Abstract
Cerebrospinal fluid (CSF) spaces include ventricles and cerebral and spinal subarachnoid spaces. CSF motion is a combined effect of CSF production rate and superimposed cardiac pulsations. Knowledge of CSF dynamics has benefited considerably from the development of phase-contrast (PC) MRI. There are several disorders such as communicating and non-communicating hydrocephalus, Chiari malformation, syringomyelic cyst and arachnoid cyst that can change the CSF dynamics. The aims of this pictorial review are to outline the PC MRI technique, CSF physiology and cerebrospinal space anatomy, to describe a group of congenital and acquired disorders that can alter the CSF dynamics, and to assess the use of PC MRI in the assessment of various central nervous system abnormalities.
During the last two decades, flow-sensitive MRI techniques have been increasingly applied to quantitatively and qualitatively assess cerebrospinal fluid (CSF) flow dynamics [1]. CSF flow MRI can be used to discriminate between communicating hydrocephalus and non-communicating hydrocephalus, to localise the level of obstruction in obstructive hydrocephalus, to determine whether arachnoid cysts communicate with the subarachnoid space, to differentiate between arachnoid cysts and subarachnoid space, to discriminate between syringomyelia and cystic myelomalacia, and to evaluate flow patterns of posterior fossa cystic malformations. This imaging method can also provide significant information in pre-operative evaluation of Chiari 1 malformation and normal pressure hydrocephalus and post-operative follow-up of patients with neuroendoscopic third ventriculostomy (NTV) and ventriculoperitoneal (VP) shunt [1-11]. In this pictorial review, we emphasise phase-contrast (PC) MRI technique, CSF physiology and cerebrospinal space anatomy, congenital and acquired disorders that can alter the cerebrospinal fluid dynamics, and the use of PC MRI in the assessment of various central nervous system (CNS) abnormalities.
CSF anatomy and physiology
CSF comprises all intracerebral ventricles, spinal and brain subarachnoid spaces, such as cisterns and sulci, and the central canal of the spinal cord. The rate of CSF formation in humans is about 0.3–0.4 ml min−1 (about 500 ml day−1). Total CSF volume is 90–150 ml in adults and 10–60 ml in neonates. Potential sites of CSF origin include the choroid plexus, parenchyma of the brain and the spinal cord, and ependymal lining of the ventricles [12].
The portion of the fluid formed in the lateral ventricles escapes by the foramen of Monro into the third ventricle and then via the aqueduct into the fourth ventricle. A little CSF occurs in the central canal of the spinal cord and may be added to the intraventricular supply. From the fourth ventricle the fluid pours into the subarachnoid spaces through the medial foramen of Magendie and the two lateral foramina of Luschka. There is no functional communication between the cerebral ventricles and the subarachnoid spaces in any region except from the fourth ventricle [13].
The absorption of the cerebrospinal fluid is a dual process. It is chiefly a rapid drainage through the arachnoid villi into the great dural sinuses, but it also escapes slowly into the true lymphatic vessels by way of an abundant but indirect perineural (ophthalmic, optic and vagal nerves) course and via the capillary bed of the CNS [13].
Two components can be distinguished in CSF circulation: (i) bulk flow (circulation) and (ii) pulsatile flow (back and forth motion). In bulk flow theory, CSF is produced by choroid plexus and absorbed by arachnoid granulations. The force, which provides CSF movement from the ventricular system to arachnoid granulation and CSF absorption, is caused by a hydrostatic pressure gradient between the site of its formation (slightly high pressure) and its site of absorption (slightly low pressure). In pulsatile flow theory, movement of the CSF is pulsatile and results from pulsations related to cardiac cycle of the choroid plexus and the subarachnoid portion of the cerebral arteries [14]. Because very little CSF water truly circulates through the subarachnoid space, pulsatile flow, rather than bulk flow, can be measured and demonstrated by PC MRI.
Phase-contrast MRI technique
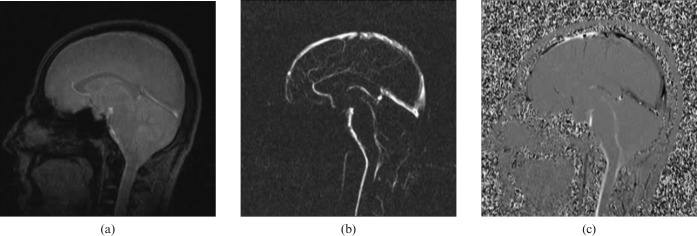
The PC MRI generates signal contrast between flowing and stationary nuclei by sensitising the phase of the transverse magnetisation to the velocity of motion [15]. Two data sets are acquired with opposite sensitisation, yielding opposite phase for moving nuclei and identical phases for stationary nuclei [16]. For stationary nuclei, the net phase is zero, and their signal is eliminated in the final image. However, flowing nuclei move from one position in the field gradient to another between the time of the first sensitisation and that of the second sensitisation. Because phase varies with position in the field, the net phase after subtraction of the two data sets is non-zero, and there is residual signal from flowing CSF [17]. When the two data sets are subtracted, the signal contribution from stationary nuclei is eliminated and only flowing nuclei are seen (Figure 1). Before PC MRI data are acquired, the anticipated maximum CSF flow velocity must be entered into the pulse sequence protocol (velocity encoding (VENC)) [18]. To obtain the optimal signal, the CSF flow velocity should be the same as, or slightly less than, the selected VENC. CSF flow velocities greater than VENC can produce aliasing artefacts, whereas velocities much smaller than VENC result in a weak signal [17,18]. The mean VENC value is 5–8 cm s−1 for standard CSF flow imaging. Low VENC values (2–4 cm s−1) can be helpful in the discrimination of communicating and non-communicating arachnoid cysts, and in the assessment of the ventriculoperitoneal shunt patency. In normal pressure hydrocephalus, significantly higher VENC values (20–25 cm s−1) should be chosen owing to hyperdynamic CSF flow within the cerebral aqueduct.
Figure 1.
(a) Re-phased image is a magnitude of flow compensated signal, in this image the flow is bright and background is visible, (b) magnitude image is a magnitude of difference signal, in this image the flow is bright and the background is suppressed and (c) phase image is a phase of difference signal, in this image forward flow is bright, reverse flow is black and background is mid-grey.
The signal initially contains phase and magnitude information. Magnitude and phase images can be generated for anatomy and velocity information, respectively. The result is that the greyscale intensity of each pixel is directly related to the velocity of CSF. Caudal flow of CSF is conventionally represented as shades of white on phase images, whereas cranial flow is by shades of black. Since it reflects the phase shifts, PC velocity image is far more sensitive to CSF flow than is the magnitude image. Two series of PC imaging techniques are applied in the evaluation of CSF flow, one in the axial plane, with through-plane velocity encoding in the craniocaudal direction for flow quantification, and one in the sagittal plane, with in-plane velocity encoding in the craniocaudal direction for qualitative assessment. Through-plane evaluation is performed in the axial oblique plane perpendicular to the aqueduct and is more accurate for quantitative analysis because the partial volume effects are minimised [17-19]. Quantitative CSF velocity and qualitative flow information can be obtained in 8–10 additional minutes in connection with routine MRI.
CSF flow is pulsatile and synchronous with the cardiac cycle, therefore cardiac gating can be used to provide increased sensitivity [19]. Cardiac gating can be provided with two different methods: prospective gating and retrospective gating. In retrospective gating, the computer follows the R wave and the data are acquired throughout the cardiac cycle. While the entire cardiac cycle can be sampled in retrospective gating, the prospectively gated acquisitions must be completed 100–200 ms before the next anticipated R wave. Thus, there appears to be large net flow of CSF in the systolic direction owing to partially sampled cardiac cycle in prospective gating. More accurate results can be obtained with retrospective gating when compared with prospective gating [20].
Clinical applications
Arachnoid cysts
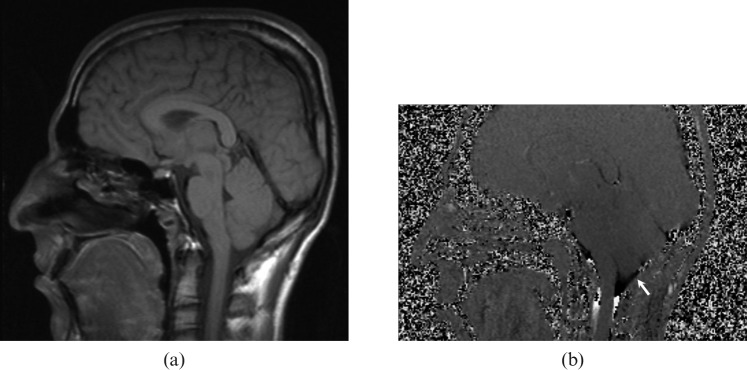
Although there has been considerable controversy regarding the indications for the surgical treatment of asymptomatic arachnoid cysts, patients with symptomatic cysts causing seizures, hydrocephalus, increased intracranial pressure or neurological impairment should be treated. Determination of whether the arachnoid cyst is communicating to the CSF spaces is important in the pre-operative evaluation [2]. Moreover, the discrimination between normal CSF spaces and intracranial cysts may not always be evident with anatomical imaging and this may be clarified with CSF flow studies. There are CSF flow patterns specific to each entity and PC MRI may improve the diagnostic confidence in differentiating communicating and non-communicating arachnoid cysts (Figure 2) and posterior fossa cystic malformations (Figures 3–6) from each other [1].
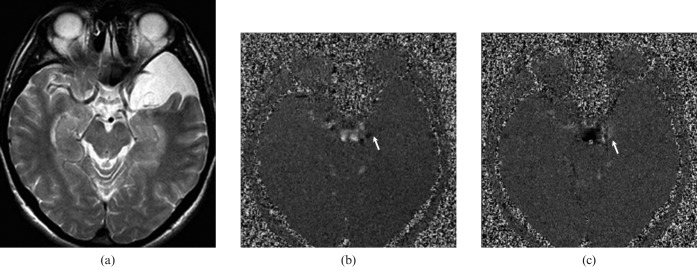
Figure 2.
A 42-year-old male with communicating left middle cranial fossa arachnoid cyst. (a) T2 weighted axial image shows left middle cranial fossa arachnoid cyst that is isointense to cerebrospinal fluid (CSF). CSF flow MRI (b) and (c) depict hypo- and hyperintensity (arrow), respectively, originating from suprachiasmatic cistern demonstrating communication between cyst and cistern. While, the pulsatile flow is seen only at the communicating site, it is not present throughout the cyst. This flow pattern is consistent with a communicating arachnoid cyst.
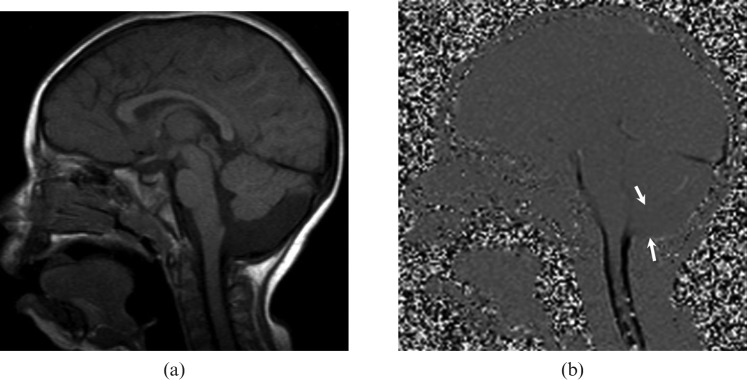
Figure 3.
A 22-year-old male with mega-cisterna magna. (a) T1 weighted sagittal image demonstrates a widened retrocerebellar cistern. (b) Cerebrospinal fluid (CSF) flow MRI shows pulsatile CSF flow that can be seen throughout the dilated cisterna magna (arrows).
Figure 6.
A 14-year-old female with Dandy-Walker malformation. (a) T1 weighted sagittal image demonstrates the dilated fourth ventricle associated with the posterior fossa cystic lesion, elevation of the tentorium, widening of the posterior fossa and dysgenesis of the vermis. (b) Cerebrospinal fluid (CSF) flow MRI shows no communication between the cyst and the posterior cervical subarachnoid space (arrow), and hyperdynamic CSF flow through the aqueduct into the cyst produces turbulent flow within the cyst.
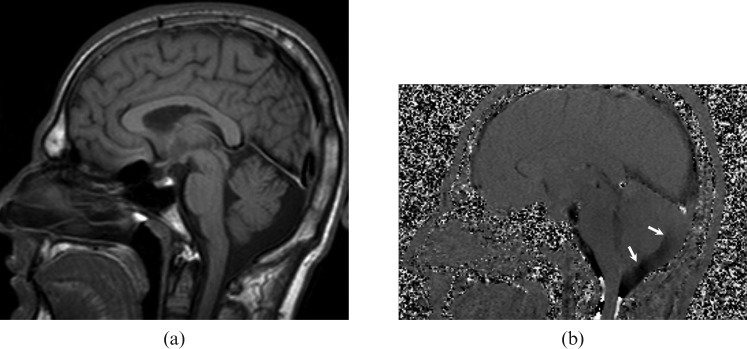
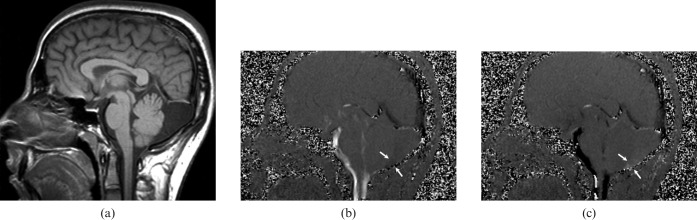
Figure 4.
A 2-year-old girl with a non-communicating posterior fossa arachnoid cyst. (a) T1 weighted sagittal image shows a retrocerebellar cystic lesion and scalloping in the occipital bone. (b) Cerebrospinal fluid (CSF) flow MRI reveals no communication between the cyst and the superior–posterior cervical subarachnoid space (arrows).
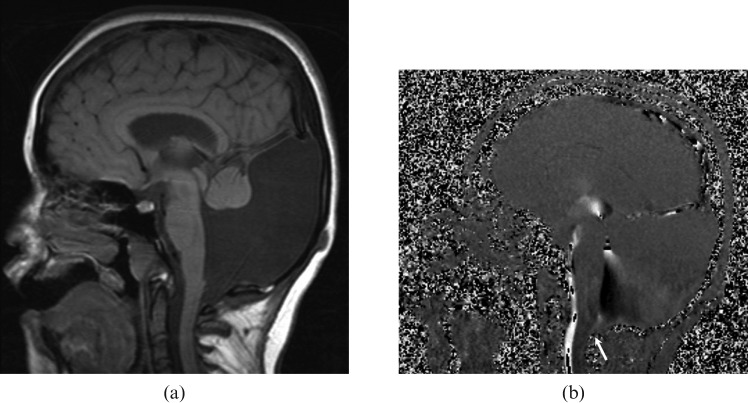
Figure 5.
A 23-year-old male with the diagnosis of communicating posterior fossa arachnoid cyst presented with a headache. (a) T1 weighted sagittal image shows retrocerebellar arachnoid cyst that is isointense to cerebrospinal fluid (CSF). The fourth ventricle is normal in configuration. CSF flow MRI (b) and (c) depict pulsatile flow (arrows) into the cyst at the level of cerebellomedullary junction. The flow is not present throughout the cyst, and different from the mega cisterna magna.
Normal pressure hydrocephalus
Normal pressure hydrocephalus (NPH) is a state of chronic hydrocephalus in which the CSF pressure is in physiological range, but a slight pressure gradient persists between the ventricles and the brain parenchyma. This pathology is described in elderly patients and has a classic symptom triad of gait disturbance, urinary incontinence and dementia [21]. The diagnosis of NPH is supported by the radiological findings of ventricular dilatation: out of proportion cortical sulcal enlargement, upward bowing of corpus callosum, flattening of the gyri against the calvarium and increased or normal CSF flow void. In properly selected patients, ventricular shunting results in resolution of symptoms and slows progressive deterioration. The aim of ventriculoperitoneal shunting is not to decrease mean pressure, but to dampen the pulse pressure by providing extra capacitance to the ventricular system [3,4]. PC MRI is useful in selection of patients for shunt placing. Caudal and rostral peak aqueduct CSF flow was significantly increased in patients with NPH. While a CSF flow measurement of less than 18 ml min−1 with a sinusoidal flow pattern is normal, a flow of greater than 18 ml min−1 suggests idiopathic NPH at the cerebral aqueduct [22]. Demonstration of increased pulsatility throughout the cerebral aqueduct has been correlated with a favourable response to shunting. CSF velocity imaging is the most sensitive method for detecting symptomatic patients with a shunt responsive NPH on the basis of hyperdynamic CSF flow (Figure 7 and 8) [4].
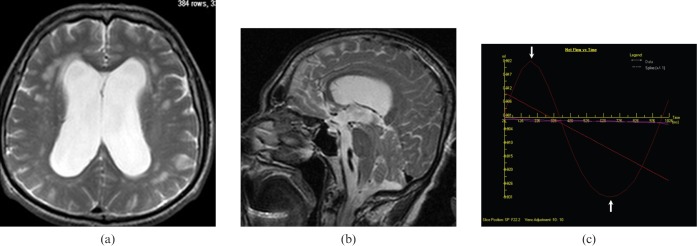
Figure 7.
A 73-year-old male with the diagnosis of normal pressure hydrocephalus presented with urinary incontinence and dementia. (a) Axial and (b) sagittal T2 weighted images show enlarged ventricles, upward bowing of corpus callosum, periventricular white matter hyperintensities consistent with transependimal CSF leakage/gliosis and normal cerebral sulci. (c) Quantitative cerebrospinal fluid (CSF) flow analysis graphic reveal hyperdynamic flow rates (arrows) in the aqueduct (average flow: 20.34 ml min−1).
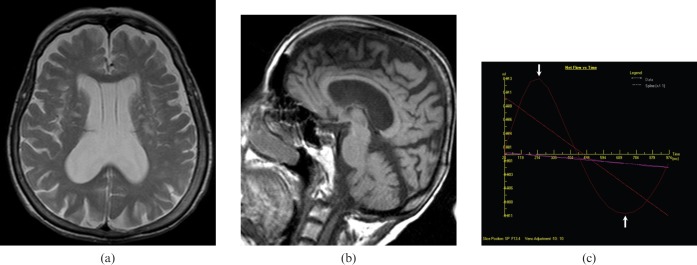
Figure 8.
A 78-year-old female with multi-infarct dementia. (a) Axial T2 weighted and (b) sagittal T1 weighted images reveal mildly dilated ventricles, periventricular white matter hyperintensities consistent with gliosis and slightly enlarged cerebral sulci. (c) Quantitative cerebrospinal fluid flow analysis graphic reveal normal flow rates (arrows) in the aqueduct (average flow: 8.28 ml min−1).
Chiari 1 malformation
The Chiari 1 malformation, also known as the Arnold–Chiari malformation, is caudal displacement of the cerebellar tonsils through the posterior foramen magnum. The suggestive symptoms of this malformation include headaches, dizziness, ataxia, fainting with a cough, weakness or numbness, episodic aural fullness, tinnitus and vertigo. CSF flow pattern may contribute the Chiari 1 symptomatology independent of tonsillar ectopia. As the herniated tonsils fill the foramen magnum in the setting of chiari, CSF flow is reduced at the craniovertebral junction, and a compensatory pulsatile descent of the cerebellar tonsils is observed during systole. This combination can effectively plug the CSF pathway at the foramen magnum [5]. Although selection criteria for surgery is based mainly on degree of tonsillar ectopia and presenting symptoms, degree of CSF flow obstruction rather than the degree of tonsillar herniation can better select patients who are most responsive to surgery (Figure 9). Improved CSF velocity profile following surgery in such patients is useful in anticipation of symptomatic improvement. After posterior fossa decompression abnormal CSF flow reverts to normal and parallels clinical improvement (Figure 10) [6].
Figure 9.
A 21-year-old male with the diagnosis of Chiari 1 malformation. (a) Sagittal T1 weighted MRI of the brain stem and cervical spinal cord shows mild cerebellar tonsillar ectopia. (b) Cerebrospinal fluid flow MRI shows normal association (arrow) between posterior cervical subarachnoid space and posterior fossa.
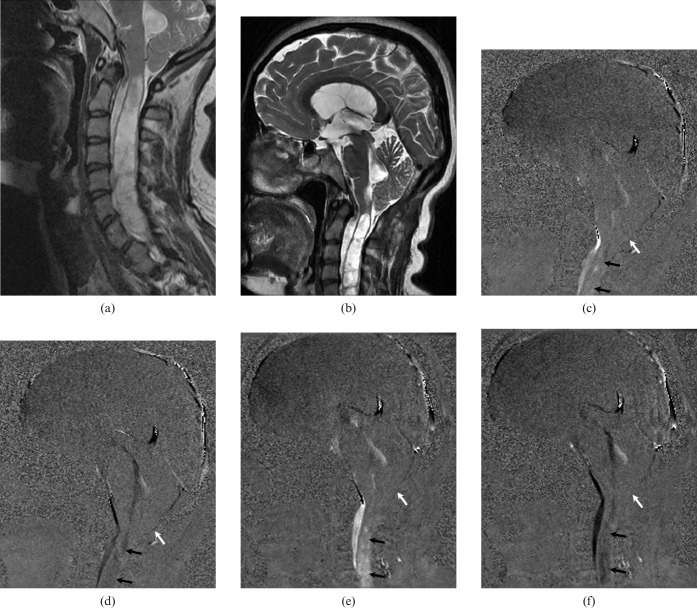
Figure 10.
A 24-year-old female with the diagnosis of Chiari 1 malformation presented with suboccipital headache. (a) Pre-operative and (b) post-operative sagittal T2 weighted MRI show a large syrinx in association with cerebellar tonsillar ectopia. (c, d) Pre- and (e, f) post-operative cerebrospinal fluid (CSF) flow MRI show no association between cervical subarachnoid space and cisterna magna (white arrow). No symptomatic improvement was observed after the surgery. Both pre-operative and post-operative CSF flow MRI (c–f) show pulsatile CSF flow in the syrinx (black arrows).
Syringomyelia
Various theories of the pathophysiology of syringomyelic cysts have been reported, but the most popular one that is formation and extension of syringomyelic cysts result from an obstruction of the spinal CSF pathways [7,8]. Syringomyelic cysts associated with a number of abnormalities including Chiari 1 malformation, spinal trauma, spinal cord tumours and arachnoiditis [8]. The detection of pulsatile CSF flow within the cystic cord lesion is both predictive of subsequent enlargement and may help discriminate a cyst from a myelomalacia (Figure 10). CSF flow imaging can provide a direct evaluation for the follow-up and the postoperative survey in patients with syringomyelic cysts [8].
Neuroendoscopic third ventriculostomy
Neuroendoscopic third ventriculostomy (NTV), which is increasingly used as alternative treatment for obstructive hydrocephalus, has become the most common neuroendoscopic procedure. This procedure restores the pulsatile bidirectional CSF motion. The indications for NTV are still controversial. Although it is an internal CSF diversionary procedure best suited to non-communicating hydrocephalus, it has had some unexplained success in communicating hydrocephalus [9]. The morbidity associated with this technique is low, and the success rates are high. A range of image parameters have been assessed to evaluate the permeability of the NTV, including ventricular size changes, flow void signal intensity and MR patency, by using cine PC MR. PC flow-sensitive MRI techniques offer more physiological data than structural MRI and qualitative assessment of the patency of ventriculostomy. In addition, the measurement of stroke volume in ventriculostomy by using cine PC MRI provides functional information about the third ventriculostomy [10].
Ventriculoperitoneal (VP) shunts
Various complications including obstruction and infection can occur in VP shunts. Although various methods including ultrasonography, radiography, thermography, radionuclide studies, CT and MRI can be used to evaluate shunt patency, clinical assessment is the primary method for assessment of the VP shunt functioning. PC MRI can also be used to evaluate VP shunt patency. In shunt catheters, because of the one-way valve mechanism, normal flow is unidirectional and rhythmic (Figure 11). Because of very low CSF flow rates in shunt catheters, minimum VENC values (2–5 cm s−1) should be used for assessing VP shunts. No signal means no flow in PC imaging [11].
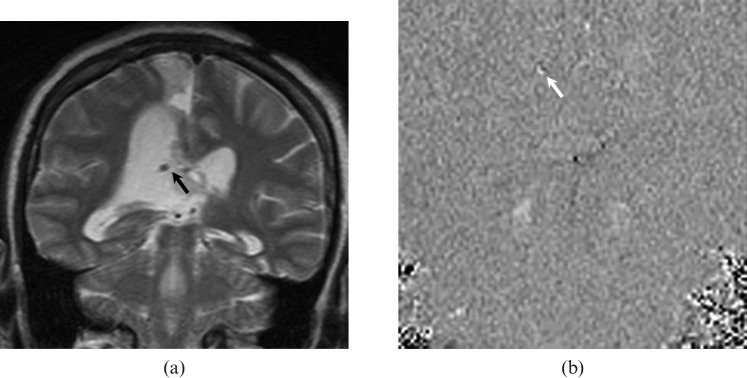
Figure 11.
A 34-year-old female patient with a ventriculoperitoneal shunt inserted for obstructive hydrocephalus. (a) Coronal T2 weighted MRI shows the ventriculoperitoneal shunt (black arrow) within the right ventricle. (b) Coronal cerebrospinal fluid flow MRI demonstrates brighter signal intensity (white arrow) than the background, suggesting patency at the site of the shunt. This flow pattern is consistent with the one-way flow in the ventriculoperitoneal shunt.
Conclusion
PC cine MR is a useful imaging technique in evaluating CSF dynamics that affects various disease processes. In evaluation, follow-up, surgical decision and post-operative survey of these disease processes, PC cine MR can provide valuable additional information to conventional MRI. CSF pulsatility and stroke volume through the aqueduct has been correlated with a positive response to shunting in patients with normal pressure hydrocephalus. CSF flow studies can be used to differentiate posterior fossa cystic malformations from each other. It also has a role in assessing the functioning of surgical interventions. Finally, pulsatile CSF flow within a cystic cord lesion may help to discriminate a syrinx from myelomalacia.
References
- 1.Yildiz H, Yazici Z, Hakyemez B, Erdogan C, Parlak M. Evaluation of CSF flow patterns of posterior fossa cystic malformations using CSF flow MR imaging. Neuroradiology 2006;48:595–605 [DOI] [PubMed] [Google Scholar]
- 2.Yildiz H, Erdogan C, Yalcin R, Yazici Z, Hakyemez B, Parlak M, et al. Evaluation of communication between intracranial arachnoid cysts and cisterns with phase-contrast cine MR imaging. AJNR Am J Neuroradiol 2005;26:145–51 [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley WG, Whittemore AR, Kortman KE, Watanabe AS, Homyak M, Teresi LM, et al. Marked cerebrospinal fluid void: indicator of successful shunt in patients with suspected normal-pressure hydrocephalus. Radiology 1991;178:459–66 [DOI] [PubMed] [Google Scholar]
- 4.Ng SE, Low AM, Tang KK, Lim WE, Kwok RK. Idiopathic normal pressure hydrocephalus: correlating magnetic resonance imaging biomarkers with clinical response. Ann Acad Med Singapore 2009;38:803–8 [PubMed] [Google Scholar]
- 5.Siddiqi NH. Chiari I Malformation: eMedicine Radiology. Updated 4 May 2009. Available from: http://emedicine.medscape.com/article/406849-overview. [Google Scholar]
- 6.McGirt MJ, Nimjee SM, Fuchs HE, George TM. Relationship of cine phase-contrast magnetic resonance imaging with outcome after decompression for Chiari I malformations. Neurosurgery 2006;59:140–6 [DOI] [PubMed] [Google Scholar]
- 7.Ball MJ, Dayan AD. Pathogenesis of syringomyelia. Lancet 1972;2:799–801 [DOI] [PubMed] [Google Scholar]
- 8.Brugieres P, Idy-Peretti I, Iffenecker C, Parker F, Jolivet O, Hurth M, et al. CSF flow measurement in syringomyelia. AJNR Am J Neuroradiol 2000;21:1785–92 [PMC free article] [PubMed] [Google Scholar]
- 9.Buxton N, Macarthur D, Mallucci C, Punt J, Vloeberghs M. Neuroendoscopic third ventriculostomy in patients less than 1 year old. Pediatr Neurosurg 1998;29:73–6 [DOI] [PubMed] [Google Scholar]
- 10.Bargalló N, Olondo L, Garcia AI, Capurro S, Caral L, Rumia J. Functional analysis of third ventriculostomy patency by quantification of CSF stroke volume by using cine phase-contrast MR imaging. AJNR Am J Neuroradiol 2005;26:2514–21 [PMC free article] [PubMed] [Google Scholar]
- 11.Castillo M, Hudgins PA, Malko JA, Burrow BK, Hoffman JC., Jr Flow-sensitive MR imaging of ventriculoperitoneal shunts: in vitro findings, clinical applications and pitfalls. AJNR Am J Neuroradiol 1991;12:667–71 [PMC free article] [PubMed] [Google Scholar]
- 12.Segal MB, Pollay M. The secretion of cerebrospinal fluid. Exp Eye Res 1977;25:127–48 [DOI] [PubMed] [Google Scholar]
- 13.Gray H. Anatomy of the Human Body. Lewis WH, editor. 20th edn. Lea & Febiger: Philadelphia, 1918. 2000. Bartleby.Com. 7 Aug. 2010. Available from: http://www.bartleby.com/107/194.html. [Google Scholar]
- 14.Barkhof F, Kouwenhoven M, Scheltens P, Sprenger M, Algra P, Valk J. Phase-contrast cine MR imaging of normal aqueductal CSF flow. Effect of aging and relation to CSF void on modulus MR. Acta Radiol 1994;35:123–30 [PubMed] [Google Scholar]
- 15.Tsuruda JS, Shimakawa A, Pelc NJ, Saloner D. Dural sinus occlusion: evaluation with phase-sensitive gradient-echo MR imaging. Am J Neuroradiol 1991;12:481–8 [PMC free article] [PubMed] [Google Scholar]
- 16.Dumoulin CL, Yucel EK, Vock P, Souza SP, Terrier F, Steinberg FL, et al. Two and three-dimensional phase contrast MR angiography of the abdomen. J Comput Assist Tomogr 1990;14:779–84 [DOI] [PubMed] [Google Scholar]
- 17.Bradley WG, Daroff RB, Fenichel GM, Jankovic J. Neurology in clinical practice: Principles of diagnosis and management, 4th edn. Philadelphia: Elsevier, 2006:306–9 [Google Scholar]
- 18.Saloner D. The AAPM/RSNA physics tutorial for residents. An introduction to MR angiography. Radiographics 1995;15:453–65 [DOI] [PubMed] [Google Scholar]
- 19.Connor SE, O'Gorman R, Summers P, Simmons A, Moore EM, Chandler C, et al. SPAMM, cine phase contrast imaging and fast spin-echo T2-weighted imaging in the study of intracranial cerebrospinal fluid (CSF) flow. Clin Radiol 2001;56:763–72 [DOI] [PubMed] [Google Scholar]
- 20.Nitz WR, Bradley WG, Jr, Watanabe AS, Lee RR, Burgoyne B, O'Sullivan RM, et al. Flow dynamics of cerebrospinal fluid: assessment with phase-contrast velocity MR imaging performed with retrospective cardiac gating. Radiology 1992;183:395–405 [DOI] [PubMed] [Google Scholar]
- 21.Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with “normal” cerebrospinal fluid pressure: A treatable syndrome. N Engl J Med 1965;273:117–26 [DOI] [PubMed] [Google Scholar]
- 22.Luetmer PH, Huston J, Friedman JA, Dixon GR, Petersen RC, Jack CR, et al. Measurement of cerebrospinal fluid flow at the cerebral aqueduct by use of phase-contrast magnetic resonance imaging: technique validation and utility in diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery 2002;50:534–43 [DOI] [PubMed] [Google Scholar]