergency department in cardiorespiratory arrest after being found unresponsive in the parents' bed. Images (a) at the level of the basal ganglia and (b) through the centrum semiovale show generalised reduction in grey/white matter differentiation. Note the dense post-mortem appearances of the dural venous sinuses (white arrows).
Figure 6.
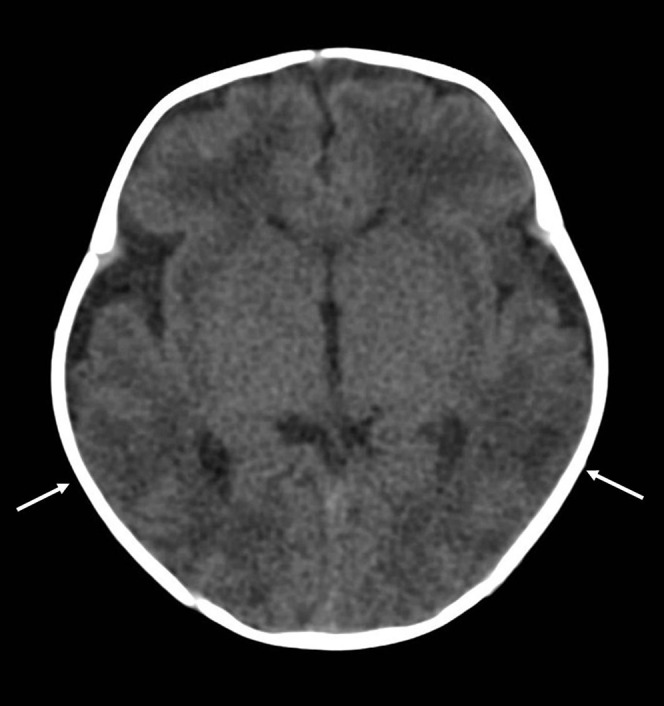
Patchy hypoxic brain injury in a 1-month-old infant following an admission for apnoea. A cardiorespiratory arrest was witnessed on the ward. Unenhanced cranial CT scan shows focal loss of grey/white matter differentiation in the posterior temporal regions bilaterally (white arrows).
Figure 7.
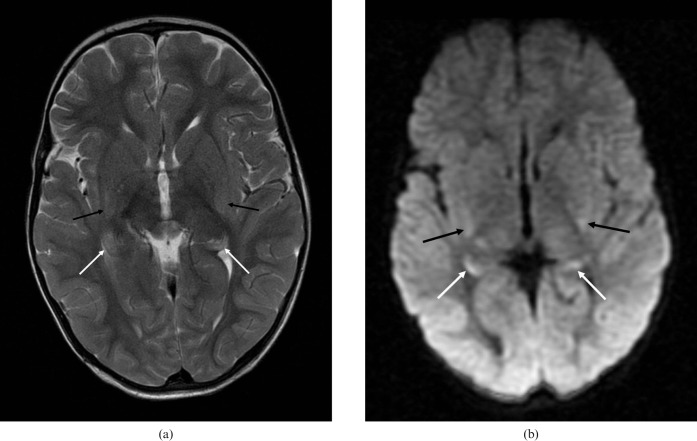
Focal hypoxic injury in a child aged 1 year and 11 months. MRI scan performed 3 days after a near-drowning incident. (a) Axial T2 image shows subtle hyperintensity of the hippocampal tails (white arrows) and posterior putamen bilaterally (black arrows), with (b) corresponding high signal on the B = 1000 diffusion-weighted images.
With respect to the presence or absence of intra- or extra-axial intracranial haemorrhage, there was complete concordance between the brain imaging and PM findings in all 14 children who underwent both imaging and PM examinations. There were therefore no false-positive or false-negative scans.
Post-mortem examination
PM examination records were examined for 40 cases in the cohort (93% of those who died and 80% of the total cohort). Two cases were part of the cohort of cases included in the paper by Byard et al [11].
Macroscopic findings
In 39 cases there was no macroscopic evidence of SDH. In one case, that of an 18-day-old male infant with suspected overlying, there was a small clot adherent to the dura. This was considered by the examining pathologist to be consistent with the sequelae of recent birth, the brain itself being normal.
In another case, a 3-month-old baby (who did not undergo brain imaging) had a left anterotemporal intracerebral haematoma resulting from an arteriovenous malformation. A third case, a 4-month-old who died from respiratory syncytial virus (RSV) bronchiolitis, had an infarct in the left caudate nucleus.
Microscopic findings
Microscopic findings were available for 31 cases. Histological examination showed no evidence of microscopic SDH in any of these cases.
In two cases (60-day-old and 88-day-old babies found unresponsive in bed), histological examination of the dura demonstrated minor foci of intradural haemorrhage but normal underlying brains. The attending pathologist was uncertain whether this was a genuine finding or an artefact caused by removing the dura from the skull. The cause of death was recorded as sudden unexpected death in infancy and sudden infant death syndrome, respectively.
Two of the cases in this cohort had a population of siderophages in the dura. One was in the case discussed above in association with a small clot that was related to birth injury. The other case was that of a 4-month-old baby who had been found face down on the bed. No evidence of macroscopic SDH was found on brain imaging or at PM.
Other findings
In three cases, extradural haemorrhage was demonstrated in the spinal canal. In all cases this was felt to be a PM artefact [12]. Histology from a 5-month-old baby dying from freshwater drowning demonstrated unifocal subarachnoid haemorrhage but no SDH.
Discussion
We present an observational, descriptive process for the investigation of the sequelae of atraumatic cardiorespiratory arrest and hypoxic–ischaemic brain injury of varying degrees of severity. Children who had a cardiorespiratory arrest warranting chest compressions, or an arrest leading to death without an associated traumatic, coagulopathic or neuropathic origin, were included.
In our cohort only one child had an identifiable SDH, and this was felt to be unrelated and separate in time to the demise of the baby. Our study agrees with the findings of a recent study of foetuses, babies and children forensically examined following atraumatic deaths [11].
The true incidence of SDH at birth is unknown, but is reported on imaging to range from 9% to 46%, irrespective of the method of delivery or presence of excessive birth trauma [13–15]. In addition, those that resolve by 4 weeks are held to be benign and clinically asymptomatic with no long-term effects. The baby with “minimal clot” in our cohort is consistent with this description.
The hypothesis paper of Geddes et al [16] included children dying from hypoxia and non-accidental injury. They noted two phenomena: microscopic intradural, subdural and retinal bleeding in those following a non-trauma-related hypoxic insult and extensive subdural haemorrhage identified at PM and brain imaging in those children who had been abused. They argued that the difference in neuropathology was one of degree.
In our cohort of a similar size, we identified three cases: one of subdural and two of microscopic intradural haemorrhage. Taken at face value, this represents an incidence of 9.7% of those cases where microscopic findings are available. However, in each of these cases there is considerable doubt about the significance of the findings in that the attending pathologist considered them to be pre-existing or an artefact, with otherwise normal brains.
The Geddes group also suggest that data from children up to 2 years of age are not relevant to their cohort and findings of infant physiology [17]. 86% (43/50) of cases in our cohort were younger than the 9-month-old threshold that the Geddes group used.
Mack et al [1] revisited the pathological anatomy, embryology and development of the meninges with specific reference to infancy. The authors note that the infant dura contains an inner vascular plexus that is likely to play a role in cerebrospinal fluid absorption, the arachnoid granulations being incompletely developed at this early stage in life. The authors note that SDHs in infancy are frequently traumatic; however, they go on to suggest that the particularly prominent inner vascular plexus of infants is the likely source of subdural bleeding in non-traumatic circumstances. Although comprehensive multisite sampling of the dura was not undertaken in all the PM cases in our study cohort, the dura was examined both macroscopically in all cases and with selected dural sampling in most; both intradural and subdural haemorrhage was notable by its absence in all but three cases.
In a recent publication by Cohen and Scheimberg [2], the authors describe a pathological study of a cohort of 25 foetuses and 30 neonates who exhibited obvious macroscopic intradural haemorrhage (IDH) and hypoxia of varying degrees of severity. The authors indicate that in two-thirds of their cases frank SDHs were present as a thin film in relation to posterior located falcine IDH. All the foetuses examined had died within 2 h of birth. Of the post-natal cases, the ages ranged from 0 to 19 days. The authors concluded that SDH and cerebral hypoxia are common associations of IDH, and that SDH almost always occurs in association with diffuse falcine IDH. This study reflects the dichotomy that exists between such pathological studies and what is encountered in common clinical and radiological practice. It is particularly notable that Cohen and Scheimberg's neuropathological study included the examination of neonates up to 19 days of age. It is now recognised that silent thin-film SDHs are relatively common within the first month of life [14, 15], occurring in the absence of hypoxic–ischaemic brain injury in the vast majority of cases. It is thus statistically likely that at least some of these cases would have had incidental birth-related IDHs and or SDHs.
Unfortunately, the full clinical details of the Cohen and Scheimberg [2] cohort of infants are not provided in the methodology of the study; however, the fact that all the infants exhibited hypoxia of varying degrees of severity suggests that they were examining a highly selected cohort of compromised foetuses and sick neonates. In the context of the older infant with no pre-existing comorbidity admitted to hospital following a cardiorespiratory arrest, such as that forming the basis of our study, the confounding element of coexisting birth-related SDHs can be discounted. All the infants in our study fulfilled the criteria of the neuropathological study, but in contradistinction there was an almost complete absence of IDH and SDH. This would suggest that hypoxia–ischaemia has no direct causal relationship to SDH formation in this age group. This lack of linkage is of vital importance in determining the causation of SDHs in an infant admitted to hospital with unexplained encephalopathy, diffuse thin-film SDH and retinal haemorrhages.
Our study is limited by a lack of histological findings for all the children. Primarily this is because seven children survived and, of the 43 children who died only 40 underwent PM, with histology results available in 31 cases.
It has not been possible to determine the minimum interval between insult and death. However, with a significant proportion of cardiorespiratory arrest likely to be from a primary respiratory cause, it is possible to speculate that the brain underwent a degree of hypoxia prior to the cardiac hypoxia causing arrest. This is consistent with findings from previous studies [18]. 18 (75%) of all cases in our series that were imaged, and a majority of the cases that underwent brain imaging while alive (7/11; 64%), demonstrated diffuse hypoxic–ischaemic injury. It is therefore likely that these children experienced a significant hypoxic–ischaemic injury and survived for a sufficient period to develop the imaging features (“black brain”) of diffuse cerebral hypodensity.
In addition, some children had only brain imaging or the accompanying PM reports were unavailable, leaving the possibility that absence of SDH on brain imaging could not be confirmed pathologically. However, the 33% of cases that had both brain imaging and PM examination act as an internal control for the study. Additionally, each radiological examination was reported by two independent neuroradiologists, with adjudication in equivocal cases by a third neuroradiologist making the possibility of misdiagnosis unlikely.
Our aim was to include all cardiorespiratory arrests occurring within or presenting to our institution during the period of interest. However, potentially a child could have been resuscitated in the emergency department at a time when the PICU was unable to take admissions, resulting in the child being retrieved to another centre and so excluded from our cohort.
It could be argued that the lack of multisite dural sampling may have led to failure to identify IDH in some of the cases in our cohort. However, the aim of this study was to determine whether macroscopic SDH, occurring as a consequence of atraumatic cardiorespiratory arrest and consequent hypoxic–ischaemic brain injury, occurred as identified by imaging, PM examination or both. This outcome was not observed in our study group.
Conclusion
We conclude that cardiopulmonary collapse per se and the attendant hypoxic–ischaemic sequelae do not cause SDH. Our findings are consistent with those of the multicentre study of Byard et al [11] and the general experience of clinicians and radiologists supporting the position that hypoxic–ischaemic brain injury does not cause macroscopic SDHs in early life. In the case of an infant presenting with a clinically significant SDH evident on brain imaging, the aetiology is very unlikely to be the collapse or resuscitative measures. In such cases an underlying cause for both the collapse and the SDHs needs to be found, and the possibility that the observed haemorrhage may be traumatically inflicted must be considered.
Footnotes
Competing interests: TJ, NSM and TS have been expert witnesses in cases of alleged NAHI.
References
- 1.Mack J, Squier W, Eastman JT. Anatomy and development of the meninges: implications for subdural collections and CSF circulation. Pediatr Radiol 2009;39:200–10 [DOI] [PubMed] [Google Scholar]
- 2.Cohen MC, Scheimberg I. Evidence of occurrence of intradural and subdural hemorrhage in the perinatal and neonatal period in the context of hypoxic ischemic encephalopathy. An observational study from two referral institutions in the United Kingdom. Pediatr Dev Pathol 2009;12:169–76 [DOI] [PubMed] [Google Scholar]
- 3.Volpe JJ. In: Neurology of the newborn (5th edn).Hypoxic-ischemic encephalopathy: clinical aspects. Saunders,Philadelphia: Volpe JJ.2008;408–27 [Google Scholar]
- 4.Levene MI, de Vries LS. Levene MI, Chervenak FA.Fetal and neonatal neurology and neurosurgery. Neonatal incranial hemorrhage (4th edn).Philadelphia Churchill Livingstone,2009;395–430 [Google Scholar]
- 5.Barkovich AJ, Westmark K, Partridge C, Sola A, Ferriero DM. Perinatal asphyxia: MR findings in the first 10 days. AJNR Am J Neuroradiol 1995;16:427–38 [PMC free article] [PubMed] [Google Scholar]
- 6.Barkovich AJ. In: Pediatric neuroimaging (4th edn) Brain and spine injuries in infancy and childhood.Philadelphia, USA:Raven Press; 226–244 [Google Scholar]
- 7.Barkovich AJ, Miller SP, Bartha A, Newton N, Hamrick SEG, Mukherjee P, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol 2006;27:533–47 [PMC free article] [PubMed] [Google Scholar]
- 8.Rao P, Carty H, Pierce A. The acute reversal sign: comparison of medical and non-accidental injury patients. Clin Radiol 1999;54:495–501 [DOI] [PubMed] [Google Scholar]
- 9.Christophe C, Fonteyne C, Ziereisen F, Christiaens F, Deltenre P, De Maertelaer V, et al. Value of MR imaging of the brain in children with hypoxic coma. AJNR Am J Neuroradiol 2002;23:716–23 [PMC free article] [PubMed] [Google Scholar]
- 10.Huang B, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics 2008;28:417–39 [DOI] [PubMed] [Google Scholar]
- 11.Byard RW, Blumbergs P, Rutty G, Sperhake J, Banner J, Krous H, et al. Lack of evidence for a causal relationship between hypoxic-ischaemic encephalopathy and subdural haemorrhage in fetal life, infancy and early childhood. Pediatr Dev Pathol 2007;10:348–50 [DOI] [PubMed] [Google Scholar]
- 12.Rutty GN, Squier WMV, Padfield CJH. Epidural haemorrhage of the cervical spinal cord: a post-mortem artefact? Neuropathol Appl Neurobiol 2005;31:247–57 [DOI] [PubMed] [Google Scholar]
- 13.Whitby EH, Griffiths PD, Rutter S, Smith M, Sprigg A, Ohadike P, et al. Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors. Lancet 2004;363:846–51 [DOI] [PubMed] [Google Scholar]
- 14.Looney CB, Smith JK, Merck LH, Wolfe HM, Chescheir NC, Hamer RM, et al. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology 2007;242:535–41 [DOI] [PubMed] [Google Scholar]
- 15.Rooks VJ, Eaton JP, Ruess L, Petermann GW, Keck-Wherley J, Pedersen RC, et al. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. AJNR Am J Neuroradiol 2008;29:1082–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geddes J, Tasker R, Hackshaw A, Nickols CD, Adams GGW, Whitwell HL, et al. Dural haemorrhage in non-traumatic infant deaths: does it explain the bleeding in ‘shaken baby syndrome’? Neuropathol Appl Neurobiol 2003;29:14–22 [DOI] [PubMed] [Google Scholar]
- 17.Geddes JF, Tasker RC, Adams GGW, Whitwell HL. Violence is not necessary to produce subdural and retinal haemorrhage: a reply to Punt et al. Pediatr Rehabil 2004;7:261–5 [DOI] [PubMed] [Google Scholar]
- 18.Sirbaugh PE, Pepe PE, Shook JE, Kimball KT, Goldman MJ, Ward MA, et al. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest. Ann Emerg Med 1999;33:174–84 [DOI] [PubMed] [Google Scholar]