Abstract
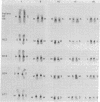
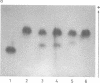
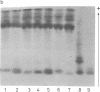
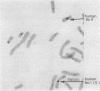
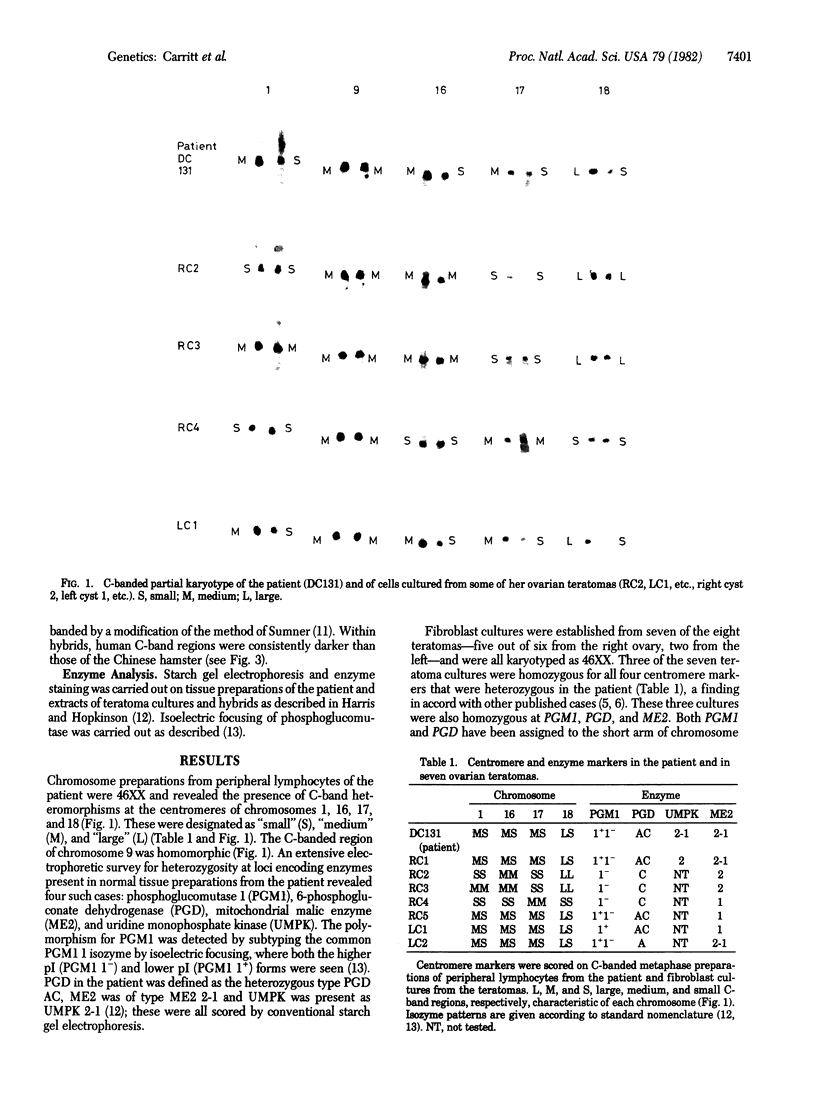
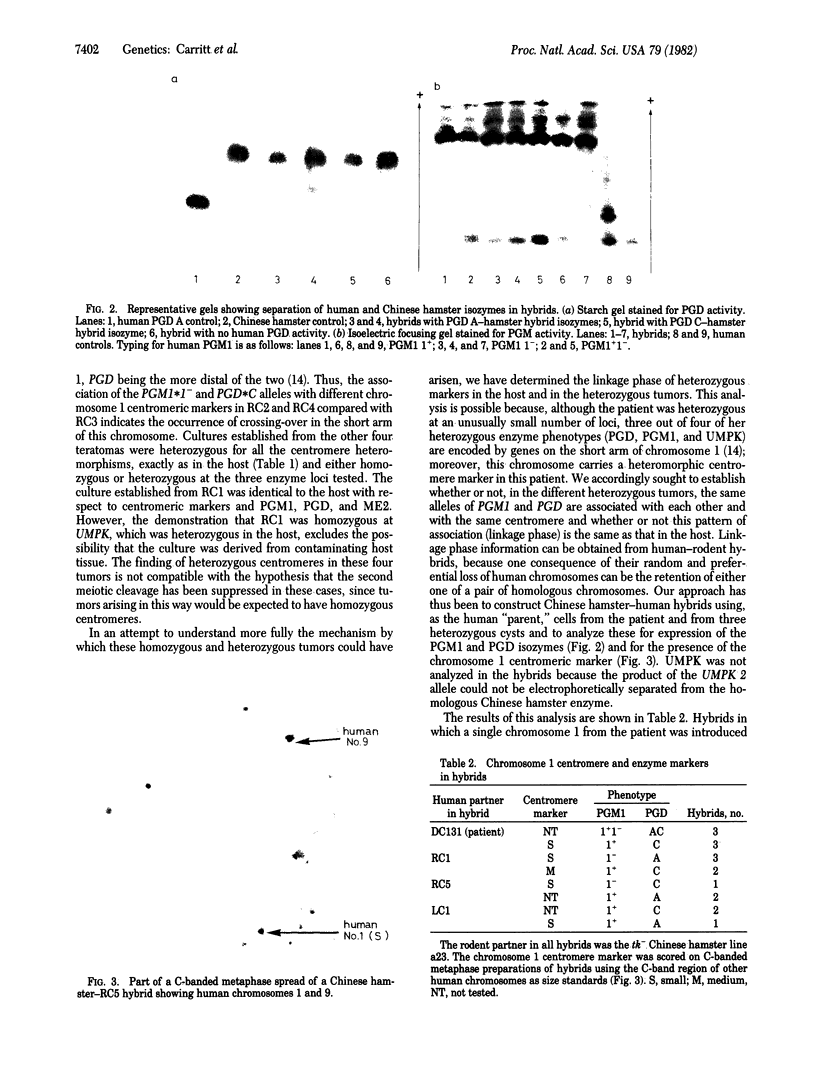
Centromere heteromorphisms and enzyme markers were examined in cells cultured from seven benign ovarian teratomas arising in a single patient. Three of the teratomas were homozygous for all eight enzyme and centromere markers found to be heterozygous in the host. The other four tumors were heterozygous at the centromeres of chromosomes 1, 16, 17, and 18, as in the patient, but were homozygous for at least one of the enzyme markers. The linkage phases of the heterozygous enzyme markers phosphogluconate dehydrogenase and phosphoglucomutase 1 and the chromosome 1 centromere heteromorphism were established for the patient and for three of the heterozygous teratomas by analysis of Chinese hamster-human somatic cell hybrids. The linkage phase of these markers in homozygous and heterozygous tumors was in every case different from that in the host. The finding of heterozygous centromeres in ovarian teratomas excludes suppression of meiosis II as a mechanism for their origin, and we suggest rather that they arise by failure of meiosis I. The linkage phases in the fully homozygous tumors are most readily derived from that in the patient, we suggest, by endoreduplication of a haploid gamete. The varied origin of ovarian teratomas has important implications for the suitability of such material for centromere-based gene mapping.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CORFMAN P. A., RICHART R. M. CHROMOSOME NUMBER AND MORPHOLOGY OF BENIGN OVARIAN CYSTIC TERATOMAS. N Engl J Med. 1964 Dec 10;271:1241–1244. doi: 10.1056/NEJM196412102712405. [DOI] [PubMed] [Google Scholar]
- Carritt B., King J., Welch H. M. Gene order and localization of enzyme loci on the short arm of chromosome 1. Ann Hum Genet. 1982 Oct;46(Pt 4):329–335. doi: 10.1111/j.1469-1809.1982.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Carritt B., Povey S. Regional asssignments of the loci AK3, ACONS, and ASS on human chromosome 9. Cytogenet Cell Genet. 1979;23(3):171–181. doi: 10.1159/000131323. [DOI] [PubMed] [Google Scholar]
- Carritt B. Somatic cell genetic evidence for the presence of a gene for citrullinemia on human chromosome 9. Cytogenet Cell Genet. 1977;19(1):44–48. doi: 10.1159/000130793. [DOI] [PubMed] [Google Scholar]
- Cook P. J., Hamerton J. L. Report of the committee on the genetic constitution of chromosome 1. Cytogenet Cell Genet. 1979;25(1-4):9–20. doi: 10.1159/000131394. [DOI] [PubMed] [Google Scholar]
- Hultén M. A., Palmer R. W., Laurie D. A. Chiasma derived genetic maps and recombination fractions: chromosome 1. Ann Hum Genet. 1982 May;46(Pt 2):167–175. doi: 10.1111/j.1469-1809.1982.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Hultén M. Chiasma distribution at diakinesis in the normal human male. Hereditas. 1974;76(1):55–78. doi: 10.1111/j.1601-5223.1974.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Linder D. Gene loss in human teratomas. Proc Natl Acad Sci U S A. 1969 Jul;63(3):699–704. doi: 10.1073/pnas.63.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder D., McCaw B. K., Hecht F. Parthenogenic origin of benign ovarian teratomas. N Engl J Med. 1975 Jan 9;292(2):63–66. doi: 10.1056/NEJM197501092920202. [DOI] [PubMed] [Google Scholar]
- Linder D., Power J. Further evidence for post-meiotic origin of teratomas in the human female. Ann Hum Genet. 1970 Jul;34(1):21–30. doi: 10.1111/j.1469-1809.1970.tb00216.x. [DOI] [PubMed] [Google Scholar]
- McCaw B. K., Hecht F., Linder D., Lovrien E. W., Wyandt H., Bacon D., Clark B., Lea N. Ovarian teratomas: cytologic data. Cytogenet Cell Genet. 1976;16(1-5):391–395. doi: 10.1159/000130640. [DOI] [PubMed] [Google Scholar]
- Ott J., Linder D., McCaw B. K., Lovrien E. W., Hecht F. Estimating distances from the centromere by means of benign ovarian teratomas in man. Ann Hum Genet. 1976 Nov;40(2):191–196. doi: 10.1111/j.1469-1809.1976.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Sumner A. T. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 1972 Nov;75(1):304–306. doi: 10.1016/0014-4827(72)90558-7. [DOI] [PubMed] [Google Scholar]
- Sutton J. G., Burgess R. Genetic evidence for four common alleles at the phosphoglucomutase-1 locus (PGM1) detectable by isoelectric focusing. Vox Sang. 1978;34(2):97–103. doi: 10.1111/j.1423-0410.1978.tb03730.x. [DOI] [PubMed] [Google Scholar]