Abstract
The purpose of this study was to evaluate intratumoral cystic lesions of pancreatic ductal adenocarcinoma (PDAC) depicted on MRI, and to correlate these cystic lesions with their histopathological findings. This study included 12 patients (7 males and 5 females; mean age, 59 years) with intratumoral cystic lesions of PDAC detected on a retrospective MRI review. We reviewed the histopathological findings of the cystic lesions within PDACs and analysed the MRI findings, focusing on the appearance of the intratumoral cystic lesions, i.e. the size, number, margin and intratumoral location, and on the ancillary findings of PDAC, i.e. peripancreatic infiltration, upstream pancreatic duct dilatation and distal parenchymal atrophy. Intratumoral cystic lesions were classified as neoplastic mucin cysts (n = 7, 58%) or cystic necrosis (n = 5, 42%) according to the histopathological findings; they ranged in greatest dimension from 0.5 cm to 3.4 cm (mean, 1.7 cm). Seven patients had only one cystic lesion each, while the remaining five had multiple cystic lesions. Most of the neoplastic mucin cysts had smooth margins (n = 6, 86%) and eccentric locations (n = 6), whereas most cystic necroses had irregular margins (n = 4, 80%) and centric locations (n = 4). The most common ancillary findings of PDAC were peripancreatic infiltration, distal pancreatic atrophy and upstream pancreatic duct dilatation (92%, 75% and 58%, respectively). The intratumoral cystic lesions of PDACs on MRI were classified as either neoplastic mucin cysts with smooth margins and eccentric locations or cystic necroses with irregular margins and centric locations.
Pancreatic cancer is the fifth leading cause of cancer-related death in both men and women and is responsible for 5% of all cancer-related deaths in the United States [1]. Despite the advances in surgical techniques, as well as the major improvements in chemotherapy and radiotherapy protocols, the prognosis of pancreatic ductal adenocarcinoma (PDAC) usually implies a 1-year survival rate of <20% and a 5-year survival rate of <5% [2].
PDAC typically presents as an irregular solid tumour with a scirrhous character resulting from a prominent desmoplastic reaction. However, recent studies have shown that PDAC may be accompanied by cystic changes within or adjacent to the mass, and that the incidence of PDAC with cystic changes ranges from <1% to 8% [3, 4]. Radiologists should be familiar with PDACs with cystic changes as they may resemble more common cystic pancreatic lesions, such as pseudocysts, intraductal papillary mucinous neoplasms (IPMNs), solid pseudopapillary tumours and non-functioning islet cell tumours, all of which are managed differently and usually have better patient survival rates [5–7].
Many studies have discussed the radiological appearance of PDAC accompanied by cystic lesions [6–11]. Most of these studies have discussed pseudocysts or retention cysts depicted adjacent to the PDAC or in the extrapancreatic area in the clinical setting of pancreatitis [8–11], whereas only a few studies have discussed intratumoral cystic lesions, such as cystic necroses, in larger ordinary PDACs [6, 7]. Some case reports have described the intratumoral cystic changes of PDAC variants, i.e. adenosquamous carcinoma [12], mucinous adenocarcinoma (colloid or mucinous non-cystic carcinoma) [13], osteoclast-like giant cell carcinoma [14] and pleomorphic giant cell carcinoma [15]. To the best of our knowledge, there have been no radiological reports regarding the intratumoral cystic lesions of ordinary PDAC. Compared with CT, MRI has the advantage of being able to detect cystic changes within pancreatic masses and to provide more accurate morphological detail on these changes [16]. Therefore, the aims of this study were to evaluate intratumoral cystic lesions of ordinary PDAC detected on MRI and to correlate the cystic lesions with their histopathological findings.
Methods and materials
Patients
This retrospective study was approved by our institutional review board, and informed consent was waived. Between December 2006 and January 1997, we reviewed retrospectively the pre-operative MR images of 186 patients with surgically resected, pathologically proven PDAC; these patients were identified using a computerised medical records system. All of these patients had undergone pre-operative MRI, including MR cholangiopancreatography (MRCP) and dual-phase contrast-enhanced three-dimensional (3D) MR angiography (MRA) (n = 161), MRCP only (n = 11) or MRI performed outside our institution (n = 14). Two radiologists retrospectively reviewed the MR images of all 186 patients in consensus to determine whether or not the PDACs had intratumoral cystic lesions. All MR images were reviewed using a local picture archiving and communication system (PACS) monitor and Digital Imaging and Communications in Medicine (DICOM) image viewing software. The criteria to identify intratumoral cystic lesions on MR images were a cerebrospinal fluid-like bright signal intensity within the mass on T2 weighted MR images and no contrast enhancement of the cystic lesions surrounded by solid masses on contrast-enhanced T1 weighted MR images. We excluded peritumoral cystic lesions, such as pseudocysts, and peripancreatic fluid, as well as dilated upstream pancreatic ducts or distal common bile ducts. Finally, this study included 12 patients (6.5%; 7 males and 5 females; mean age, 59 years; age range, 44–74 years) with PDAC and intratumoral cystic lesions detected on MR images. All of these patients underwent pre-operative MRCP with 3D MRA (n = 11) or MRCP only (n = 1) in our institution. The histopathological diagnosis of PDAC was made from the surgical specimens obtained from Whipple's operation (n = 6), distal pancreatectomy (n = 5) or total pancreatectomy (n = 1).
Histopathological review
In all 12 study patients, the histopathological specimens were reviewed retrospectively by a gastrointestinal pathologist (S.J.J. with 18 years’ clinical pathology experience) from our institution. Nine gross and histopathological specimens were evaluated while focusing on the features of the intratumoral cystic lesions. The remaining three histopathological specimens were not available. Therefore, only these patients’ histopathological reports and photographs of the gross specimens were reviewed.
MRI techniques
MRI was performed on a 1.5 T MR imaging system (Magnetom Vision; Siemens Medical Solutions, Erlangen, Germany) with a phased-array body coil with four elements and included (i) T1 and T2 weighted images with and without fat suppression, (ii) MRCP using both thick-slab single-shot rapid acquisition with relaxation enhancement (RARE), and multislice half-Fourier acquisition single-shot turbo spin-echo (HASTE) and (ii) dual-phase contrast-enhanced coronal 3D MRA. T1 weighted axial gradient-echo MRI (repetition time/echo time (TR/TE), 149/4.1 ms; flip angle, 80°; section thickness, 6–8 mm; 18–20 sections; intersection gap, 1.6 mm) and HASTE T2 weighted axial MRI (TR/TE, infinite/134 ms; flip angle, 150°; section thickness, 8 mm; 18–20 sections; intersection gap, 1.6 mm) were obtained during a single breath-hold. The parameters for single-shot RARE and multislice HASTE MRCP are shown in Table 1. Post-processing of multislice HASTE images was not performed. In 11 of 12 patients, 0.1 mmol kg–1 body weight gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany) was injected intravenously as a rapid bolus after acquiring unenhanced MR images, and was followed by 10 ml of normal saline flush. After MR arteriography and MR portography were performed, single-phase MR images were obtained 50 s after contrast injection with the same parameters used for fat-saturated unenhanced T1 weighted images.
Table 1. Specific Sequence Parameters for MRCP.
| RARE | HASTE | |
| TR/TE (ms) | Infinite/1080 | Infinite/144 |
| Flip angle (°) | 150 | 150 |
| Matrix | 512×240 | 512×128 |
| ETL | 256 | 128 |
| FOV (cm2) | 30×32 | 30×32 |
| Acquisition time (s) | 3.32 | 18–23 |
| Slice thickness (mm) | 50–70 | 4 |
| No. of slices | 1 | 15–19 |
MRCP, magnetic resonace cholangiopancreatography; RARE, rapid acquisition with relaxation enhancement; HASTE, half-Fourier acquisition single-shot turbo spin echo; TR, repetition time; TE, echo time; ETL, echo train length; FOV, field of view.
MRI analysis
Two board-certified gastrointestinal radiologists (J.H.B. and S.E.Y.), each with seven years’ clinical experience, reviewed retrospectively and in consensus the pre-operative MR images, including those taken in the axial and coronal planes, in the same room and at the same time; they focused on the appearance of the tumour, including its size, nature and location. The tumour size was defined as the longest dimension on the axial contrast-enhanced T1 weighted MR image in which the lesion appeared the largest. The nature of the tumour was grossly classified as complex, i.e. total cystic portions ≤75% of the tumour, or cystic, i.e. total cystic portions >75% of the tumour. The tumour location was identified as being in the head, body or tail of the pancreas. We reviewed the appearance of the intratumoral cystic lesions, i.e. the size, number, margin (smooth vs irregular) and location. The location of intratumoral cystic lesions was grossly classified as (i) centric, i.e. the cyst primarily occupying the inner half of the tumour; (ii) eccentric, i.e. the cyst primarily occupying the outer half of the tumour; or (iii) combined, i.e. the cyst equally occupying the inner and outer halves of the tumour. The size of the intratumoral cystic lesions was measured only for the largest cyst with the longest diameter. The ancillary findings of ordinary PDAC, such as peripancreatic infiltration, distal pancreatic parenchymal atrophy and upstream pancreatic duct dilatation, were also evaluated. When the tumour was located in the pancreatic head, the presence or absence of the “double-duct sign”, i.e. dilatation of the common bile duct and the main pancreatic duct, was evaluated. Both reviewers were blinded to the descriptive findings on both the surgery and pathology reports.
One author (H.J.K. with 5 years’ clinical experience) reviewed all 12 MR images and made a radiological diagnosis of the pancreatic mass. The reviewer was blinded to both the patient information and the final diagnoses. All images were reviewed using a local PACS monitor and DICOM image viewing software.
Statistical analysis
We evaluated any statistical differences in the mean tumour size and mean size of the intratumoral cystic lesions according to the histological type of the intratumoral cystic lesions using the Mann–Whitney test. A p-value <0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS for Windows version 11.5 (SPSS, Chicago, IL).
Results
The radiological differential diagnoses, as well as the MRI and histopathological findings of PDAC with intratumoral cystic lesions, are summarised in Table 2.
Table 2. Radiological differential diagnoses and MRI and histopathological findings in 12 patients with PDAC with intratumoral cystic lesions.
| Case no./sex/age (years) | Radiological differential diagnosis | MRI findings |
Histopathological findings | |
| Tumour: size/nature/location/ancillary findings | Intratumoral cyst: size/number/margin/location | |||
| 1/F/59 | PDAC | 1.8 × 1.8 cm/complex/body/PI, PDD and PA | 0.8 × 0.7 cm/1/smooth/eccentric | Neoplastic mucin cyst |
| 2/M/51 | PDAC | 5.0 × 3.6 cm/complex/head/PI, PDD, PA and DDS | 1.6 × 1.5 cm/1/smooth/eccentric | Neoplastic mucin cyst |
| 3/M/74 | PDAC, iIPMN | 2.6 × 1.5 cm/complex/head/PDD, PA and DDS | 0.5 × 0.4 cm/1/irregular/centric | Neoplastic mucin cyst |
| 4/M/51 | PDAC, iIPMN | 4.5 × 2.5-cm/cystic/tail/PI and PA | 2.1 × 1.4 cma/3/smooth/eccentric | Neoplastic mucin cyst |
| 5/F/74 | PDAC, iIPMN | 2.1 × 1.8-cm/complex/tail/PI, PDD, and PA | 1.0 × 0.8 cma/5/smooth/eccentric | Neoplastic mucin cyst |
| 6/F/49 | PDAC | 2.9 × 2.1 cm/complex/head/PI, PDD and PA | 1.4 × 0.8 cm/1/smooth/eccentric | Neoplastic mucin cyst |
| 7/M/50 | PDAC, MCA, iIPMN | 6.0 × 4.7-cm/cystic/tail/PI | 3.0 × 2.1 cma/4/smooth/eccentric | Neoplastic mucin cyst |
| 8/F/70 | PDAC | 2.5 × 2.0 cm/complex/head/PI, PDD, PA and DDS | 1.0 × 1.0 cm/1/irregular/centric | Cystic necroses |
| 9/M/71 | PDAC, IPMN | 2.5 × 2.0-cm/cystic/head/PI and PA | 2.0 × 1.8 cm/1/irregular/centric | Cystic necroses |
| 10/M/56 | PDAC, NICT | 4.7 × 3.7 cm/cystic/tail/PI | 3.4 × 1.7 cma/2/smooth/centric | Cystic necroses |
| 11/M/44 | PDAC, NICT | 4.5 × 4.1 cm/complex/tail/PI | 1.3 × 1.4 cma/4/irregular/combined | Cystic necroses |
| 12/F/58 | SPT, NICT | 5.4 × 5.3 cm/complex/head/PI, PDD, PA and DDS | 2.2 × 0.9 cm/1/irregular/centric | Cystic necroses |
PDAC, pancreatic ductal adenocarcinoma; iIPMN, invasive carcinoma of intraductal papillary mucinous neoplasm; MCA, mucinous cystadenocarcinoma; NICT, non-functioning islet cell tumour; SPT, solid pseudopapillary tumour; PI, peripancreatic infiltration; PDD, pancreatic duct dilatation; PA, parenchymal atrophy; DDS, double duct sign.
aThe size of the intratumoral cystic lesion is the same as that of the largest main cyst.
Histopathological findings
In the gross specimens, six tumours (Cases 1–6 in Table 2) had mucoid cystic change (n = 5) or focal cystic change (n = 1) within the yellowish, infiltrative, firm mass. In the microscopic specimens, the cystic lesions were partly or completely lined by mucin-producing neoplastic cells and were embedded in the fibrous stroma of the ordinary PDAC (Figures 1 and 2). We referred to the cystic lesions as “neoplastic mucin cysts”. Another tumour (Case 7 in Table 2) revealed mainly multifocal mucoid cysts and some necrotic foci on a histopathological specimen. In the remaining five tumours (Cases 8–12 in Table 2), the gross specimens showed cystic changes with necroses within the solid masses (Figure 3). In two of these five tumours, the histological specimens had tumour necroses without lining cells. Therefore, according to the histopathological findings, we were able to classify the intratumoral cystic lesions as either neoplastic mucin cysts (n = 7; 58%) or cystic necroses (n = 5; 42%).
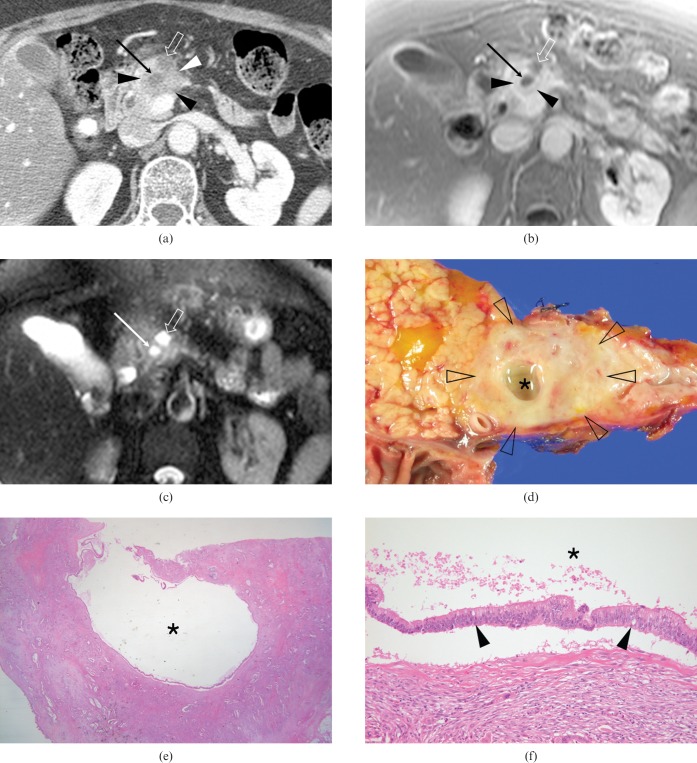
Figure 1.
A 59-year-old woman with pancreatic ductal adenocarcinoma (PDAC) and an intratumoral neoplastic mucin cyst in the pancreatic body (Case 1). (a) Axial contrast-enhanced CT scan shows a low-attenuation mass (arrowheads) containing an eccentric cyst (arrow) with upstream ductal dilatation (open arrow). (b) Axial contrast-enhanced fat-suppressed T1 weighted fast-low angle shot (FLASH) image (repetition time/echo time (TR/TE), 149/4.1 ms; flip angle, 80°) shows a slightly hypointense mass (arrowheads) in the background of the more enhanced pancreatic parenchyma. Note the markedly hypointense lesion with a smooth margin, representing an intratumoral cyst (arrow), as well as the upstream ductal dilatation (open arrow). (c) Axial fat-suppressed T2 weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE) image (TR/TE, 4.4/134 ms; flip angle, 150°) shows an intratumoral cyst (arrow) with a smooth margin and bright signal intensity similar to that of the cerebrospinal fluid, as well as upstream ductal dilatation (open arrow). (d) Photograph of a surgical specimen shows a yellowish-gray infiltrative mass (open arrowheads) and an eccentrically located mucoid cystic change (asterisk). (e, f) Photomicrographs of a histological specimen show a cyst (asterisks) lined by mucin-secreting malignant cells (arrowheads in (f)) within the fibrous stroma of an ordinary PDAC (haematoxylin and eosin stain, ×10 (e) and ×200 (f)).
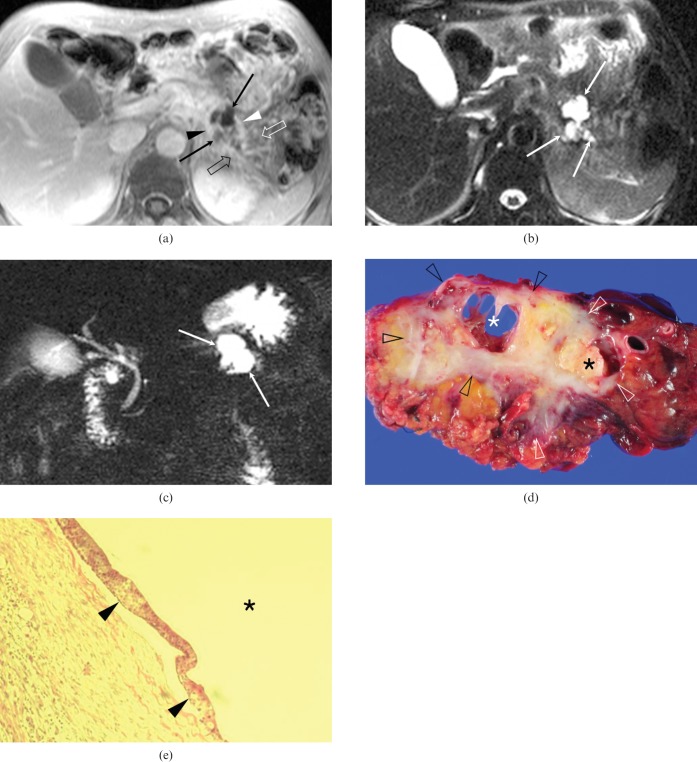
Figure 2.
A 51-year-old man with pancreatic ductal adenocarcinoma and intratumoral neoplastic mucin cysts in the pancreatic tail (Case 4). (a) Axial contrast-enhanced fat-suppressed T1 weighted fast-low angle shot (FLASH) image shows two eccentrically located cysts (arrows) surrounded by a peripheral, irregular, rim-like enhancing mass (arrowheads). Note the infiltration of the peripancreatic fat planes (open arrows). (b) Axial fat-suppressed T2 weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE) image shows three eccentrically located cysts with smooth margins (arrows). (c) Thick-slab coronal oblique MR cholangiopancreatography image with single-shot rapid acquisition with relaxation enhancement (RARE) shows a cyst (arrows). Note that there is no dilatation of the downstream main pancreatic duct. (d) Photograph of a surgical specimen shows an ill-defined, greyish to yellowish, firm mass (open arrowheads) with two eccentrically located cystic lesions (asterisks). (e) Photomicrograph of a histological specimen shows a cyst (asterisk) lined by mucin-producing neoplastic cells (arrowheads) (haematoxylin and eosin stain, ×100).
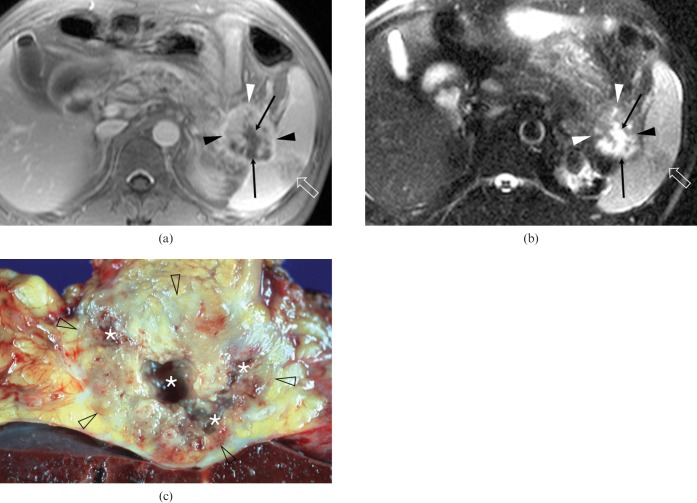
Figure 3.
A 44-year-old man with pancreatic ductal adenocarcinoma and intratumoral cystic necroses in the pancreatic tail (Case 11). (a) An axial contrast-enhanced fat-suppressed T1 weighted fast-low angle shot (FLASH) image and (b) an axial fat-suppressed T2 weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE) image show a relatively well-demarcated solid and cystic mass surrounded by irregular rim enhancement (arrowheads). The four irregularly marginated, cystic lesions are located in the centric and eccentric, i.e. combined, portions within the tumour (arrows). Note that the tumour invades the splenic hilum and produces ischaemic change in the spleen (open arrows). (c) Surgical specimen shows an inhomogeneously yellowish-white mass (open arrowheads) with multifocal necroses (asterisks).
MRI features
In seven PDACs with neoplastic mucin cysts, the mean size in the longest dimensions was 3.6 × 2.6 cm (range, 1.8 × 1.8 cm to 6.0 × 4.7 cm). These tumours appeared as either complex (n = 5) or cystic (n = 2) and were located in the pancreatic head (n = 3), tail (n = 3) or body (n = 1). The mean size of the greatest dimensions of these intratumoral cystic lesions was 1.5 × 1.1 cm (range, 0.5 × 0.4 cm to 3.0 × 2.1 cm). There was one intratumoral cystic lesion in four patients, whereas five, four and three cystic lesions were each seen in three patients. In six PDACs with neoplastic mucin cysts (86%), the cysts had smooth margins and eccentric locations (Figures 1 and 2).
In five PDACs with cystic necroses, the mean size of the longest dimensions was 3.9 × 3.4 cm (range, 2.5 × 2.0 cm to 5.4 × 5.3 cm). These tumours appeared as either complex (n = 3) or cystic (n = 2) and were located in the pancreatic head (n = 3) or tail (n = 2). The mean size of the greatest dimensions of these intratumoral cystic lesions was 2.0 × 1.4 cm (range, 1.0 × 1.0 cm to 3.4 × 1.7 cm). There was one cystic lesion in three patients, and four and two cystic lesions each in two patients. In 4 of 5 PDACs with cystic necroses, the cystic changes (80%) had irregular margins (Figures 3 and 4) and centric locations (Figure 4). The remaining PDAC had a smooth margin and a combined location (Figure 3).
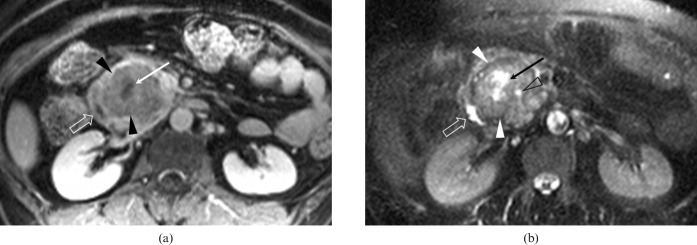
Figure 4.
A 58-year-old woman with pancreatic ductal adenocarcinoma and intratumoral cystic necroses in the pancreatic head (Case 12). (a) An axial contrast-enhanced fat-suppressed T1 weighted fast low angle shot (FLASH) image and (b) an axial fat suppressed T2 weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE) image show a relatively large well-demarcated solid mass (arrowheads) with an irregularly marginated central area of necrosis (arrows). The mass laterally displaces the second portion of the duodenum (open arrows). The mass is accompanied by minimal dilatation of the common bile duct (open arrowhead in (b)) and of the main pancreatic duct (not shown).
There was no significant difference between the mean size of the PDACs with neoplastic mucin cysts and that of PDACs with cystic necroses (p<0.29). There was also no significant difference in the mean size of the intratumoral cystic lesions in either group (p<0.808).
The most common ancillary finding of PDAC was peripancreatic infiltration (n = 11; 92%). 9 PDACs (75%) showed distal pancreatic parenchymal atrophy. 7 PDACs (58%) located in the pancreatic head (n = 5), body (n = 1) and tail (n = 1) showed upstream main pancreatic duct dilatation. Four of the six PDACs in the pancreatic head had the positive double-duct sign.
Radiological differential diagnoses
All seven patients with neoplastic mucin cysts, and four of the five patients with cystic necroses, were radiologically diagnosed with PDAC. The remaining patient with cystic necroses (Case 12 in Table 2) was diagnosed with a solid pseudopapillary tumour (Figure 4). 4 (57%) of 7 PDACs with neoplastic mucin cysts mimicked invasive carcinoma of IPMN (Figure 2) or mucinous cystadenocarcinoma, whereas 3 (60%) of 5 PDACs with cystic necroses mimicked non-functioning islet cell tumours.
Discussion
PDACs with cystic lesions are uncommon and may occur by means of the four following mechanisms:
1. Obstruction of the pancreatic duct by the tumour may result in the formation of retention cysts localised outside the tumour periphery [3, 17].
2. Duct obstruction may also trigger acute pancreatitis resulting in pseudocyst formation localised outside the tumour [3, 6].
3. A microcystic appearance caused by marked ectasia of the invasive gland and referred to as the “large-duct type” [17, 18].
4. A tumour is large and cystic necroses may occur within a tumour [3, 17].
Retention cysts or pseudocysts accompanied by PDAC result from pancreatic duct obstruction caused by tumour and are followed by subsequent dilatation of the duct or pancreatitis. They may thus resemble IPMN [3, 17]. However, this study focused on cystic changes within the tumour and excluded retention cysts and pseudocysts accompanied by PDAC.
In a recent study on cystic neoplasms and lesions of the pancreas, Kosmahl et al [18] were able to show that PDAC with cystic features accounted for 7% out of all 30 cases. In the recent large PDAC series reported by the same authors [3], 38 (8%) of 483 PDACs showed cystic features. Among these 38 PDACs, 24 were a large-duct type with small cysts, 8 had cystic necroses, 4 had retention cysts and the remaining 2 exhibited closely attached pseudocysts. As the retention cysts and pseudocysts were not located within the mass but were adjacent to the tumour, the intratumoral cystic changes were proven to occur in 32 (6.6%) of 483 PDACs. The present study showed that 12 (6.5%) of 186 PDACs had intratumoral cystic lesions with 7 neoplastic mucin cysts and 5 cystic necroses; these results are similar to those of Kosmahl et al [3]. As PDACs with intratumoral cystic lesions are uncommon but are not rare, they should be considered as part of the differential diagnosis when a tumorous cystic lesion of the pancreas is depicted on the radiological images.
According to previous reports [3, 17, 18], the aforementioned intratumoral cysts of “large-duct-type” PDAC are usually 0.5–0.7 cm in diameter and only rarely exceed 1 cm. They are difficult to detect on imaging studies because of their small diameter. In addition, as large-duct-type PDACs usually appear in the region of the tumour infiltrating overlying duodenal muscularis propria [17], they show different features from our cases of neoplastic mucin cysts. In the present study, seven PDACs on microscopy were noted to have intratumoral cystic lesions, which were partly or completely lined by mucin-producing neoplastic cells surrounded by the fibrous stroma of ordinary PDAC; we referred to them as “neoplastic mucin cysts”. To the best of our knowledge, this is a new mechanism of observing the intratumoral cystic changes of PDACs.
Interestingly, four (57%) of the seven PDACs with neoplastic mucin cysts detected in this study should be distinguished from mucin-producing neoplasms, such as invasive carcinoma of IPMN, on MR images. Is the pathogenesis of PDACs with neoplastic mucin cysts related to IPMNs? Yamada et al [19] reported on invasive carcinomas derived from IPMNs of the pancreas during long-term follow-up, and suggested that, when a solid mass in the pancreatic parenchyma is surrounded by dilated pancreatic ducts, invasive carcinoma of IPMNs should be considered. However, the neoplastic mucin cysts detected in our patients did not appear around a solid mass but within a solid mass. Therefore, we believe that the pathogenesis of PDAC with neoplastic mucin cysts differs from that of invasive IPMN of the pancreas. Indeed, mucin is produced in the greatest quantities by the duct cells and serves as a defensive barrier against the catalytic activity of the acinar enzymes in the pancreas. Therefore, it is not surprising that all ductal tumours of the pancreas are mucinous, as most pancreatic neoplasms of presumed ductal origin also display some degree of mucin production [20].
In general, PDAC with cystic necrosis is known to be a large tumour [3, 17]; however, in our study, there was no difference between the tumour size of PDACs with cystic necroses and that of PDACs with neoplastic mucin cysts. In other words, a large PDAC with intratumoral cystic lesions could have neoplastic mucin cysts rather than cystic necroses. In the present study, neoplastic mucin cysts within PDAC had a tendency towards smooth margins and eccentric locations, whereas cystic necroses within PDAC had irregular margins and centric locations.
In this study, PDACs with neoplastic mucin cysts, which were located in the pancreatic tail and had multiple intratumoral cysts, mimicked other mucin-producing neoplasms of the pancreas, such as invasive carcinoma of IPMNs. Conversely, PDACs with cystic necroses should be differentiated from other solid pancreatic neoplasms with intratumoral cystic necroses, such as non-functioning islet cell tumours and solid pseudopapillary tumours. Although surgical resection or biopsy is generally mandatory to confirm the diagnosis of these tumours, 11 of the 12 patients in this study were radiologically diagnosed as having PDAC because their lesions had the typical features of ordinary PDAC on MRI, including dilatation of the upstream pancreas duct, distal parenchymal atrophy and infiltration of the peripancreatic fat planes. These ancillary findings of ordinary PDACs help to differentiate PDACs with intratumoral cystic lesions from other pancreatic tumours, including cystic neoplasms.
One patient with PDAC with cystic necroses was radiologically diagnosed with a solid pseudopapillary tumour or non-functioning islet cell tumour involving the pancreatic head because the patient was a female and the tumour showed a relatively well-defined margin, central focal necroses within the solid mass and minimal dilatation of the pancreatic and common bile duct, as well as minimal distal parenchymal atrophy despite the relatively large size of the tumour (5.4 × 5.3 cm).
This study has several limitations. Firstly, it included only a small number of patients because of the low incidence of PDACs with intratumoral cystic lesions on MR images among resected PDACs. Secondly, we could not completely evaluate all histological specimens because of the lack of three specimens of PDACs with cystic necroses and because it was a retrospective study. We believe, however, that these three cases represented PDACs with cystic necroses, as they were easily distinguished from the PDACs with neoplastic mucin cysts on the gross specimens and we reviewed their gross photographs and histopathological reports. Thirdly, as we used a new term — neoplastic mucin cysts — an international pathological consensus regarding this term is needed.
In conclusion, radiologically detectable intratumoral cystic lesions accompanied by PDACs on MR images are uncommon but not rare (6.5% in this study). Intratumoral cystic lesions within PDACs result from either neoplastic mucin cysts or cystic necroses. Neoplastic mucin cysts tend to have smooth margins and eccentric locations, whereas cystic necroses tend to have irregular margins and centric locations. The ancillary findings of ordinary PDACs are helpful for differentiating PDAC with intratumoral cystic lesions from other neoplasms, including cystic tumours, of the pancreas.
Acknowledgments
The authors thank Yong Ho Auh, MD, Professor of Radiology, Cornell University Weill Medical College, New York, NY for his valuable suggestions and Bonnie Hami, MA, for her editorial assistance in preparing the manuscript.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin 2000;50:7–33 [DOI] [PubMed] [Google Scholar]
- 2.Evans DB, Abbruzzese JL, Rich TA. Cancer of the pancreas. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 5th edn. Philadelphia, PA: Lippincott-Raven, 1997:1054–87 [Google Scholar]
- 3.Kosmahl M, Pauser U, Anlauf M, Kloppel G. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Mod Pathol 2005;18:1157–64 [DOI] [PubMed] [Google Scholar]
- 4.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med 2004;351:1218–26 [DOI] [PubMed] [Google Scholar]
- 5.Gasslander T, Arnelo U, Albiin N, Permert J. Cystic tumors of the pancreas. Dig Dis 2001;19:57–62 [DOI] [PubMed] [Google Scholar]
- 6.Choi EK, Park SH, Kim DY, Kim KW, Byun JH, Lee MG, et al. Unusual manifestations of primary pancreatic neoplasia: radiologic-pathologic correlation. J Comput Assist Tomogr 2006;30:610–7 [DOI] [PubMed] [Google Scholar]
- 7.Park MS, Kim KW, Lim JS, Lee JH, Kim JH, Kim SY, et al. Unusual cystic neoplasms in the pancreas: radiologic-pathologic correlation. J Comput Assist Tomogr 2005;29:610–6 [DOI] [PubMed] [Google Scholar]
- 8.Grogan JR, Saeian K, Taylor AJ, Quiroz F, Demeure MJ, Komorowski RA. Making sense of mucin-producing pancreatic tumors. AJR Am J Roentgenol 2001;176:921–9 [DOI] [PubMed] [Google Scholar]
- 9.Kimura W, Sata N, Nakayama H, Muto T, Matsuhashi N, Sugano K, et al. Pancreatic carcinoma accompanied by pseudocyst: report of two cases. J Gastroenterol 1994;29:786–91 [DOI] [PubMed] [Google Scholar]
- 10.Nugent CE, Lehman GA, Madura JA, Kopecky K. Pancreatic cancer presenting with resolving pseudocyst during octreotide therapy. Pancreas 1993;8:506–9 [DOI] [PubMed] [Google Scholar]
- 11.Itai Y, Moss AA, Goldberg HI. Pancreatic cysts caused by carcinoma of the pancreas: a pitfall in the diagnosis of pancreatic carcinoma. J Comput Assist Tomogr 1982;6:772–6 [DOI] [PubMed] [Google Scholar]
- 12.Myung SJ, Kim MH, Lee SK, Seo DW, Kim YS, Min YI. Adenosquamous carcinoma of the pancreas: differentiation from pancreatic pseudocyst. Gastrointest Endosc 1998;47:410–3 [DOI] [PubMed] [Google Scholar]
- 13.Demos TC, Posniak HV, Harmath C, Olson MC, Aranha G. Cystic lesions of the pancreas. AJR Am J Roentgenol 2002;179:1375–88 [DOI] [PubMed] [Google Scholar]
- 14.Oehler U, Jurs M, Kloppel G, Helpap B. Osteoclast-like giant cell tumour of the pancreas presenting as a pseudocyst-like lesion. Virchows Arch 1997;431:215–8 [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Hirohashi K, Tanaka H, Uenishi T, Shuto T, Kubo S, et al. Resectable pleomorphic giant cell carcinoma of the pancreas. Int J Gastrointest Cancer 2001;29:63–8 [PubMed] [Google Scholar]
- 16.Lopez Hänninen E, Pech M, Ricke J, Denecke T, Amthauer H, Lehmkuhl L, et al. Magnetic resonance imaging in the assessment of cystic pancreatic lesions: differentiation of benign and malignant lesion status. Acta Radiol 2006;47:121–9 [DOI] [PubMed] [Google Scholar]
- 17.Adsay NV, Klimstra DS. Cystic forms of typically solid pancreatic tumors. Semin Diagn Pathol 2000;17:81–8 [PubMed] [Google Scholar]
- 18.Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch 2004;445:168–78 [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y, Mori H, Matsumoto S, Kamei N, Hongo N. Invasive carcinomas derived from intraductal papillary mucinous neoplasms of the pancreas: a long-term follow-up assessment with CT imaging. J Comput Assist Tomogr 2006;30:885–90 [DOI] [PubMed] [Google Scholar]
- 20.Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, Conlon KC, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol 2001;25:26–42 [DOI] [PubMed] [Google Scholar]