Abstract
Objective
The purpose of this study was to assess the technical feasibility and local efficacy of biplane fluoroscopy-guided percutaneous radiofrequency (RF) ablation combined with transcatheter arterial chemoembolisation (TACE) for hepatocellular carcinoma (HCC).
Method
Our retrospective study was approved by the institutional review board and informed consent was waived. 18 patients with 19 HCCs (mean 2.5 cm diameter; range 2–4.2 cm) were treated with percutaneous RF ablation combined with TACE. After segmental TACE, 18 (95%) of 19 HCCs were visible on fluoroscopy. Shortly (median 2 days; range 1–4 days) after TACE, percutaneous RF ablation was performed under real-time biplane fluoroscopic guidance. We evaluated major complications, rate of technical success at immediate post-RF ablation CT images and local tumour progression at follow-up CT images.
Results
Major complication was not observed in any patients. Technical success was achieved for all 18 visible HCCs. During the follow-up period (median 20 months; range 5–30 months), no local tumour progression was found.
Conclusion
Biplane fluoroscopy-guided RF ablation combined with TACE is technically feasible and effective for treatment of HCC.
Percutaneous radiofrequency (RF) ablation has been widely implemented in the management of hepatocellular carcinoma (HCC) with promising results. Although its local efficacy for small tumours (i.e. <2 cm) is similar to surgical outcomes [1], results for medium-sized and large tumours are less robust. Thus, multimodal treatments such as combined percutaneous RF ablation with transarterial chemoembolisation (TACE) have been explored for medium or large HCCs in order to enhance the therapeutic effect. In a recent study, RF ablation combined with TACE was similar to surgical resection in patients with early-stage disease [2].
Percutaneous RF ablation shortly following TACE has been usually performed under guidance of either ultrasonography or CT/CT fluoroscopy. Since intratumoural retention of radio-opaque iodised oil induced by TACE conveniently provides radiographic contrast to the index lesion, biplane fluoroscopy (anterior posterior and lateral projections) can be used as an alternative guiding modality for RF ablation combined with TACE. Easier targeting of dome lesions, often difficult to visualise on ultrasound, through an oblique approach without pleural transgression is one potential advantage of biplane fluoroscopy guidance. Also unlike on ultrasound, microbubble formation during ablation would not obscure the index lesion on biplane fluoroscopy, allowing easier and more spatially accurate application of overlapping ablations that are often needed for larger tumours. However, to our knowledge, there have been no studies investigating the role of biplane fluoroscopy as a guidance modality in this clinical setting. The purpose of our study was to retrospectively assess the technical feasibility and local efficacy of biplane fluoroscopy-guided percutaneous RF ablation combined with TACE for HCC ≥2 cm.
Methods and materials
Patients and enrolment criteria
Our retrospective study was approved by the institutional review board with waiver of informed consent. From April 2006 to January 2008, patients with HCC were assessed for the following inclusion criteria: (1) three or fewer HCCs with a diameter ranging from 2 cm to 5 cm; (2) Child–Pugh class A or B; (3) absence of vascular invasion or extrahepatic metastases; (4) absence of severe coagulopathy (i.e. prothrombin activity <40% or platelet count <40 000 ml–1); (5) tumour located at least 1 cm away from the colon, gallbladder or main bile duct. All referred patients meeting the above criteria were treated with a combination of TACE and RF ablation.
All patients suspected of having HCC had undergone a routine physical examination, blood laboratory tests and dynamic CT and/or MRI. The diagnosis of HCC was based on American Association for the Study of Liver Diseases (AASLD) guidelines [3] as follows: typical vascular pattern (hypervascular in the arterial phase, and wash-out in the portal/delayed phase) of liver nodule in at least one of the dynamic CT or MRI, or a serum α-fetoprotein value exceeding 200 ng ml–1. Histopathological confirmation was not obtained in any patient. Tumour size was defined as the maximum diameter measured on CT or MRI, and the segmental location of the tumour was also recorded in the data sheet according to the Couinaud nomenclature.
Transhepatic arterial chemoembolisation
All TACE procedures were performed on an inpatient basis by an interventional radiologist (SWP) with 10 years of experience of TACE. After coeliac and superior mesenteric arteriography using a 5-French catheter (Cook, Bloomington, IN), the hepatic artery was catheterised. The tumour feeding branch off either the right or left hepatic artery was selectively cannulated using a 3-French microcatheter (Microferret; Cook). Thus, selective embolisation of the tumour-supplying artery was performed, sparing the majority of hepatic parenchymal arterial supply, using an emulsion of iodised oil (Lipiodol; Andre Gurbet, Aulnay-sous-Bois, France) and doxorubicin hydrochloride (Adriamycin RDF; Ildong Pharmaceutical, Seoul, Korea). Infusion of this emulsion was performed until arterial flow stasis was achieved and/or iodised oil was visualised in the portal branches. Further embolisation with gelatin sponge particles (1–2 mm in diameter; Gelfoam; Upjohn, Kalamazoo, MI) was also performed. After embolisation, angiography was performed to determine the extent of vascular occlusion and presence of any residual tumour staining.
Radiofrequency ablation
Shortly (median 2 days; range 1–4 days) after TACE, percutaneous RF ablation was performed by one of two radiologists (YJK, MWL) with 6 and 4 years of experience in RF ablation. The RF ablation procedure was performed in the interventional suite equipped with a flat-panel biplane fluoroscopy/angiography instrument (Axiom Artis dBA; Siemens, Erlangen, Germany) if the index tumour was visible on both anteroposterior and lateral projections secondary to retained iodised oil from prior TACE. RF electrode advancement toward the index tumour through non-tumourous hepatic tissue was guided by biplane fluoroscopy, with concurrent use of ultrasound to avoid the traversal of critical structures such as large vessels and the gallbladder. If the index tumour was not clearly demonstrated on fluoroscopic images, another conventional guiding modality such as ultrasound or CT was used for the procedure.
The patients were treated under local anaesthesia with conscious sedation or general anaesthesia. For local anaesthesia, 1% lidocaine was injected from the insertion site to the peritoneum and liver capsule, along the anticipated needle track. In all patients, a 17-gauge cooled-tip electrode with a 3 cm exposed tip (Cool-tip; Valleylab, Boulder, CO) was used for the ablation. RF energy was delivered using impedance-based control algorithm of the generator. To achieve adequate ablative margin (at least 0.5 cm), multiple overlapping ablations were applied as needed, depending on tumour size, shape and location. At the end of the procedure, tract ablation was performed to prevent bleeding or tumour seeding. For liver dome lesions, a transthoracic approach was avoided in favour of an oblique approach from the lower intercostal space (Figure 1). The targeting time, from the electrode puncturing the patient’s skin to placement of the electrode within the index tumour, was typically within 1 min.
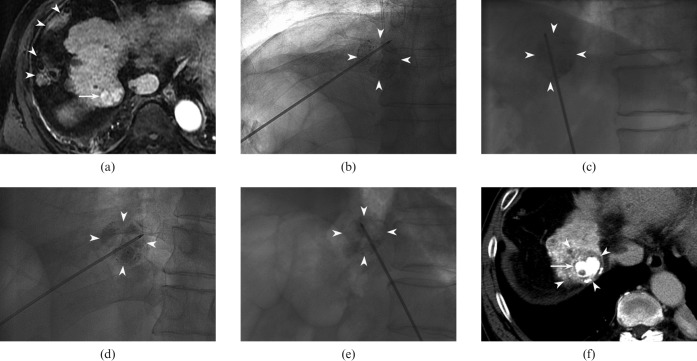
Figure 1.
64-year-old man with hepatocellular carcinoma (HCC) directly contacting the right hemidiaphragm (segment VIII). (a) Transverse arterial phase T1 weighted image (repetition time/echo time, 4.3/2.0 ms; flip angle, 12°) reveals a hyperintense lesion (arrow). Note that overlying colon (arrowheads) in the right perihepatic space precludes transverse approach of radiofrequency (RF) electrode to the index tumour. The tumour was invisible on ultrasound because of poor acoustic window. (b, c). 3 days after transarterial chemoembolisation (TACE), he underwent RF ablation under fluoroscopic guidance. Anteroposterior (b) and lateral (c) fluoroscopic views show obliquely angled approach of RF electrode into the index tumour with lipiodol retention (arrowheads) without traversing the thorax. (d, e) Overlapping treatments were performed in order to achieve adequate ablative margin. For the second ablation cycle, electrode placement was adjusted to cover a different portion (d and e) of the index tumour (arrowheads). (f) Transverse portal phase CT image obtained immediately after ablation shows index tumour (arrow) surrounded by non-enhancing ablative zone (arrowheads).
Follow-up after RF ablation
CT was performed immediately after RF ablation to assess technical success, which was defined as presence of a non-enhancing area surrounding the index tumour with an ablative margin of 0.5 cm or larger on portal phase CT images. In cases of insufficient ablative margin, a second session RF ablation was performed within 24 h. 1 month follow-up CT was used for evaluation of technical effectiveness. Thereafter, the patients were followed up every 3 months using liver function tests, serum α-fetoprotein and dynamic liver CT. The presence of major complications were also evaluated based on a previous guideline [4], defined as any event leading to substantial morbidity and disability, increasing the level of care, lengthening hospital stay or requiring blood transfusion or interventional drainage procedure. Local tumour progression was defined as new enhancing lesion within or adjacent to the ablation site on dynamic liver CT [4]. Distant metastasis was defined as new HCC in the liver distant from the index tumour or in extrahepatic regions.
Results
During the study period, 18 consecutive patients with 19 HCC nodules met the inclusion criteria and were included in our analysis (Table 1). Of 18 patients, 4 (22%) had undergone previous hepatic resection (n = 2) or percutaneous RF ablation (n = 2) for HCC. The 19 nodules averaged 2.5 cm in diameter, with a range of 2–4.2 cm (median 2.3 cm). 10 (53%) nodules were located in the liver dome, either directly contacting the diaphragm (n = 6) or within 1 cm of the diaphragm (n = 4).
Table 1. Baseline characteristics of 18 patients with hepatocellular carcinoma (HCC) nodules.
| Age (years) | |
| Range (mean) | 39–70 (53) |
| Sex | |
| Males n (%) | 16 (89%) |
| Females n (%) | 2 (11%) |
| Child–Pugh class | |
| A | 15 (83%) |
| B | 3 (17%) |
| Aetiology of cirrhosis | |
| HBV | 14 (78%) |
| HCV | 2 (11%) |
| HBV–HCV | 1 (5.5%) |
| Alcohol | 1 (5.5%) |
| Serum AFP level | |
| <20 ng ml–1 | 7 (39%) |
| 20–200 ng ml–1 | 7 (39%) |
| >200 ng ml–1 | 4 (22%) |
AFP, α-fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus.
After TACE, 18 (95%) of 19 HCCs were clearly visible on biplane fluoroscopy and thus were ablated with fluoroscopy guidance (Table 2). The mean number of overlapping ablations was 2.2 per HCC nodule (range 1–3). The mean ablation time was 21 min (range 10–50 min). After biplane fluoroscopy-guided single-session RF ablation, technical success was achieved in all 18 nodules (Figures 1 and 2). Thus, based on intention-to-treat analysis, technical success of single-session RF ablation using biplane fluoroscopy was achieved in 18 (95%) of 19 nodules after TACE. Primary technical effectiveness based on 1 month follow-up CT was also achieved in all 18 nodules. The remaining one nodule invisible on biplane fluoroscopy was initially ablated with ultrasound guidance. However, incomplete ablation was depicted on CT performed immediately after RF ablation, prompting a second session of ablation under CT guidance.
Table 2. Characteristics of lesions treated with combined transcatheter arterial chemoembolisation (TACE) and percutaneous radiofrequency (RF) ablation.
| Nodule no. | Location (segment) | Liver dome location | Size (cm) | Time interval after TACE (day) | Guiding modality | No. of sessions | Overlapping no. | Total ablation time (min) | Technical success | Local tumour progression | Follow-up duration (months) |
| 1 | 8 | Y | 2 | 1 | Biplane fluoroscopy | 1 | 2 | 11 | Y | N | 30 |
| 2 | 8 | Y | 2.6 | 3 | Biplane fluoroscopy | 1 | 3 | 30 | Y | N | 28 |
| 3* | 6 | N | 2.2 | 2 | US/CT | 2 | 2 | 38 | N | N | 22 |
| 4* | 4 | Y | 3.9 | 2 | Biplane fluoroscopy | 1 | 2 | 15 | Y | N | 22 |
| 5 | 8 | Y | 2.4 | 2 | Biplane fluoroscopy | 1 | 1 | 20 | Y | N | 23 |
| 6 | 6 | N | 2.1 | 2 | Biplane fluoroscopy | 1 | 2 | 15 | Y | N | 21 |
| 7 | 8 | Y | 2.0 | 1 | Biplane fluoroscopy | 1 | 2 | 10 | Y | N | 22 |
| 8 | 8 | Y | 2.3 | 2 | Biplane fluoroscopy | 1 | 1 | 15 | Y | N | 20 |
| 9 | 2 | Y | 2.4 | 1 | Biplane fluoroscopy | 1 | 3 | 30 | Y | N | 5 |
| 10 | 6 | N | 2.3 | 3 | Biplane fluoroscopy | 1 | 1 | 10 | Y | N | 15 |
| 11 | 6 | N | 2.3 | 2 | Biplane fluoroscopy | 1 | 1 | 15 | Y | N | 21 |
| 12 | 8 | N | 2.0 | 2 | Biplane fluoroscopy | 1 | 3 | 20 | Y | N | 20 |
| 13 | 4 | Y | 4.2 | 2 | Biplane fluoroscopy | 1 | 3 | 50 | Y | N | 19 |
| 14 | 2 | N | 2.7 | 4 | Biplane fluoroscopy | 1 | 2 | 20 | Y | N | 18 |
| 15 | 5 | N | 2.0 | 2 | Biplane fluoroscopy | 1 | 2 | 22 | Y | N | 17 |
| 16 | 7 | N | 3.1 | 3 | Biplane fluoroscopy | 1 | 3 | 33 | Y | N | 12 |
| 17 | 8 | Y | 2.6 | 1 | Biplane fluoroscopy | 1 | 3 | 30 | Y | N | 14 |
| 18 | 8 | N | 2.4 | 2 | Biplane fluoroscopy | 1 | 3 | 20 | Y | N | 9 |
| 19 | 8 | Y | 2 | 1 | Biplane fluoroscopy | 1 | 2 | 15 | Y | N | 9 |
*Nodules no. 3 and no. 4 were in the same patient. Nodule no. 3 could not be targeted under biplane fluoroscopic guidance due to invisibility. Therefore, ultrasound (US)-guided RF ablation was performed initially. However, a second session of treatment was required under CT guidance because of incomplete ablation noted on immediate post-ablation CT images.
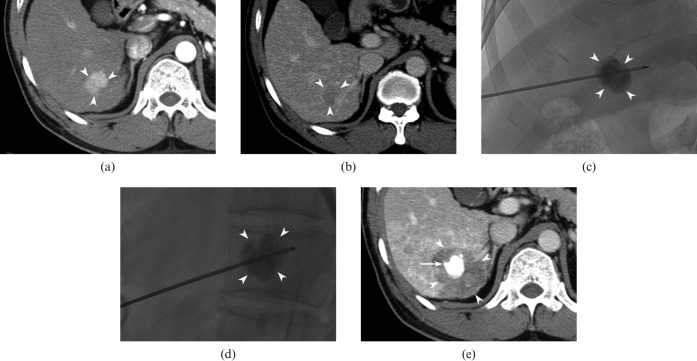
Figure 2.
46-year-old man with hepatocellular carcinoma (HCC) in the liver. (a, b) Transverse CT scan shows a 2.3 cm nodule (arrowheads) in segment VI that is hypervascular in the arterial phase (a) and washes out in the delayed phase (b), consistent with HCC. (c, d) 2 days after transarterial chemoembolisation (TACE), he underwent radiofrequency (RF) ablation. Anteroposterior (c) and lateral (d) fluoroscopic images show the RF electrode properly placed through the centre of the index tumour (arrowheads). (e) Transverse portal phase CT image obtained immediately after ablation reveals that the index tumour with retained iodised oil (arrow) is ablated with a sufficient ablative margin (arrowheads).
There were no deaths related to combined treatment. Major complication was also not observed in our study. No local tumour progression was found during follow-up period (median 20 months; range 5–30 months). New distant intrahepatic HCC developed in five patients, treated with RF ablation (n = 4) or surgical resection (n = 1). No patients died during follow-up period.
Discussion
Sufficient ablative margin (0.5–1.0 cm) around the index tumour is critical for achieving sustained local response following RF ablation. Therefore, a tumour of 2.0 cm diameter requires an ablation zone of 3.0–4.0 cm. Given that single electrode insertion can produce an ablation zone up to approximately 3 cm in short diameters [1], tumours 2 cm or larger would potentially require multiple overlapping ablations. This overlapping technique is, however, often time-consuming and technically difficult. In addition, geometric considerations predict relatively minor gains in total effective ablation volume with increasing number of overlapping ablations, as shown by a study using computer modelling [5]. For instance, 6 overlapping ablations theoretically yields only a modest 25% increase in the effective ablation volume compared with a single ablation [5]. Not surprisingly, the local tumour progression rate after RF ablation alone for HCCs larger than 2 cm is still suboptimal whereas that of tumours smaller than 2 cm is almost similar to surgical resection [1]. Therefore, combined treatment such as RF ablation plus TACE rather than RF ablation alone may be warranted for HCCs larger than 2 cm.
Ultrasound has been most widely implemented as a guiding modality for RF ablation. The virtues of ultrasound are numerous and include easy availability, lower cost and real-time multiplanar imaging capability. However, ultrasound also has serious drawbacks. For multiple overlapping ablations, the characteristic hyperechoic area of microbubbles generated by previous ablation cycles often obscures the index tumour and may hinder accurate placement of the electrode for subsequent ablation cycles. Ultrasound-guided targeting is difficult for tumours in sonographic blind spots such as the liver dome. In addition, for combined treatment, prior chemoembolisation may alter sonographic conspicuity of the index tumour owing to variable uptake of iodised oil and chemotherapeutic agent in the tumour and the adjacent hepatic parenchyma.
CT fluoroscopy has also been used to guide RF ablation alone or RF ablation combined with TACE [2,6]. It provides several contiguous axial images through near real-time image reconstruction during the interventional procedure. When the CT plane includes both the electrode path and the index tumour, the needle advance into the tumour can be monitored in a real-time manner. One major drawback of this guiding modality is the high radiation dose to both patient and operator, which is on the order of centigrays per second of exposure whereas conventional fluoroscopy is on the order of centigrays per minute of exposure [7]. This concern is obviously magnified for cases requiring multiple overlapping ablations. In addition, targeting the index tumour tends to be more time-consuming than for other modalities such as ultrasound and conventional fluoroscopy. For a tumour in the dome of the liver, oblique advance of the electrode is preferred to avoid violation of the thorax or the pleura. However, such an oblique approach might have been technically cumbersome with CT fluoroscopy guidance owing to a limited range of CT gantry tilting. Although the transthoracic approach for dome lesion has been generally accepted to be safe, pneumothorax can complicate 38–70% of cases, of which 18–40% will require chest tube placement [8-10]. Alternatively, the oblique approach for a dome lesion under CT guidance with coronal/sagittal reformatted imaging is also useful to avoid this complication.
In our study, RF ablation guided by biplane fluoroscopy following TACE was technically feasible for almost all HCC nodules (18/19, 95%). Both technical success and primary technique effectiveness were obtained in all cases treated by using fluoroscopy guidance. In all 18 HCC nodules treated under fluoroscopic guidance, technical success was obtained after single session ablation. Despite the small sample size of our study, this result compares favourably to that (single session, 77%; two or three sessions, 23%) of a previous investigation using combined treatment in which CT fluoroscopy was used for guiding modality [2]. As there was no significant difference in lesion diameter between the two studies (2.5 cm vs 2.5 cm), our results imply that biplane fluoroscopy deserves strong consideration as another viable guiding option for RF ablation combined with TACE.
Biplane fluoroscopy guided RF ablation procedure for combined treatment has several potential strengths. First, the fluoroscopically visible index tumour can be easily targeted regardless of location. Liver dome lesions are particularly challenging for RF ablation with other guiding modalities. In our series, 53% (10/19) of the tumours were located in the liver dome, and all could be easily targeted and successfully ablated with a single session procedure. Pneumothorax or haemothorax was not observed in any patient. Recently, registration and fusion of intraprocedural ultrasound with pre-procedural CT, MRI or positron emission tomography (PET) images have been reported to be feasible for thermal ablation and biopsy of liver tumours [11,12]. With these fusion techniques, dome HCCs inconspicuous on ultrasound could be targeted for percutaneous RF ablation. Second, proper needle placement into the tumour can be more confidently made because biplane fluoroscopy provides real-time orthogonal projectional imaging for the simultaneous delineation of the electrode and the index tumour. Enhanced confidence for targeting may shorten overall procedure time especially for cases needing multiple overlapping ablations. In addition, fluoroscopy provides much higher resolution, artefact-free images than does CT or CT fluoroscopy, allowing for more precise overlapping ablations. To achieve an adequate ablation zone, we used multiple overlapping ablations with a single cool-tip electrode — rather than single ablation with a multitine or a cluster type electrode which can induce larger ablation zones — since multiple overlapping ablations could be easily performed under biplane fluoroscopy guidance, which allows fast and spatially accurate electrode repositioning. Unlike on ultrasound, microbubbles generated by previous cycles of ablation do not obscure the radio-opaque index tumour on fluoroscopy. Third, this method allows a greater degree of freedom for electrode insertion than ultrasound or CT guidance. This is pertinent not only for liver dome tumours as described above, but subcapsular tumours may also be better accessed through oblique approach with biplane fluoroscopy than with ultrasound or CT to avoid direct puncture and thereby minimise the risk of bleeding or seeding [13]. However, major drawback of this guiding method is obviously that fluoroscopy cannot provide cross-sectional imaging with soft-tissue contrast of ultrasound or CT. Moreover, unlike with CT or contrast enhanced ultrasound, immediate post-ablation status cannot be assessed with biplane fluoroscopy. Therefore, ultrasound as an accessory guidance modality was required to avoid traversal of critical intrahepatic or extrahepatic structures during targeting and to estimate ablation zone during ablation. In addition, very faint iodised oil uptake into the index tumour will limit fluoroscopic visualisation and preclude fluoroscopic guidance. In our study, 5% (1/19) of HCC nodule was invisible on biplane fluoroscopy.
Recently, Kang et al [14] reported on single-session combined TACE and RF ablation of HCC ≤5 cm (mean diameter, 2.4 cm) with promising results. In this paper, however, ultrasound guidance was the main guiding modality if the index tumour was sonographically visible (54%, 31 of 57 HCCs); monoplane fluoroscopy guidance was used only for tumours not visible on ultrasound (46%, 26 of 57 HCCs). In contrast, we used biplane fluoroscopy as a primary guiding modality with adjuvant ultrasound guidance. Monoplane fluoroscopy guidance requires multiple projections of fluoroscopic imaging for electrode placement into the tumours and is therefore more cumbersome and time-consuming than biplane fluoroscopy guidance. This drawback of monoplane guidance would be more accentuated in multiple overlapping ablations. Another difference between the two studies is the time interval between TACE and RF ablation. We implemented the combined treatment with time intervals of 1–4 days (median 2 days), whereas Kang et al [14] performed RF ablation immediately after TACE. Three cases (6%) of major complications including two cases of hepatic infarction were encountered in their series, whereas no major complications were observed in our study. Although single-session combined treatment seems to be safe in general, it seems reasonable that risk of certain complications, such as hepatic infarction, might be decreased by imposing a gap of a few days between the two treatments.
Some limitations of our study should be mentioned. First, retrospective nature as well as small number of patients are limiting. Second, the diagnosis of HCC was based on imaging characteristics without pathological proof. However, current guideline [3] indicates that a non-invasive diagnosis of HCC can be made when a nodule larger than 2 cm has typical enhancing features on dynamic imaging. In addition, a negative biopsy of a nodule in a cirrhotic liver can never be regarded to definitively exclude HCC. Third, the median follow-up of only 20 months limits evaluation of local efficacy.
Conclusion
Our study demonstrates the use of biplane fluoroscopy as a feasible and effective guidance modality for RF ablation following TACE of HCC ≥2 cm. Biplane fluoroscopy should be considered a viable guiding option for combined treatment.
Acknowledgment
This work was supported by the Konkuk University Medical Center Research Grant 2008.
References
- 1.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008;47:82–9 [DOI] [PubMed] [Google Scholar]
- 2.Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology 2008;247:260–6 [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36 [DOI] [PubMed] [Google Scholar]
- 4.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 2005;235:728–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodd GD, 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol 2001;177:777–82 [DOI] [PubMed] [Google Scholar]
- 6.Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, et al. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol 2002;13:1225–32 [DOI] [PubMed] [Google Scholar]
- 7.Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT. CT fluoroscopy–guided interventional procedures: techniques and radiation dose to radiologists. Radiology 2001;220:161–7 [DOI] [PubMed] [Google Scholar]
- 8.Kato T, Yamagami T, Hirota T, Matsumoto T, Yoshimatsu R, Nishimura T. Transpulmonary radiofrequency ablation for hepatocellular carcinoma under real-time computed tomography-fluoroscopic guidance. Hepatogastroenterology 2008;55:1450–3 [PubMed] [Google Scholar]
- 9.Shibata T, Maetani Y, Kubo T, Itoh K, Togashi K, Hiraoka M. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol 2004;15:1323–7 [DOI] [PubMed] [Google Scholar]
- 10.Toyoda M, Kakizaki S, Horiuchi K, Katakai K, Sohara N, Sato K, et al. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol 2006;12:608–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crocetti L, Lencioni R, Debeni S, See TC, Pina CD, Bartolozzi C. Targeting liver lesions for radiofrequency ablation: an experimental feasibility study using a CT-US fusion imaging system. Invest Radiol 2008;43:33–9 [DOI] [PubMed] [Google Scholar]
- 12.Wood BJ, Zhang H, Durrani A, Glossop N, Ranjan S, Lindisch D, et al. Navigation with electromagnetic tracking for interventional radiology procedures: a feasibility study. J Vasc Interv Radiol 2005;16:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YJ, Raman SS, Yu NC, Busuttil RW, Tong M, Lu DS. Radiofrequency ablation of hepatocellular carcinoma: can subcapsular tumors be safely ablated? AJR Am J Roentgenol 2008;190:1029–34 [DOI] [PubMed] [Google Scholar]
- 14.Kang SG, Yoon CJ, Jeong SH, Kim JW, Lee SH, Lee KH, et al. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol 2009;20:1570–7 [DOI] [PubMed] [Google Scholar]