Abstract
The differentiation of idiopathic normal-pressure hydrocephalus (INPH) from other types of dementia is a clinical challenge. The aim of this prospective study was to evaluate the role of proton MR spectroscopy (MRS) and white matter hyperintensities (WMH) in the diagnosis of INPH, predicting response to therapy and differentiating INPH from other dementias. The study included 18 patients with INPH (Group 1), 11 patients with other types of dementia (Group 2) and 20 control patients (Group 3). The value of WMH scores and MRS findings in diagnosis, evaluation of response to therapy and in the differentiation of INPH from other dementias was statistically evaluated. The level of statistical significance was set at p<0.05 (Kruskal–Wallis and Mann–Whitney U-test). In both Groups 1 and 2, N-acetylaspartate (NAA)/choline-NAA/creatine ratios were significantly less than in the control group (p<0.05). The WMH and MRS findings of Groups 1 and 2 demonstrated no statistically significant correlation (p>0.05). No correlation was found between the outcome of shunt operations and WMH and MRS findings (p>0.05). In conclusion, neither WMH nor MRS were useful in differentiating INPH from other types of dementia. WMH and MRS showed no additional benefit in identifying INPH patients who will better respond to shunt therapy.
Idiopathic normal-pressure hydrocephalus (INPH) is a rare disease affecting the elderly [1]. The exact aetiology of the disease is unknown and the most common symptoms are dementia, gait apraxia and urinary incontinence [1, 2]. INPH differs from other dementias in that the symptoms can show regression with cerebrospinal fluid (CSF) diversion [2, 3]. This opportunity for treatment makes it important to differentiate INPH from other dementias that cause senile changes, vascular disease and Alzheimer's [3, 4].
Many tests have been employed in the diagnosis of INPH, including CSF pressure measurements, intrathecal saline infusion tests, intermittent CSF drainage, cerebral blood flow (CBF) measurements and brain biopsy [3]. In addition, imaging methods such as radionuclide cisternography, CT, MRI, CT cisternography, phase-contrast cine MRI and perfusion MRI have also been used [5–7]. Treatment options for INPH include third ventriculostomy, ventriculoperitoneal shunt (VPS) or lumboperitoneal shunt procedures [8]. The success rate of shunt therapies varies between 30% and 65% [9–12].
Some reports have emphasised that subcortical and deep white matter hyperintensities (WMH) on T2 weighted images are more common in patients with INPH [2, 11]. This finding is attributed to ischaemia of small vessels owing to low CBF [2, 13]. Also, recent studies have reported a relationship between WMH and vascular compliance and pulsation defects [14]. Some authors suggest that the response to shunt therapy is worse in patients with WMH, while others propose the opposite [2, 7, 11, 15].
Proton MR spectroscopy (MRS) is a non-invasive technique that images some of the metabolites in brain tissue [16, 17]. Although MRS is commonly used in differentiating a variety of brain lesions, the number of articles evaluating its efficacy in the diagnosis of INPH is limited [10, 18–21]. MRS can aid in analysing the severity of neuronal injury before the treatment and effects of shunt therapy [21]. N-acetylaspartate (NAA) is a metabolite mainly found in neurons and is accepted as the neuronal marker [4, 16, 17]. A decrease in NAA levels shows neuronal injury and loss, as the regeneration capacity of the neurons is limited [18, 20]. In the other dementia syndromes, the NAA peak decreases irreversibly [18–21]. By contrast, in INPH, although cerebral functions can deteriorate because of ventriculomegaly, minimal NAA decrease or neuronal loss is observed [21]. This finding implies that cerebral injury is reversible.
The aim of this study was to evaluate both the efficacy of MRS and the quantification of WMH in the differential diagnosis of INPH from other causes of dementia. We also hoped to assess the ability of these approaches to predict response to therapy.
Methods and materials
Patient population
Patients suffering from dementia who were sent to our department for routine MRI were included in the study. MRS was added to the imaging protocol and both MRS and routine MRI findings were prospectively evaluated. Three groups of patients were identified. Group 1 comprised patients diagnosed as having probable INPH on the basis of clinical, laboratory and radiological findings, as well as on spinal tap tests. Group 2 patients were those on routine clinical follow-up owing to other dementias (senile, vascular, dementia due to Alzheimer's disease) [12]. The diagnosis of dementia was confirmed in both groups with neuropsychological tests. An experienced neurologist (OT) and neuroradiologist (BH) decided the grouping of patients in consensus based on their diagnoses according to clinical guidelines for INPH [12]. The control group (Group 3) comprised patients of the same age who had MRI scans in response to headache.
The number of patients included in each group was as follows: Group 1, 18 patients (8 males, 10 females; mean age 66 years, age range 50–75 years); Group 2, 11 patients (6 males, 5 females; mean age 64 years, age range 45–79 years); Group 3, 20 patients (10 males, 10 females; mean age 53 years, age range 40–75 years) (Table 1). The patients included in the control group had no pathological findings or any additional illnesses. Patients under 40 years of age were not included in the control group, as INPH mainly affects the elderly. Patients with trauma, depression, malignancy, intracranial mass lesion, bleeding, obstructive hydrocephalus or intracranial infectious disease were also excluded from the study. All of the patients in Groups 1 and 2 had at least two of the following symptoms: urinary incontinence, dementia or apraxia. The Evans index was calculated for each patient by dividing the maximum width between the frontal horns of by the lateral ventricles to the length between the two inner tabulae [6]. Patients with an Evans index <0.30 were not included in either Group 1 or 2. Informed consent was taken from all patients before the examination. The study was approved by the ethics committee of our university.
Table 1. Demographic characteristics of the three patient groups.
| INPH |
OD |
Controls |
|
| Group 1 | Group 2 | Group 3 | |
| Number of cases (female/male) | 18 (10/8) | 11 (5/6) | 20 (10/10) |
| Mean age, years (range) | 66 (50–75) | 64 (45–79) | 53 (40–75) |
INPH, idiopathic normaly-pressure hydrocephalus; OD, other dementias.
Imaging procedures
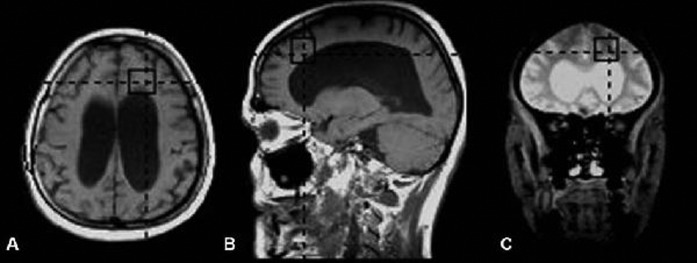
Brain MRI examinations were performed in a 1.5 T MR device (Magnetom Vision Plus; Siemens, Erlangen, Germany) with a standard head coil according to the following MRI protocol: fluid attenuated inversion recovery (FLAIR) axial plane (time to repeat (TR)/time to echo (TE) 8400/114; time interval (TI) 2150 msn; field of view (FOV) 230; matrix 256×256), T1 weighted spin-echo (SE) axial and sagittal planes (TR/TE 550/18; matrix 192×256; FOV 230; 4 mm slice thickness and 1 mm slice gap) and T2 weighted turbo spin-echo (TSE) axial and coronal planes (TR/TE 5400/99; FOV 230 mm; matrix 345×512; slice thickness 2 mm) were applied. Following these sequences, MRS using position resolved spectroscopy (PRESS) sequence was performed by placing an 8 cm3 VOI (volume of interest) in the frontal lobe neighbouring the frontal horn of lateral ventricle (TE/TR 135/2000; NEX 136) (Figure 1). The total examination duration of all sequences was about 20 min.
Figure 1.
Representative (a) axial, (b) sagittal T1 weighted and (c) coronal T2 weighted MR spectroscopy images. The black rectangles indicate the region of interest for MR spectroscopy. To achieve a reproducible position, the voxels were placed in the same regions in all patients and controls.
Following the acquisition of all images, MRS findings in addition to routine MRI findings were evaluated by two radiologists (OA, MP) blind to the clinical and laboratory data at the workstation of our MR unit. Ratios of NAA, choline (Cho) and creatine (Cr) peaks were calculated. WMH at the lateral ventricular and supraventricular levels detected on FLAIR and T2 weighted images were scored visually according to the grading system:
Grade 1: less than 25% of white matter affected.
Grade 2: 25–50% of white matter affected.
Grade 3: 50–75% of white matter affected.
Grade 4: more than 75% of white matter affected.
Patients in Group 1 were followed clinically for 1 year to assess shunt responsiveness. To evaluate predictors of outcome, treatment response to CSF diversion was defined as improvement in at least one symptom of INPH (definite INPH). MR and MRS findings of all patients were compared with clinical and laboratory examinations, as well as post-operative outcomes. The contribution of the findings to the diagnosis and therapy was statistically analysed.
Statistical analysis
All statistical analysis was performed with SPSS 13.0 software (SPSS Inc., Chicago, IL). The concordance of the data to the normal variation was evaluated with the Shapiro–Wilk test. The results from all three groups were compared with Kruskal–Wallis χ2 tests. The relationship between two groups was evaluated with the Mann–Whitney U-test. The level of statistical significance was set at p<0.05.
Results
The Evans indices of all patients in Groups 1 and 2 were >0.3. The mean Mini-mental State Examination (MMSE) scores in Groups 1 and 2 were 18.6 (range 15–22) and 17.3 (range 15–20), respectively. All patients in Groups 1 and 2 had dementia. All patients in Group 1 and 9 out of 11 patients in Group 2 had gait apraxia. Urinary incontinence was present in 14 out of 18 patients in Group 1, whereas 10 out of 11 patients in Group 2 were incontinent. There was no statistically significant difference between the presence of symptoms and each group (p>0.05). In Group 1, 12 (67%) patients improved following VPS operation; the remaining 6 patients (33%) did not improve (Table 2). There was no statistical significance between the presence of symptoms and the response to the shunt surgery in INPH patients (p>0.05).
Table 2. Symptoms and shunt outcomes of patients with probable INPH (Group 1).
| No. (M/F) | Age (years) | Gait apraxia | Urinary incontinence | Dementia | Shunt outcome |
| 1 (F) | 75 | + | + | + | + |
| 2 (F) | 63 | + | + | + | − |
| 3 (M) | 65 | + | + | + | − |
| 4 (M) | 70 | + | − | + | + |
| 5 (M) | 66 | + | + | + | + |
| 6 (M) | 66 | + | + | + | + |
| 7 (F) | 68 | + | + | + | + |
| 8 (F) | 50 | + | − | + | − |
| 9 (M) | 50 | + | − | + | + |
| 10 (F) | 75 | + | + | + | + |
| 11 (F) | 70 | + | − | + | + |
| 12 (M) | 73 | + | + | + | − |
| 13 (M) | 59 | + | + | + | − |
| 14 (F) | 60 | + | + | + | − |
| 15 (M) | 73 | + | + | + | + |
| 16 (F) | 57 | + | + | + | + |
| 17 (F) | 74 | + | + | + | + |
| 18 (F) | 72 | + | + | + | + |
INPH, idiopathic normal-pressure hydrocephalus; M/F, male/female.
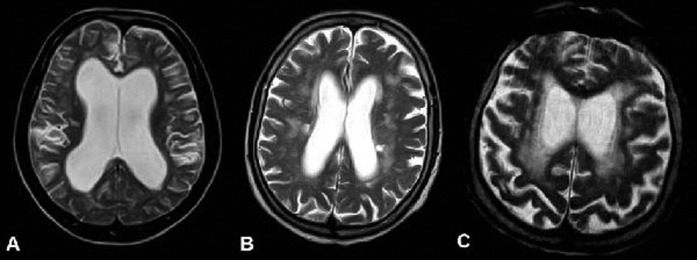
WMH was increased in both Groups 1 and 2, although no statistically significant difference was found between all three groups or between two of the groups (p>0.05) (Figure 2). There was no correlation between WMH and the response to shunt operation (p>0.05) (Table 3).
Figure 2.
Scoring of the white matter hyperintensities (WMH) in axial T2 weighted images of three patients: (a) Grade 1, (b) Grade 3 and (c) Grade 4 (case with vascular dementia). Cases in (a, b) were diagnosed as idiopathic normal pressure hydrocephalus.
Table 3. MR spectroscopy findings and WMH of the three patient groups.
| INPH (Group 1) | OD (Group 2) | Controls (Group 3) | Among groups | INPH vs controls | INPH vs OD | OD vs controls | Shunt response | |
| NAA/Cho | 1.38±0.47 | 1.33±0.25 | 1.68±0.38 | p<0.05 | p<0.05 | NS | p<0.05 | NS |
| NAA/Cr | 1.51±0.27 | 1.78±0.65 | 2.2±0.9 | p<0.05 | p = 0.001 | NS | p<0.05 | NS |
| Cho/Cr | 1.31±0.65 | 1.54±0.68 | 1.17±0.37 | NS | NS | NS | NS | NS |
| WMH | 1.61±0.97 | 2±0.89 | 1.2±0.95 | NS | NS | NS | NS | NS |
Cho, choline; Cr, creatine; INPH, idiopathic normal pressure hydrocephalus; OD, other dementias; NAA, N-acetylaspartate; NS, not significant; WMH, white matter hyperintensities.
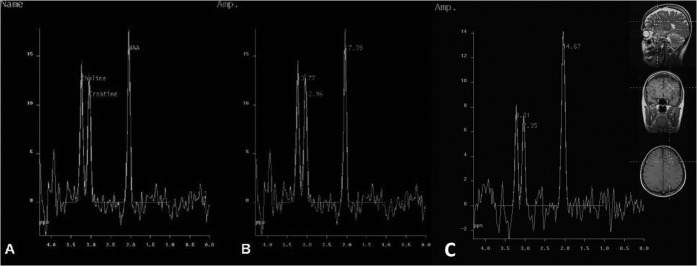
In Groups 1 and 2, NAA/Cho ratios were significantly less than for the control group (p<0.05) (Figure 3). In patients with INPH, NAA/Cr ratios were significantly less than for the control group (p = 0.001). NAA/Cr ratios in the other patients with dementia were less than the control group (p<0.05). In Groups 1 and 2 no statistically significant difference between NAA/Cho and NAA/Cr ratios was detected (p>0.05). Likewise, no significant correlation was detected between the NAA/Cho and NAA/Cr ratios and response of patients with INPH to the shunt procedure (p>0.05) (Table 3).
Figure 3.
Proton MR spectra in (a, b) a patient with idiopathic normal pressure hydrocephalus and (c) the control. N-acetylaspartate (NAA), choline (cho) and creatine peaks are observed in (a). The NAA/Cho ratio (1.51, b) in this patient was decreased compared with the control (1.67, c).
Although Cho/Cr ratios in Groups 1 and 2 were increased compared with controls, this increase was not statistically significant. No correlation was found between Cho/Cr ratios and the response to shunt procedure (p>0.05) (Table 3).
Discussion
INPH is a rare disease usually affecting the elderly [1, 13]. This condition can be either idiopathic or secondary (SNPH) to subarachnoid haemorrhage, meningitis, cranial trauma or intracranial surgery [1–3]. INPH is characterised by gait disturbance, dementia and/or urinary incontinence. Normal opening pressure was observed at lumbar puncture in patients without causative disorders and ventricular enlargement was seen owing to disturbed CSF circulation [9–15].
Many pathophysiological changes occur in INPH in addition to ventriculomegaly [2, 22]. Other findings are increased resistance to CSF reabsorption, hyperdynamic aqueductal CSF flow, decrease in intracranial compliance, increased CSF pulse pressure with normal CSF pressure and decreased CBF [5, 14]. As yet, no theory has been proposed to explain all these changes [23]. It is assumed that the decrease in CBF forms the basis of the pathophysiological changes [24–26]. By contrast, alternative theories support the decrease in intracranial compliance or the changes in spread of pulse waves [14, 22, 27–29]. As a result, INPH can be accepted as a complex pathology with many different contributing factors [2, 23].
MRS is a technique commonly used in the differentiation of brain tumours, cerebrovascular diseases, post-radiotherapy changes, intracranial abscesses and degenerative diseases [4, 16, 17]. The NAA concentration in the brain is used as a neuronal marker [19]. Only a limited number of published studies have evaluated the role of MRS in INPH [10, 18]. It has been reported that NAA levels decrease in patients with INPH [20, 27]. Our study supports this observation. Shiino et al [10] postulated that the effectiveness of shunt procedures could be predicted by NAA/Cr and NAA/Cho ratios; in this study, it was reported that patients with decreased NAA in the white matter show poor response to shunt therapy, owing to irreversible neuronal damage. By contrast, patients with high NAA/Cr and NAA/Cho ratios prior to treatment responded well. In our study, decreased NAA/Cr and NAA/Cho ratios were detected in INPH patients, but no statistically significant correlation with the response to shunt therapy was found. Our MRS findings also show that there is neuronal loss of the brain parenchyma in patients with INPH; this could be a consequence of various factors such as ischaemia, degeneration or mechanical stress. MRS, although not sufficient alone to diagnose INPH or to differentiate it from other causes of dementia, can be used as an adjunct tool to other MRI techniques.
Although in many studies the pathophysiology of INPH has centred on cerebral ischaemia, ischaemia is not present in all cases [22, 24–28]. Mathew et al [30] proposed that dilation of lateral ventricles decreases the flow in anterior cerebral arteries owing to stretching of the vessels. Ventricular expansion forms a pressure over venous structures and capillaries by increasing parenchymal pressure. It can also be postulated that narrowing of arterioles due to ageing can increase white matter ischaemia and the frequency of INPH [2, 14, 28–31]. As a result of decreasing CBF, venous return and CSF absorption via the transependymal–transvenous route decreases [28, 31]. In their study with MRI and positron emission tomography (PET), Owler et al [32] reported that CBF is decreased by 19% in patients with INPH when compared with the control group; however, the standard deviation of the data is high and CBF is normal in 16% of patients with INPH. The CBF measurements in patients with INPH and the control group suggest that ischaemia is not a prerequisite for the generation of INPH [5, 28]. It is not known if the ischaemia is the cause or the effect of the disease [23, 27]. The general concept is that decreased CBF causes neuronal loss [2, 26]. In the literature, it is reported that CBF is normal in 15% of patients with INPH [5, 33]. In patients with low CBF (global ischaemia), shunt procedures do not always increase CBF and no significant correlation has been shown between the relief of symptoms and CBF [5]. In our study, the decrease in NAA/Cho and NAA/Cr ratios could be a result of neuronal loss owing to various factors (e.g. cerebral ischaemia). As the same findings can be interpreted in the other dementia patients, this finding is not specific to INPH.
The WMH encountered in T2 weighted and FLAIR images of patients with INPH can be evaluated in two groups: hyperintensities of the periventricular area (PVH) and deep white matter hyperintensities (DWMH) [34, 35]. PVH and DWMH are related to periventricular oedema and ischaemic white matter degeneration [2, 11, 28]. The predictive value of PVH and DWMH in the diagnosis of INPH is not clear and no direct statistical relationship has been detected [35, 36]. Our results are in good correlation with the literature and no statistically significant relationship was detected between INPH and WMH. This result shows that the evaluation of WMH is not useful in differentiating INPH from other causes of dementia. As reported in the literature, WMH can be encountered in the normal ageing process [2, 11, 14, 35]. In our study, Grade 2 and above WMH was found in 7 of 20 cases of the control group. As a result, the detection of WMH in a patient is not helpful for excluding the INPH diagnosis. In the literature it is reported that there is a negative correlation between the presence of PVH and DWMH and that their presence cannot be used as a determinant to abandon the shunt procedure [15, 34, 36]. In our study, we did not find any correlation between WMH and response to shunt procedure. As a result, we propose that the presence of WMH cannot be used as a criterion to preclude the shunt procedure.
The major limitation of our study is that there is no gold standard method for definite INPH diagnosis [12]. As a result, false-negative and false-positive values for the parameters evaluated in this study could not be detected. TE values of MRS examinations were relatively long and we could not measure values for all metabolites (e.g. myoinositol). Myoinositol/Cr levels are elevated in dementias that are pathologically characterised by gliosis, such as Alzheimer's disease [4]. The use of a longer TE in the MRS acquisition (rather than TE 30–35) precludes the possibility of observing myoinisitol. Another limitation of our study is that the response to shunt therapy is evaluated with subjective criteria. As MRS images were acquired using the single-voxel technique, and only in frontal lobes, other brain areas and basal ganglia were not evaluated. Taking the aetiology of INPH into consideration, the neuronal injury in these other anatomical locations should also be assessed. We could not perform multivoxel spectroscopy in all cases owing to technical limitations. New studies evaluating the brain in a more global fashion with multivoxel spectroscopy are warranted.
One reason for the many conflicting findings in INPH diagnosis could be the difficulty in differentiating more acute and treatable cases from chronic cases with irreversible neuronal loss. Most of the patients included in the study were referred to our department from other hospitals; thus, we could not obtain previous detailed clinical and laboratory data. For this reason, it was not possible to classify patients with dementia as acute–chronic onset or minimal–moderate–severe. Multidisciplinary large studies correlating these data with MRS and WMH are needed.
Conclusion
Despite increasing efforts in this area over the past few years, the development, hydrodynamic properties, imaging findings, diagnosis and treatment of INPH are not fully understood. Unfortunately, in many healthcare centres, the differential diagnosis of INPH from other causes of dementia by clinical characteristics is established according to the results of the invasive shunt operation. WMH was not useful in differentiating INPH from other types of dementia. MRS can demonstrate some pathological changes in patients with INPH, but is not sufficient alone to establish the differential diagnosis. MRS can be used as an adjunct tool to other imaging modalities. WMH and MRS showed no extra benefit in identifying INPH patients who will better-respond to shunt therapy. New studies aimed at developing non-invasive tests for both the diagnosis and evaluation of response to therapy of INPH are warranted.
Footnotes
This study has been presented as a poster presentation at the 33rd European Society of Neuroradiology Annual Meeting held in Crakow in September 18–21, 2008.
References
- 1.Kitagaki H, Mori E, Ishii K, Yamaji S, Hirono N, Imamura T. CSF spaces in idiopathic normal pressure hydrocephalus: morphology and volumetry. AJNR Am J Neuroradiol 1998;19:1277–84 [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman GA, Levi CR, Schofield P, Wang Y, Lowett EC. The pathophysiology of the aqueduct stroke volume in normal pressure hydrocephalus: can co-morbidity with other forms of dementia be excluded? Neuroradiology 2005;47:741–8 [DOI] [PubMed] [Google Scholar]
- 3.Holodny AI, Waxman R, George AE, Rusinek H, Kalnin AJ, De Leon M. MR differential diagnosis of normal-pressure hydrocephalus and Alzheimer disease: significance of perihippocampal fissures. AJNR Am J Neuroradiol 1998;19:81319 [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarci K, Petersen RC, Boeve BF, Knopman DS, Tang-Wai DF, O'Brien PC, et al. 1H MR spectroscopy in common dementias. Neurology 2004;63:1393–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman GA. The pathophysiology of idiopathic normal pressure hydrocephalus: cerebral ischemia or altered venous hemodynamics? AJNR Am J Neuroradiol 2008;29:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki M, Honda S, Yuasa T, Iwamura A, Shibata E, Ohba H. Narrow CSF space at high convexity and high midline areas in idiopathic normal pressure hydrocephalus detected by axial and coronal MRI. Neuroradiology 2008;50:117–22 [DOI] [PubMed] [Google Scholar]
- 7.Brecknell JE, Brown JIM. Is idiopathic normal pressure hydrocephalus an independent entity? Acta Neurochir 2004;146:1003–7 [DOI] [PubMed] [Google Scholar]
- 8.Gangemi M, Maiuri F, Buonamassa S, Colella G, Divitiis E. Endoscopic third ventriculostomy in idiopathic normal pressure hydrocephalus. Neurosurgery 2004;55:129–34 [DOI] [PubMed] [Google Scholar]
- 9.Bradley WC, Scalzo D, Queralt J, Nitz WN, Atkinson DJ, Wong P. Normal pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 1996;198:523–9 [DOI] [PubMed] [Google Scholar]
- 10.Shiino A, Nishida Y, Yasuda H, Suzuki M, Matsuda M, Inubushi T. Magnetic resonance spectroscopic determination of a neuronal and axonal marker in white matter predicts reversibility of deficits in secondary normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 2004;75:1141–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tullberg M, Hultin L, Ekholm S, Mansson JE, Fredman P, Wikkelso C. White matter changes in normal pressure hydrocephalus and Binswanger disease: specificity, predictive value and correlations to axonal degeneration and demyelination. Acta Neurol Scand 2002;105:417–26 [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa M. Clinical guidelines for idiopathic normal pressure hydrocephalus. Neurol Med Chir 2004;44:222–3 [DOI] [PubMed] [Google Scholar]
- 13.Bradley WG. Normal pressure hydrocephalus: new concepts on etiology and diagnosis. AJNR 2000;21:1586–90 [PMC free article] [PubMed] [Google Scholar]
- 14.Bateman GA, Levi CR, Schofield P, Wang Y, Lovett EC. The venous manifestations of pulse wave encephalopathy: windkessel dysfunction in normal aging and senile dementia. Neuroradiology 2008;50:491–7 [DOI] [PubMed] [Google Scholar]
- 15.Krauss JK, Droste DW, Vach W, Regel JP, Orszagh M, Borremans JJ, et al. Cerebrospinal fluid shunting in idiopathic normal pressure hydrocephalus of the elderly: effect of periventricular and deep white matter lesions. Neurosurgery 1996;39:292–9 [DOI] [PubMed] [Google Scholar]
- 16.Fayed N, Dávila J, Oliveros A, Castillo J, Medrano JJ. Utility of different MR modalities in mild cognitive impairment and its use as a predictor of conversion to probable dementia. Acad Radiol 2008;15:1089–98 [DOI] [PubMed] [Google Scholar]
- 17.Petrou M, Sundgren PC, Pang Y, Rohrer S, Foerster B, Chenevert TL. Manually adjusted versus vendor-preset definition of metabolite boundaries impact on proton metabolite ratios. Acad Radiol 2007;14:340–3 [DOI] [PubMed] [Google Scholar]
- 18.Capizzano AA, Schuff N, Amend DL, Tanabe JL, Norman D, Maudsley AA, et al. Subcortical ischemic vascular dementia: assessment with quantitative MR imaging and 1H MR spectroscopy. Am J Neuroradiol 2000;21:621–30 [PMC free article] [PubMed] [Google Scholar]
- 19.Kizu O, Yamada K, Nishimura T. Proton chemical shift imaging in normal pressure hydrocephalus. AJNR Am J Neuroradiol 2001;22:1659–64 [PMC free article] [PubMed] [Google Scholar]
- 20.Kubas B, Kulak W, Sobaniec W, Walecki J, Lewko J. Proton magnetic rasonance spectroscopy in patients with normal pressure hydrocephalus. Neuroradiol J 2006;19:597–602 [DOI] [PubMed] [Google Scholar]
- 21.Matarin M, Pueyo R, Poca MA, Falcon C, Mataro M, Bargallo N, et al. Post-surgical changes in brain metabolism detected by magnetic resonance spectroscopy in normal pressure hydrocephalus: results of a pilot study. J Neurol Neurosurg Psychiatry 2007;78:760–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greitz D. Radiological assessment of hydrocephalus: new theories and implications for theraphy. Neurosurg Rev 2004;27:147–65 [DOI] [PubMed] [Google Scholar]
- 23.Krauss JK, Havle B. Normal pressure hydrocephalus: survey of contemporary diagnostic algorithms and therapeutic decision-making in clinical practice. Acta Neurochir 2004;146:379–88 [DOI] [PubMed] [Google Scholar]
- 24.Edwards RJ, Dombrowski SM, Luciano MG, Pople IK. Chronic hydrocephalus in adults. Brain Pathol 2004;14:325–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momjain S, Owler BK, Czosnyka Z, Czosnyka M, Harris NG, Smielewski P, et al. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain 2004;127:965–72 [DOI] [PubMed] [Google Scholar]
- 26.Brooks DJ, Beaney RP, Powell M, Leenders KL, Crockard HA, Thomas DG, et al. Studies on cerebral oxygen metabolism, blood flow and blood volume, in patients with hydrocephalus before and after surgical decompression, using positron emission tomography. Brain 1986;109:613–28 [DOI] [PubMed] [Google Scholar]
- 27.Bradley WG. Normal pressure hydrocephalus and deep white matter ischemia: which is the chicken, and which is the egg? AJNR Am J Neuroradiol 2001;22:1638–40 [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley WG. Idiopathic normal pressure hydrocephalus: new findings and thoughts on etiology. AJNR Am J Neuroradiol 2008;29:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Marco G, Peretti II, Poncelet AD, Baledent O, Onen F, Feugeas MCH. Intracranial fluid dynamics in normal and hydrocephalic states. Systems analysis with phase-contrast magnetic resonance imaging. J Comput Assist Tomogr 2004;28:247–54 [DOI] [PubMed] [Google Scholar]
- 30.Mathew NT, Meyer JS, Hartmann A, Ott EO. Abnormal cerebrospinal fluid-blood flow dynamics: implications in diagnosis, treatment, and prognosis in normal pressure hydrocephalus. Arch Neurol 1975;32:657–64 [DOI] [PubMed] [Google Scholar]
- 31.Bradley WG. Cerebrospinal fluid dynamics and shunt responsiveness in patients with normal-pressure hydrocephalus. Mayo Clin Proc 2002;77:507–8 [DOI] [PubMed] [Google Scholar]
- 32.Owler BK, Momjain S, Czosnyka Z, Czosnyka M, Pena A, Harris NG, et al. Normal pressure hydrocephalus and cerebral blood flow: a PET study of baseline values. J Cereb Blood Flow Metab 2004;24:17–23 [DOI] [PubMed] [Google Scholar]
- 33.Fukuhara T, Luciano MG. Clinical features of late-onset idiopathic aqueductal stenosis. Surg Neurol 2001;55:132–7 [DOI] [PubMed] [Google Scholar]
- 34.Tullberg M, Jensen C, Ekholm S, Wikkels C. Normal pressure hydrocephalus: vascular white matter changes on MR images must not exclude patients from shunt surgery. AJNR Am J Neuroradiol 2001;22:1665–73 [PMC free article] [PubMed] [Google Scholar]
- 35.Krauss JK, Regel JP, Vach W, Orszagh M, Jüngling FD, Bohus M, et al. White matter lesions in patients with idiopathic normal pressure hydrocephalus and in an age-matched control group: a comparative study. Neurosurgery 1997;40:491–5 [DOI] [PubMed] [Google Scholar]
- 36.Tamaki N, Shirakuni T, Ehara K, Matsumoto S. Characterization of periventricular edema in normal-pressure hydrocephalus by measurement of water proton relaxation times. J Neurosurg 1990;73:864–70 [DOI] [PubMed] [Google Scholar]