Abstract
Objective
The purpose of this study was to analyse retrospectively the intensity-modulated radiotherapy (IMRT) results in patients with head and neck cancer (HNC) treated between November 2003 and June 2007.
Methods
Patients with early and locoregionally advanced HNC were treated with inverse-planned step-and-shoot IMRT. The prescribed dose varied from 66 Gy to 70 Gy in those receiving IMRT as definitive treatment and from 60 Gy to 70 Gy in the post-operative setting. IMRT was given alone, after induction chemotherapy (ICT), with concomitant chemotherapy (CRT) or with both. Acute and late toxicities are reported; locoregional control (LRC), locoregional relapse-free survival (LRRFS) and overall survival (OS) were calculated from the start of radiation.
Results
IMRT was used in 78 patients (48 as definitive treatment, 30 post-operatively), of whom 20 also received ICT and 35 CRT. Three patients stopped IMRT early, one for toxicity (mucosa). Acute toxicity scoring revealed 5 cases (6%) of severe skin toxicity and 65 cases (83%) of severe mucosal toxicity. After a median follow-up of 18.7 months, late toxicities included xerostomia (44%), loss of taste (14%) and fibrosis of the neck (9%). 16 patients had died, of whom 10 due to tumour recurrence/progression and 2 due to treatment (but not IMRT related). The LRC, LRRFS and OS at 3 years are 66.1%, 48.5% and 60.3% in the definitive IMRT group and 85.4%, 82.5% and 85.9% in the post-operative setting, respectively.
Conclusion
We consider IMRT for locoregional HNC feasible not only as a single modality but also after surgery, after induction chemotherapy and concurrently with chemotherapy.
Locoregional head and neck cancer (HNC) poses a major therapeutic and technological challenge. Different strategies have been applied to improve treatment outcome, such as altered fractionation radiotherapy [1,2], concurrent chemoradiotherapy [3-8], bioradiation (i.e. concurrent use of radiation and cetuximab [9]) and also, recently, the use of a more effective induction chemotherapy followed by (chemo-) radiation (i.e. sequential therapy) [10,11].
Radiotherapy techniques have evolved strongly during the last decade with the implementation of intensity-modulated radiotherapy (IMRT). The sharp dose fall-off gradient of this technique permits the administration of a highly conformal and more homogeneous dose to the planning target volume (PTV) [12] than conventional and conformal radiotherapy. This allows better sparing of the organs at risk (e.g. parotid glands, submandibular and minor salivary glands, larynx and swallowing structures), leading to a decrease in acute and late side effects [13-16]. This may open a window for treatment intensification of radiotherapy alone or combined with chemotherapy and/or targeted therapy. In addition, IMRT permits the administration of different doses to different adjacent risk zones at the same time, so-called “dose painting”.
For a long time, however, a major problem of IMRT was the lack of hard evidence of its superiority over the more classic irradiation techniques. Kam et al [17] showed in a prospective randomised study, without concurrent chemotherapy, significantly less observer-rated severe xerostomia and a significantly higher stimulated parotid and whole saliva flow rate after IMRT treatment for early stage nasopharyngeal carcinoma than two-dimensional radiotherapy. Interestingly, this was not in concordance with patient-reported outcome. Very recently, Nutting et al [18] reported the first phase III multicentre randomised controlled trial in patients with HNC showing significantly less Grade 2 or more xerostomia at 12 and at 18 months in the IMRT arm than the conventional radiotherapy arm, both without concurrent chemotherapy. No differences in acute mucositis or pain scores were found, although the IMRT group suffered from significantly more acute fatigue of Grade 2 or more.
However, a clear survival benefit of IMRT over the more classic three-dimensional conformal radiation therapy has not been shown as yet [19,20], and there are some concerns about the theoretically higher risk of induction of secondary cancers by IMRT because of the increased low-dose irradiated volume [21,22]. IMRT might also lead to unexpected higher toxicity in areas that were not in the classic two-dimensional beam path but that are irradiated in the IMRT set-up, especially in combination with concurrent chemotherapy [23]. Therefore, experiences of IMRT, with and without induction and/or concurrent chemotherapy, should be reported and shared.
In this article we review the results of IMRT in patients with early and locoregionally advanced HNC treated at our hospital between November 2003 and June 2007.
Methods and materials
Patients
Since 2003 selected patients with newly diagnosed early and locoregionally advanced HNC according to the 2002 American Joint Committee on Cancer staging system [24] have been treated with IMRT. All patients were treated with IMRT either after surgery or as definitive treatment modality, with or without sequential and/or concurrent chemotherapy.
The pre-treatment evaluation included a complete history and physical examination, pan-endoscopy, chest X-ray, complete blood count, liver and renal biochemistry, CT scan of the head and neck region and for most patients also a baseline MRI scan of this region.
Treatment planning and delivery
For each patient a contrast-enhanced CT scan with 3 mm slice thickness was made in the treatment position with an immobilisation mask.
Volumes
The gross tumour volume (GTV), the clinical target volume (CTV) and the nearby organs at risk (OAR) were delineated on the Pinnacle 6.2b and 7.6c planning system (Philips Medical Systems, Eindhoven, the Netherlands). OAR contoured routinely were the spinal cord, brain stem, brain, parotid glands, larynx (when not involved) and the mucosa of the mouth outside the PTV. In cases of paranasal sinus tumours and nasopharyngeal tumours, we also delineated the optical nerves, optical chiasm and lacrymal glands. Lymph nodes were delineated according to the Danish Head and Neck Cancer Group (DAHANCA), European Organisation for Research and Treatment of Cancer (EORTC), Oncology and Radiotherapy Group for Head and Neck Cancer (GORTEC), National Cancer Institute of Canada (NCIC), Radiation Therapy Oncology Group (RTOG) consensus guidelines as described by Gregoire et al [25]. The PTV was defined as the CTV plus a 3 mm margin. A planning risk volume (PRV) was created 3 mm around the critical OAR (spinal cord, brain stem, optical nerves and optical chiasm).
Neck region
Most patients had bilateral lymph node irradiation while lymph node regions were irradiated unilaterally in case of tumours of the oral cavity or oropharynx located more than 2 cm from the midline. No lymph node irradiation was done in case of paranasal sinus tumours and after neck dissection without lymph node involvement.
Irradiation techniques
IMRT plans were made with the inverse step-and-shoot treatment planning module of Pinnacle 6.2b at the beginning and 7.6c afterwards. The dose to the PTV was prescribed according to the International Commission on Radiation Units and Measurements (ICRU) report 62 [26]. The prescribed dose to the non-involved lymph node levels was 50 Gy in 25 fractions in 5 weeks. The PTV of the tumour and of the high-risk lymph node levels was planned to receive 60–66 Gy in 6–6.5 weeks in the post-operative setting [8] and 70 Gy in 7 weeks in case of incomplete resection and in patients who received definitive IMRT, all in fractions of 2 Gy. For some non-operated patients in poor condition or with a rapidly proliferating tumour a simultaneous integrated boost technique was planned, 54 Gy (1.8 Gy/fraction) to the non-involved lymph node levels and 66 Gy (2.2 Gy/fraction) to the high-risk zone, in 30 fractions over 6 weeks.
The dose to the PRV of the spinal cord was limited to 50 Gy and a maximum of 59 Gy with 50% of the volume (D50) under 55 Gy was tolerated to the PRVs of the brain stem, the optical nerves and the optical chiasm. The dose to the other OAR was kept as low as possible, respecting the prescription to the PTV.
For unilateral neck irradiation we used 4 beams (340°–30°–100°–170° for the left side and 40°–330°–260°–190° for the right side), whereas for bilateral neck irradiation and paranasal sinus irradiation 5 (220°–290°–0°–70°–140°) or 7 (206°–258°–309°–0°–51°–102°–154°) equidistant beams were calculated.
For the overall treatment planning the number of segments varied from 50 to 80 in patients without cervical lymph node irradiation or with only unilateral neck irradiation, and from 80 to 115 segments in case of bilateral neck irradiation. The latter was reduced to 60–100 segments after dosimetric verifications on a humanoid phantom showing important dose differences above 100 segments [27].
IMRT treatment was delivered with 6 MV photons by an Electa SLi accelerator (Electa, Stockholm, Sweden) with electronic portal imaging device (EPID). Patients were treated on five consecutive days per week.
Quality assurance
We performed a patient-specific dosimetric verification on a humanoid phantom for the first 50 patients in addition to the standard machine-specific quality control [28,29]. Analysis of these phantom verifications showed high reliability of dose calculations and delivery. Based on these data the verifications on a humanoid phantom were continued randomly.
Verification of patient positioning was done by EPID for the first three fractions, after which the systematic error was calculated and corrected on the mask. Online corrections were made for errors of more than 3 mm in any direction. The electronic portal imaging (EPI) was performed daily until three consecutive days with random errors of less than 3 mm. Afterwards EPI was repeated once a week with the same evaluation criteria.
Concomitant treatment
Patients with an operable HNC received IMRT after surgery with or without concomitant chemotherapy depending on prognostic factors; patients with inoperable disease were often treated in a study protocol [10,30] with platinum-based induction chemotherapy followed by IMRT with or without concomitant chemotherapy (cisplatin, carboplatin or gemcitabine).
Follow-up and assessment
Patients were seen weekly during radiotherapy. Acute radiation toxicity was graded according to the RTOG radiation morbidity scoring criteria [31].
After the completion of their treatment, patients were seen every month during the first year, every 2 months in the second year and every 3–6 months thereafter. Late toxicity was always mentioned but not properly graded.
The first post-treatment CT scan was obtained 2–6 months after the completion of radiotherapy; thereafter, regular CT or MRI studies were obtained every 6 months or earlier on indication.
Statistics
Locoregional control (LRC) and survival rates (locoregional relapse-free survival (LRRFS) and overall survival (OS)) at 3 years have been calculated separately for those receiving IMRT as definitive treatment and those in the post-operative setting by using the Kaplan–Meier method [32]. The duration of LRC is defined as the time from the start of radiotherapy until the first documented progression or recurrence of locoregional disease or until death by any cause. LRRFS is calculated from the start of IMRT until the first documented locoregional recurrence or until death of any cause. OS is calculated from the start of IMRT until death of any cause.
All statistical analyses were performed with SPSS 15.0.0 for Windows (September 2006; SPSS Inc., Chicago, IL).
Results
Between November 2003 and June 2007, 1099 patients with HNC were irradiated at our department. Of these 78 patients were treated with IMRT for their primary disease. 63 of the 78 patients had squamous cell carcinoma (SCC), 9 had adenocarcinoma and 6 had an undifferentiated carcinoma. Primary tumour site included the oral cavity in 19 patients, the oropharynx in 21, the nasopharynx in 13, the larynx in 7, the hypopharynx in 3, the maxillary sinus in 4, the ethmoidal sinus in 1, unknown (carcinoma of unknown primary; CUP) in 2, the nose in 1, the external auditory canal in 1 and the salivary glands in 6. Further information on patient and tumour characteristics is given in Tables 1 and 2.
Table 1. Patient characteristics (n = 78).
| Age (years) | Median 60 (range 34–82) | |
| Gender | 54 male / 24 female | |
| Performance stage | 0 in 34, 1 in 43, unknown in 1 | |
| Primary tumour stage | I | 7 |
| II | 20 | |
| III | 16 | |
| IV | 34 | |
| Unknown | 1 | |
Table 2. Tumour and lymph node staging [24].
| T1 | T2 | T3 | T4 | Tx | All | |
| N0 | 7 | 20 | 9 | 8 | 0 | 44 |
| N1 | 2 | 3 | 2 | 2 | 0 | 9 |
| N2 | 5 | 4 | 4 | 3 | 1 | 17 |
| N3 | 0 | 3 | 0 | 1 | 2 | 6 |
| Nx | 0 | 0 | 0 | 1 | 1 | 2 |
| All | 14 | 30 | 15 | 15 | 4 | 78 |
Induction chemotherapy (all platinum based) was given to 20 patients and 35 patients received chemotherapy concurrently with IMRT (CRT). In those receiving CRT this was applied with cisplatin in 17 cases, with carboplatin in 12 cases and with gemcitabine in 6 cases. Further details on treatment and treatment delivery are given in Tables 3–6. 60 patients had bilateral neck irradiation, 13 had unilateral neck irradiation and in 5 patients the lymph nodes were not irradiated. Patients who were treated with definitive IMRT received a median dose to the PTV of 70 Gy (range 44–70 Gy), given in 35 fractions over 7 weeks. Five patients in this group were treated with a simultaneous integrated boost (54 Gy (1.8 Gy/fr.) to the non-involved lymph node areas and 66 Gy (2.2 Gy/fr.) to the high-risk zone in 30 fractions over 6 weeks). In the post-operative setting the median dose to the PTV was 66 Gy (range 18–70 Gy, the latter in case of macroscopic incomplete resection), given in fractions of 2 Gy. Details on radiotherapy doses in relation to tumour characteristics and treatment are given in Table 3.
Table 3. Treatment options and radiotherapy dose in relation to stage and primary tumour site.
| n | Primary site | St I | St II | St III | St IV | St X | n × radiation dose (Gy)/n of fractions (reason of protocol violation) |
| Definitive IMRT (48 patients) | |||||||
| 6 | Oral cavity | 1 | 3 | 2 | 6 × 70/35 | ||
| 15 | Oropharynx | 2 | 6 | 3 | 4 | 1 × 44/22 (toxic megacolon), 4 × 66/30, 2 × 66/33 (T1N0), 8 × 70/35 | |
| 12 | Nasopharynx | 1 | 1 | 10 | 1 × 62/31 (patient refusal), 1 × 66/33 (T1N0), 2 × 68/34, 8 × 70/35 | ||
| 3 | Hypopharynx | 3 | 1 × 66/30, 2 × 70/35 | ||||
| 6 | Larynx | 1 | 4 | 1 | 1 × 50/25 (prior supraclavicular RT), 3 × 66/33, 2 × 70/35 | ||
| 4 | Sinus | 1 | 3 | 1 × 50/25 (pre-operative), 1 × 60/30, 2 × 70/35 | |||
| 2 | 1 nose, 1 CUP | 1 | 1 | 2 × 70/35 | |||
| Post-operative IMRT (30 patients) | |||||||
| 13 | Oral cavity | 2 | 3 | 3 | 5 | 2 × 60/30, 11 × 66/33 | |
| 6 | Oropharynx | 2 | 2 | 2 | 1 × 60/30, 1 × 66/33, 1 × 68/34 (pos SM), 3 × 70/35 (pos SM) | ||
| 1 | Nasopharynx | 1 | 1 × 70/35 (after adenoidectomy) | ||||
| 1 | Larynx | 1 | 1 × 56/28 (prior RT for lymphoma) | ||||
| 1 | Sinus | 3 | 3 × 66/33 | ||||
| 6 | Salivary gland | 2 | 2 | 2 | 1 × 18/9 (intercurrent death), 3 × 66/33, 1 × 68/34 (pos SM), 1 × 70/35 (pos SM) | ||
| 2 | 1 CUP, 1 EAC | 1 | 1 | 1 × 60/30, 1 × 66/33 | |||
St, stage; CUP, carcinoma of unknown primary; EAC, external auditory canal; IMRT, intensity-modulated radiotherapy; pos SM, positive resection margins; RT, radiotherapy.
Table 6. Treatment options and outcome in relation to tumour stage for patients with a squamous cell carcinoma of oral cavity, oropharynx, hypopharynx and larynx.
| n | TRT option | St I | St II | St III | St IV | Recurrence (recurr)/progression (progr) | n death/cause |
| Definitive IMRT (30 patients) | |||||||
| 17 | RT | 4a | 9b | 4cde | 0 | 2 Tu recurrab, 1 LN recurrc | 1 LN recurrc, 1 postop complicationd |
| 8 | CRT | 0 | 3fgh | 1i | 4jkl | 1 Tu recurrf, 1 Tu and LN recurrg, 1 Tu progrj | 1 Tu progrj, 2 × 2nd primaryhk, 1 toxic deathl |
| 5 | ICT→CRT | 0 | 1m | 0 | 4no | 1 Tu recurrm, 2 Tu progrno | 1 Tu recurrm, 1 Tu progrn, 1 Tu progr & M+o |
| Post-operative IMRT (18 patients) | |||||||
| 13 | RT | 2 | 4 | 4p | 3q | 1 LN recurrp, 1 Tu & LN recurrq | |
| 5 | CRT | 0 | 1 | 0 | 4r | 1 Tu recurrr | 1 Tu recurrr |
TRT, treatment; postop, post-operative; CRT, concurrent chemotherapy and IMRT; ICT, induction chemotherapy; RT, radiotherapy; IMRT, intensity modulated radiotherapy; St, stage; M+, metastasis; d,e,f,i, salvage surgery; Tu, primary tumour; LN, lymph node.
The overall treatment time of the radiotherapy in all treatment categories is given in Table 4.
Table 4. Toxicities and overall treatment time in relation to different treatment approaches.
| Treatment |
Acute toxicity |
Late toxicity |
OTT of CRT |
|||||||||||||||
| Categories | No. | Skin |
Mucosa |
Xerostomia | Fibrosis of the neck | Loss of taste | Bone problems | Dysphagia | Teeth problems | Median (range) in days | ||||||||
| G0 | G1 | G2 | G3 | G4 | G0 | G1 | G2 | G3 | G4 | |||||||||
| RT | 18 | 3 | 8 | 6 | 1 | 0 | 0 | 0 | 1 | 17 | 0 | 7 | 3 | 3 | 1 | 1 | 0 | 49 (40–53) |
| CRT | 10 | 1 | 6 | 3 | 0 | 0 | 0 | 0 | 3 | 7 | 0 | 6 | 1 | 3 | 0 | 0 | 1 | 51 (30–54) |
| Surgery→RT | 23 | 1 | 13 | 5 | 4 | 0 | 0 | 0 | 4 | 19 | 0 | 10 | 2 | 1 | 2 | 0 | 0 | 46 (13–53) |
| Surgery→CRT | 7 | 0 | 5 | 2 | 0 | 0 | 0 | 0 | 2 | 5 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 46 (37–51) |
| ICT→RT | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 46 (46–46) |
| ICT→CRT | 18 | 2 | 10 | 6 | 0 | 0 | 0 | 0 | 3 | 14 | 1 | 9 | 1 | 4 | 0 | 1 | 1 | 50 (36–54) |
| All | 78 | 7 | 44 | 22 | 5 | 0 | 0 | 0 | 13 | 64 | 1 | 34 | 7 | 11 | 3 | 2 | 3 | 48 (13–54) |
IMRT, intensity-modulated radiotherapy; CRT, concurrent chemotherapy and IMRT; ICT, induction chemotherapy; OTT, overall treatment time; RT, radiotherapy.
Three patients discontinued IMRT, one owing to development of a toxic megacolon, one patient developed a fatal pulmonary infection and one patient refused treatment after 31 fractions (62 Gy) because of mucosal toxicity.
Acute and late toxicities according to treatment are given in Table 4. One patient still had a feeding tube 2 years after the end of radiation. This patient had been treated with definitive IMRT (without additional chemotherapy) for a T2N0M0 tonsil carcinoma.
Of the 48 patients who received definitive IMRT, 35 (73%) developed a complete response (CR), 8 (17%) a partial response (PR) and 4 a disease stabilisation (SD). One patient died during treatment from a toxic megacolon. Five patients (four with PR and one with SD) underwent salvage surgery and became free of disease.
After a median follow-up of 18.7 months (range 4 days to 51.7 months) 16 patients had died: 10 from tumour recurrence/progression, 1 from a toxic megacolon, 1 as result of a post-operative complication after salvage surgery, 3 from a second primary and 1 from a pulmonary infection. Details of treatment and outcome in relation to treatment setting and tumour stage are given in Table 5 and 6.
Table 5. Treatment options and outcome in relation to tumour stage for all patients.
| n | TRT option | St I | St II | St III | St IV | St X | Recurrence (recurr)/progression (progr) | n death/cause |
| Definitive IMRT (48 patients) | ||||||||
| 18 | RT | 4 | 9 | 4** | 0 | 1 | 2 Tu recurr, 1 LN recurr | 1 postop complic, 1 LN recurr, 2nd primary |
| 10 | CRT | 0 | 3* | 2** | 5 | 0 | 1 Tu recurr, 1 Tu & LN recurr, 1 Tu progr | 1 Tu progr, 2 × 2nd primary, 1 toxic death |
| 2 | ICT→RT | 1 | 0 | 0 | 1 | 0 | ||
| 18 | ICT→CRT | 0 | 1 | 1 | 16 | 0 | 1 Tu recurr, 2 LN recurr, 1 Tu & LN recurr, | 1 Tu recurr, 1 LN recurr & liver M+, |
| 4 Tu progr, 1 LN progr | 3 Tu progr, 1 Tu progr & skin/lung M+ | |||||||
| Post-operative IMRT (30 patients) | ||||||||
| 23 | RT | 2 | 6 | 8 | 7 | 0 | 1 Tu & LN recurr, 1 Tu progr | 1 Tu progr & lung M+, 1 intercurrent death |
| 7 | CRT | 0 | 1 | 1 | 5 | 0 | 1 Tu recurr | 1 Tu recurr |
TRT, treatment; postop, post-operative; CRT, concurrent chemotherapy and IMRT; ICT, induction chemotherapy; RT, radiotherapy; IMRT, intensity modulated radiotherapy; St, stage; M+, metastasis; Tu, primary tumour; LN, lymph node; complic, complication.
*Salvage surgery in 1 patient; **salvage surgery in 2 patient.
After 3 years the LRC was 66.1% (standard error (SE) 7.7%) in those receiving definitive IMRT (Figure 1), and 85.4% (SE 6.8%) in those who received IMRT in the post-operative setting (Figure 2). The 3 year LRRFS and OS were 48.5% (SE 9.6%) and 60.3% (SE 9.8%) in the definitive IMRT group; these figures were 82.5% (SE 7.1%) and 85.9% (SE 8.1%) for those treated in the post-operative setting, respectively (Figures 1 and 2).
Figure 1.
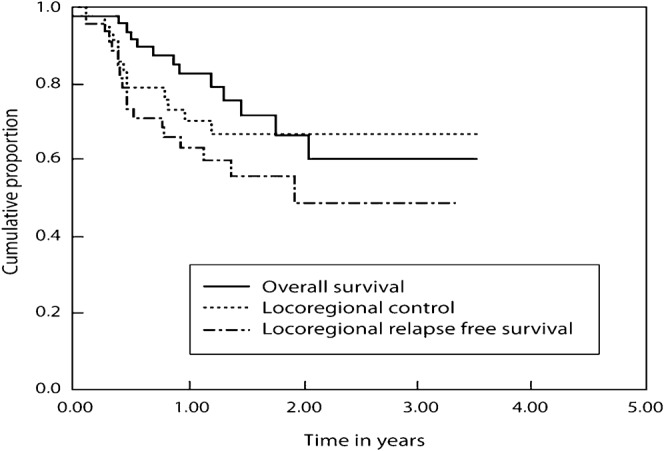
Treatment results in patients receiving intensity-modulated radiotherapy as a definitive non-surgical approach.
Figure 2.
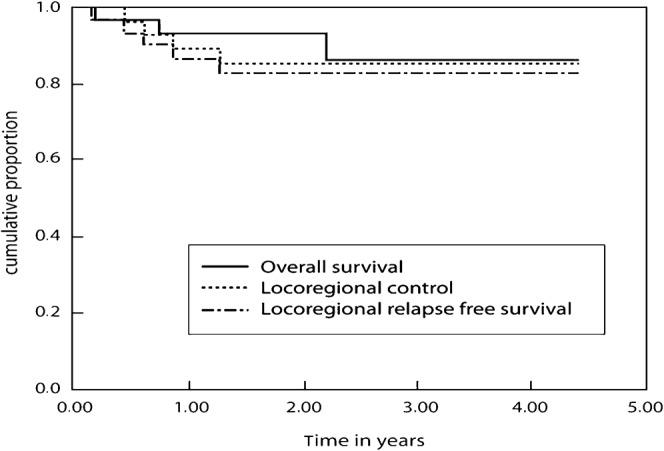
Treatment results in patients treated with intensity-modulated radiotherapy in the post-operative setting.
Further analysis of the patients in the definitive IMRT group showed for the 30 (62.5%) Stage 3/4 patients at 3 years an LRC of 64.7% (SE 9.9%), an LRRFS of 40.7% (SE 12.4%) and an OS of 45.3% (SE 12.8%).
A separate analysis was done for the patients (n = 48) who had squamous cell cancer (SCC) in the four disease sites: oral cavity (n = 19), oropharynx (n = 19), larynx (n = 7) and hypopharynx (n = 3). Overall, toxicity data in this SCC subgroup were quite similar to those observed in the total population. RTOG Grade 3 dermatitis occurred in 2 patients (4%), RTOG Grade 3 mucositis was observed in 41 patients (85%), but there was no Grade 4 toxicity. None of the patients in this subgroup discontinued IMRT as result of intolerance. Of the 30 patients in this subgroup who were treated with definitive IMRT, 21 (70%) developed a CR, 5 (17%) a PR and 2 an SD; the 3 year LRC was 66.8% (SE 10.0) and the 3 year LRRFS and OS were 42.6% (SE 14.3%) and 54.5% (SE 13.6%), respectively (Figure 3). For the 18 patients of this subgroup who were treated with IMRT in the post-operative setting, the 3 year LRC was 82.2% (SE 9.3%), the LRRFS was 82.2% (SE 9.3%) and the OS was 90.0% (SE 9.5%) (Figure 4).
Figure 3.
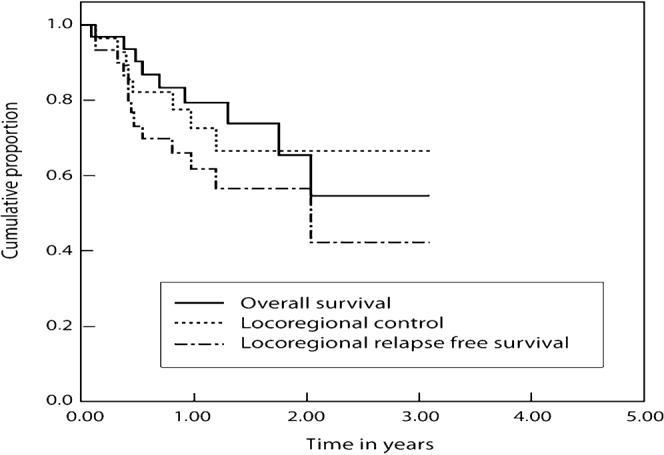
Treatment results with intensity-modulated radiotherapy in primarily non-operated patients with a squamous cell carcinoma of oral cavity, oropharynx, hypopharynx and larynx.
Figure 4.

Treatment results with intensity-modulated radiotherapy in patients with a squamous cell carcinoma of oral cavity, oropharynx, hypopharynx and larynx in the post-operative setting.
Discussion
In our retrospective audit, IMRT could be delivered without important interruptions, even when given with concurrent chemotherapy and/or after induction chemotherapy. The overall treatment time for the radiotherapy part, which has been reported to be a major prognostic factor [33,34], was maximally 54 days, although most patients could be irradiated within the foreseen timeframe (median 47 days; range 13–54).
The different treatment strategies within each stage group (Table 4) can be explained by the diversity of tumour sites and by the specific treatment protocols in the different referring hospitals.
We considered the acute toxicity acceptable: skin toxicity was mild with RTOG grade 3 dermatitis in only five patients (7%) and no grade 4; mucosal toxicity was more pronounced with an RTOG Grade 3 mucositis in 64 patients (82%) and only 1 RTOG Grade 4 toxicity. Only one (1.3%) patient refused to continue IMRT after 62 Gy of the planned 70 Gy owing to mucositis. Similar acute toxicity data were reported by Seung et al [35].
After a median follow-up of 18.7 months we observed xerostomia, although not properly graded, in 34 patients (44%), loss of taste in 11 patients (14%), fibrosis of the neck in 7 (9%), dysphagia in 2 patients (of whom 1 needed a permanent feeding tube) and bone and teeth problems in less than 5%.
16 patients (21%) had died, 10 (13%) as a result of treatment failure.
The LRC, LRRFS and OS at 3 years for the whole population in this retrospective audit are difficult to interpret because of the variable patient population, the different disease sites and the different treatment protocols. However, when we focus on the SCCs in the four disease sites, oral cavity, oropharynx, larynx and hypopharynx, then the results do not differ much from those observed in total patient cohort, i.e. LRC, LRRFS and OS were 66.8%, 42.6% and 54.5%, respectively, for those who received definitive IMRT, and 82.2%, 82.2% and 90.0%, respectively, for those receiving IMRT in the post-operative setting.
Our results are clearly inferior to those reported by several American groups [35-42] but are in line with those reported from institutions in Europe [43-45]. The causes of these differences are merely speculative, but most likely they are both patient and tumour related. In a subset analysis of the international Phase III trial in which patients were treated with radiotherapy alone or with radiation plus cetuximab, survival curves for those patients treated outside the USA were clearly inferior to those treated in the USA, with an absolute difference in 3 year survival of more than 30% [9,46]. Of interest in this analysis was the fact that only about 50% of the non-USA patients participating in the trial had a performance status more than 80%, while in the USA patients this percentage was much higher (±75–80%). It is well known that cross-trial comparisons are notoriously difficult. This may be even more so in case of trials performed in different continents.
Conclusion
We consider IMRT feasible and safe in our patient population not only as single modality for locoregional HNC but also after surgery, induction chemotherapy and when given concurrently with chemotherapy. Acute and late toxicity were considered acceptable.
Acknowledgments
The authors wish to thank the surgeons at the different centres: Paul Van de Heyning, Carl Van Laer and Jos Claes from the Antwerp University Hospital; Eric Fossion, Marc Dom, Bert Schmelzer, Lieve Vidts, Guy Cools and Sophie De Schepper from the Middelheim Hospital; Kristof Hendrickx, Gerry Orye and Herman Van De Vijver from the Saint Nikolaas Hospital. They also wish to thank Professor Dr Vincent Gregoire from the St-Luc University Hospital in Brussels for his support, ideas and critical remarks and Brenda Dom for the acquisition of data.
References
- 1.Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006;368:843–54 [DOI] [PubMed] [Google Scholar]
- 2.Budach W, Her T, Budach V, Belka C, Dietz K. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer 2006;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to loco-regional treatment for head and neck squamous cell carcinoma: three meta-analyses of update individual data. Lancet 2000;355:949–55 [PubMed] [Google Scholar]
- 4.Pignon JP, Le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14 [DOI] [PubMed] [Google Scholar]
- 5.Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E, Daly-Schveitzer N, et al. Combined post-operative radiotherapy and weekly cisplatin infusion for locally advanced head and neck cancer carcinoma. Final report of a randomized trial. Int J Radiat Oncol Biol Phys 1996;36:999–1004 [DOI] [PubMed] [Google Scholar]
- 6.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced Head and Neck Cancer. N Engl J Med 2004;350:1945–52 [DOI] [PubMed] [Google Scholar]
- 7.Cooper JS, Pajak TF, Forastiere A, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high risk squamous cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–44 [DOI] [PubMed] [Google Scholar]
- 8.Bernier J, Cooper YS. Chemoradiation after surgery for high risk head and neck cancer patients: how strong is the evidence? Oncologist 2005;10:215–24 [DOI] [PubMed] [Google Scholar]
- 9.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567–78 [DOI] [PubMed] [Google Scholar]
- 10.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 2007;357:1695–704 [DOI] [PubMed] [Google Scholar]
- 11.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007;357:21–31 [DOI] [PubMed] [Google Scholar]
- 12.Clark CH, Bidmead AM, Mubata CD, Harrington KJ, Nutting CM. Intensity-modulated radiotherapy improves target coverage, spinal cord sparing and allows dose escalation in patients with locally advanced cancer of the larynx. Radiother Oncol 2004;70:189–98 [DOI] [PubMed] [Google Scholar]
- 13.Grégoire V, De Neve W, Eisbruch A, Lee N, Van denWeyngaert D, Van Gestel D. Intensity-modulated radiation therapy for head and neck carcinoma. Oncologist 2007;12:555–64 [DOI] [PubMed] [Google Scholar]
- 14.Eisbruch A, ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose volume and function relationships in parotid glands following conformal and intensity modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999;45:577–87 [DOI] [PubMed] [Google Scholar]
- 15.Gregoire V, Maingon P. Intensity modulated radiation therapy in head and neck squamous cell carcinoma: state of art and future challenges. Cancer Radiother 2005;9:42–50 [DOI] [PubMed] [Google Scholar]
- 16.Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J Clin Oncol 2006;24:2618–23 [DOI] [PubMed] [Google Scholar]
- 17.Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 2007;25:4873–9 [DOI] [PubMed] [Google Scholar]
- 18.Nutting C, A'Hern R, Rogers MS, Sydenham MA, Adab F, Harrington K. First results of a phase III multicenter randomized controlled trial of intensity modulated (IMRT) versus conventional radiotherapy (RT) in head and neck cancer (PARSPORT: ISRCTN48243537; CRUK/03/005) (abstract LBA6006). J Clin Oncol 2009;27:18s [Google Scholar]
- 19.Corvo R. Evidence-based radiation oncology in head and neck squamous cell carcinoma. Radiother Oncol 2007;85:156–70 [DOI] [PubMed] [Google Scholar]
- 20.Van denSteen D, Hulstaert F, Camberlin C. Intensiteitsgemoduleerde radiotherapie (IMRT). Health Technology Assessment (HTA). KCE reports 62A (D2007/10.273.32). Brussels, Belgium: Federaal Kenniscentrum voor de gezondheidszorg (KCE), 2007 [Google Scholar]
- 21.Verellen D, Vanhavere F. Risk assessment of radiation-induced malignancies based on whole-body equivalent estimates for IMRT treatment in the head and neck region. Radiother Oncol 1999;53:199–203 [DOI] [PubMed] [Google Scholar]
- 22.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys 2006;65:1–7 [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal D, Chambers M, Fuller CD, Rebueno NC, Garcia J, Kies MS, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2008;72:747–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC cancer staging manual, 6th edn, New York, NY: Springer, 2002 [Google Scholar]
- 25.Gregoire V, Levendag P, Ang KK, Bernier J, Braaksma M, Budach V, et al. Selection and delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol 2003;69:227–36 [DOI] [PubMed] [Google Scholar]
- 26.International Commission on Radiation Units and Measurements ICRU Report, 62. Prescribing, recording and reporting photon beam therapy (Supplement to ICRU report 50) Bethesda, MD: ICRU Publications, 2000 [Google Scholar]
- 27.Schaeken B, De Ost B, Van Gestel D, Janssens H, Van denWeyngaert D. Verification of the integral delivered dose for IMRT treatments in the head & neck region with alanine/ESR dosimetry. Proceedings of the 216th PTB seminar Alanine Dosimetry for Clinical Applications, 8–9 May 2006, Braunschweig: PTB. Bericht PTB-DOS-51. [Google Scholar]
- 28.Venselaar J, Welleweerd H, Mijnheer B. Tolerances for the accuracy of photon beam dose calculations of treatment planning systems. Radiother Oncol 2001;60:191–201 [DOI] [PubMed] [Google Scholar]
- 29.Venselaar J, Welleweerd H. Application of a test package in an intercomparison of photon dose calculation performance of treatment planning systems used in clinical setting. Radiother Oncol 2001;60:203–13 [DOI] [PubMed] [Google Scholar]
- 30.Specenier PM, Weyler J, Van Laer C, Van denWeyngaert D, Van denBrande J, Huizing MT, et al. A non-randomized comparison of gemcitabine-based chemoradiation with or without induction chemotherapy for locally advanced squamous cell carcinoma of the head and neck. BMC Cancer 2009;9:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) [editorial]. Int J Radiat Oncol Biol Phys 1995;31:1341–6 [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81 [Google Scholar]
- 33.Fowler JF, Lindstrom MJ. Loss of local control with prolongation in radiotherapy. Int J Radiat Oncol Biol Phys 1992;23:457–67 [DOI] [PubMed] [Google Scholar]
- 34.Peters LJ, Withers HR. Applying radiobiological principles to combined modality treatment of head and neck cancer. The time factor. Int J Radiat Oncol Biol Phys 1997;13:403–26 [DOI] [PubMed] [Google Scholar]
- 35.Seung S, Bae J, Solhjem M, Bader S, Gannett D, Hansen EK, et al. Intensity-modulated radiotherapy for head-and-neck cancer in the community setting. Int J Radiat Oncol Biol Phys 2008;72:1075–81 [DOI] [PubMed] [Google Scholar]
- 36.Saba NF, Edelman S, Tighiouart M, Gaultney J, Davis LW, Khuri FR, et al. Concurrent chemotherapy with intensity-modulated radiation therapy for locally advanced squamous cell carcinoma of the larynx and oropharynx: a retrospective single-institution analysis. Head Neck 2009;31:1447–55 [DOI] [PubMed] [Google Scholar]
- 37.Traynor Am, Richards GM, Hartig GK, Khuntia D, Cleary JF, Wiederholt PA, et al. Comprehensive IMRT plus weekly cisplatin for advanced head and neck cancer: The University of Wisconsin experience. Head Neck 2010;32:599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenfeld GO, Amdur RJ, Morris CG, Li JG, Hinerman RW, Mendenhall WM. Patterns of failure and toxicity after intensity-modulated radiotherapy for head and neck cancer. Int J. Radiat Oncol Biol Phys 2008;71:377–85 [DOI] [PubMed] [Google Scholar]
- 39.Yao M, Dornfeld KJ, Buatti JM, Skwarchuk M, Tan H, Nguyen T, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma – the University of Iowa experience. Int J Radiat Oncol Biol Phys 2005;63:410–21 [DOI] [PubMed] [Google Scholar]
- 40.Klem ML, Mechalakos JG, Wolden Sl, Zelefsky MJ, Singh B, Kraus D, et al. Intensity-modulated radiotherapy for had and neck cancer of unknown primary: toxicity and preliminary efficacy. Int J Radiat Oncol Biol Phys 2008;70:1100–7 [DOI] [PubMed] [Google Scholar]
- 41.Lee NY, O'Meara W, Chan K, Della-Bianca C, Mechalakos JG, Zhung J, et al. Concurrent chemotherapy and intensity-modulated radiotherapy for locoregionally advanced laryngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys 2007;69:459–68 [DOI] [PubMed] [Google Scholar]
- 42.Chao KS, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patters of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. 2003;55:312–21 [DOI] [PubMed] [Google Scholar]
- 43.Richetti A, Cianchetti M, Fogliata A, Cozzi L, Ciernik I, Bernier J. Intensity modulated radiation therapy in patients with head and neck cancer: the experience of the oncology institute of southern Switzerland. Radiother Oncol 2007;82:S61–2 [Google Scholar]
- 44.Lambrecht M, Dirix P, Van denBogaert W, Nuyts S. Incidence of isolated regional recurrence after definitive (chemo-)radiotherapy for head and neck squamous cell carcinoma. Radiother Oncol 2009;93:498–502 [DOI] [PubMed] [Google Scholar]
- 45.Dirix P, Nuyts S. doi: 10.1016/j.ijrobp.2009.10.018. Value of intensity-modulated radiotherapy in stage IV head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys (in press) [DOI] [PubMed] [Google Scholar]
- 46.EMEA (Dataonfile) Erbitux-H-558-II-05. Available at: http://www.ema.europa.eu/docs/eu_GB/document_library/EPAR_-_scientific_discussion_-_variation/human/000558/wc500029116.pdf. [Google Scholar]


