Abstract
Nodular regenerative hyperplasia (NRH) is an uncommon liver disease characterised histologically by numerous small hyperplastic nodules that are not separated by fibrotic tissue. It is thought to be the result of obliterative vasculopathy, and it has been associated with chronic use of medications, toxic substances and a wide variety of systemic diseases. Imaging diagnosis of early-stage NRH remains problematic. The nodules are rarely discerned and their appearance and behaviour before and after contrast medium administration are heterogeneous and not specific. A review of the literature shows that ultrasound has succeeded on occasion in revealing small focal liver lesions in patients with NRH. To our knowledge, there has been no published data on the performance in this setting of last-generation ultrasound scanners and techniques such as contrast-enhanced ultrasound (CEUS). The question is an important one because abdominal ultrasound is widely used as a first-line imaging technique for the evaluation of liver disease, and this makes it particularly suitable as a potential tool for the early diagnosis of NRH. Owing to the prolonged subclinical period and the limited help provided by imaging, the diagnosis in vivo of NRH is currently frequently missed, and it is still made exclusively on the basis of liver biopsy. In conclusion, this report describes 4 cases of biopsy-proven NRH that have been diagnosed over the past 2 years by our group. All were characterised by known comorbidities that confer a predisposition to NRH and by a peculiar parenchymal ultrasound pattern that we refer to as the “atoll sign”.
Nodular regenerative hyperplasia (NRH) is an uncommon liver disease characterised histologically by numerous small (usually 1–3 mm) hyperplastic nodules that are not separated by fibrotic tissue [1]. It is thought to be the result of obliterative vasculopathy, and it has been associated with chronic use of certain medications, toxic substances and a wide variety of systemic diseases that disturb hepatic blood flow, particularly myeloproliferative and immunological disorders. NRH rarely affects protein synthesis in the liver, so most patients remain asymptomatic with normal liver function for years. For this reason, as late as 1990 virtually all reported cases of NRH had been diagnosed on autopsy [1]. However, nodular compression of the sinusoids can cause portal hypertension with upper gastrointestinal bleeding, generally related to oesophageal varices.
Imaging diagnosis of early-stage NRH remains problematic. The nodules are usually quite small, and none of the major imaging techniques, including ultrasound, CT scanning, MRI and scintigraphy, reveal significant changes in the appearance of the liver, other than enlargement and/or parenchymal inhomogeneity. The nodules themselves are rarely discerned and, on such occasions, their appearance and behaviour before and after contrast medium administration are heterogeneous and not specific. Moreover, there is also the possibility that previous reports [2,3] had grouped together patients with NRH and large regenerative nodules, so that the rare nodules evidenced in those series by CT and MRI could only be the latter. Large regenerative nodules are larger (diameter ranging from 5 to 50 mm) and usually enhance after contrast medium administration [4]. A review of the literature shows that ultrasound has succeeded on occasions in revealing small focal liver lesions in patients with NRH. The appearance of these lesions varied from hypoechoic [5,6] to isoechoic [2] or hyperechoic [2,5,7]. However, these reports date back to the 1980s and 1990s, when ultrasound technology was far less refined than it is today. To our knowledge, there are no published data on the performance in this setting of last-generation ultrasound scanners and techniques like contrast-enhanced ultrasound (CEUS). The question is an important one because abdominal ultrasound is widely used as a first-line imaging technique for the evaluation of liver disease (and a variety of other conditions as well), and this makes it particularly suitable as a potential tool for the early diagnosis of NRH, particularly in predisposed patient groups. In fact, owing to the prolonged subclinical period and to the limited help given from imaging, the diagnosis in vivo of NRH is currently frequently missed, and it is still made exclusively on the basis of liver biopsy [8].
This report describes four cases of biopsy-proven NRH that have been diagnosed over the past 2 years by our group. All were characterised by known comorbidities that confer a predisposition to NRH and by a peculiar parenchymal ultrasound pattern that we refer to as the “atoll sign”.
All ultrasound examinations were performed with an Aloka Prosound SSD 5500 ePHD (extended pure harmonic detection) scanner (Aloka, Tokyo, Japan) and multifrequency convex array transducers (3.0–6.0 MHz).
All biopsy were performed with a cutting technique using a 21-gauge needle (Biomol, Hospital Service, Milan, Italy).
Case report 1
A 36-year-old male presented with intense epigastric pain. He had a 5 year history of type 2 diabetes, which he treated on an irregular basis with a biguanide/sulfonylurea combination. 4 years before admission, he had undergone open cholecystectomy for gallstones. Admission laboratory tests revealed markedly elevated serum lipase (2346 U l−1; normal values, 23–300) and amylase levels (668 U l−1; normal values, 30–110), a γ-glutamyl-transpeptidase level of 101 Mu ml−1 (normal values, 8–78), mildly elevated transaminase levels and hyperglycaemia (256 g l−1). The white blood cell count was 13 000 mm−3. Total bilirubin, alkaline phosphatase levels and other routine blood chemistry parameters were within normal limits.
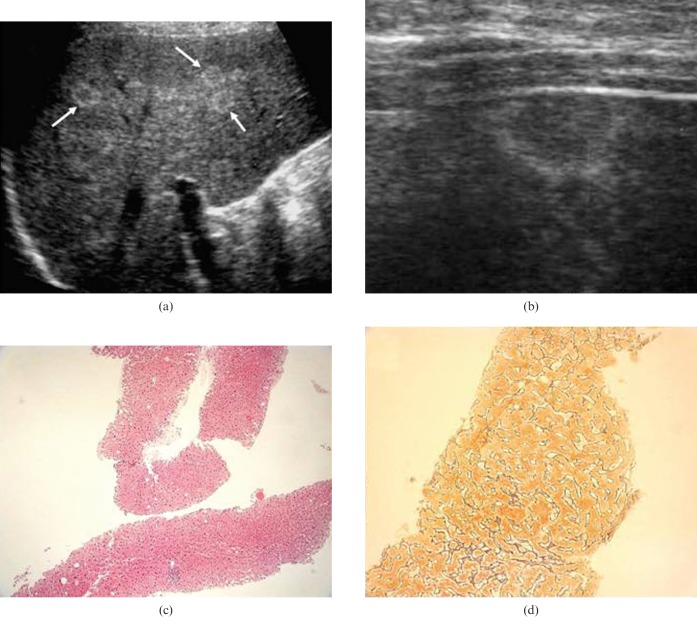
An upper abdominal ultrasound scan revealed multiple round focal lesions, up to 11 mm in diameter, scattered throughout both lobes. All appeared isoechoic with thin hyperechoic rims and no mass effect (Figure 1a,b). The intrahepatic biliary tree and the common bile duct were not dilated, and the pancreas was normal in size. No fluid collections were observed. CEUS and contrast-enhanced CT scans showed no signs of focal liver lesions in the vascular phases. The CT scan confirmed the normal appearance of the biliary tree and pancreas.
Figure 1.
Case 1, (a) upper abdominal ultrasound scan of the right hepatic lobe performed with a 3.5 MHz convex probe. The scan revealed an atoll-like pattern consisting of clusters of small (11 mm) round isoechoic focal lesions with thin hyperechoic rims (arrows). (b) Upper abdominal ultrasound scan of the left hepatic lobe (performed with a 7.5 MHz linear probe). Detail of one of the focal lesions, which produces no “mass effect” at the liver surface. (c) Histological appearance of one of the focal lesions in the right lobe (ultrasound-guided fine-needle biopsy). Regenerative hyperplastic hepatocytes are arranged in plates more than one cell thick that in turn form micronodules. Some of hepatocytes display nuclear hypertrophy (HE 4×). (d) With reticulin staining, the regenerative hyperplasic nodules and their compression of surrounding tissues are easily visualised (20×). Note the absence of fibrosis between the nodules.
The patient was diagnosed with acute pancreatitis, probably owing to the passage of sludge through the common bile duct. His condition improved rapidly, and 5 days after admission an ultrasound-guided fine-needle biopsy was obtained from an area of the liver characterised by confluent nodules. The histological diagnosis was NRH (Figure 1c,d), and the long-standing presence of uncontrolled diabetes was thought to have functioned as a predisposing factor in the development of the disease.
Case report 2
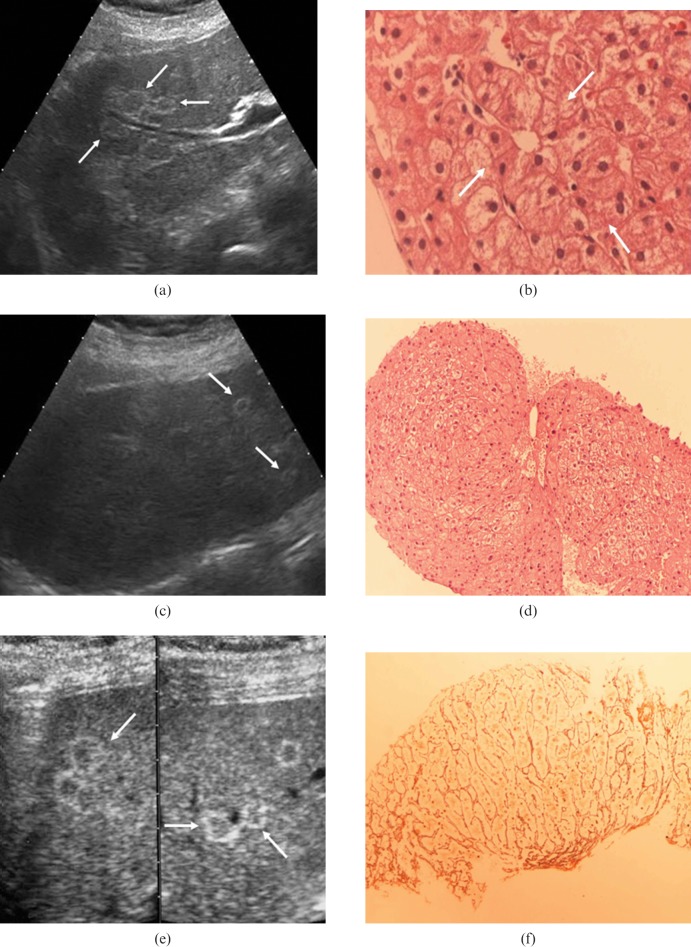
A 74-year-old female had a 12 year history of essential thrombocythaemia. She was taking hydroxyurea and allopurinol orally and her platelet counts ranged from 400 000 to 520 000 mm−3. The complete blood count was otherwise normal, as were all routine blood chemistry parameters (including those reflecting liver function). An annual abdominal ultrasound scan (performed as part of the disease surveillance protocol) revealed that both liver lobes contained multiple isoechoic lesions with thin hyperechoic rims and a maximum diameter of around 12 mm (Figure 2a). No focal lesions were seen on the CEUS scan. Our experience with the previous case prompted us to obtain an ultrasound-guided fine-needle biopsy, which confirmed our suspicion of NRH (Figure 2b).
Figure 2.
(a) Case 2, upper abdominal ultrasound scan on the right hepatic lobe (using a 3.5 MHz convex probe). Multiple lesions, isoechoic with a thin hyperechoic rim, are visible (arrows), the larger diameter of such lesions is 12 mm. (b) Case 2, histological sample from ultrasound-guided fine-needle biopsy on one of the focal lesions of the right liver lobe. Regenerative hyperplastic hepatocytes are arranged in micronodules with microacinar appearance (arrows) (HE stain, 40×). (c) Case 3, upper abdominal ultrasound scan on the right hepatic lobe (using a 3.5 MHz convex probe). Some round isoechoic lesions with a thin hyperechoic rim, with a larger diameter of 10 mm, are present (arrows). (d) Case 3, histological sample from ultrasound-guided fine-needle biopsy on one of the focal lesions of the right liver lobe. The nodular arrangement of multilayered regenerative hyperplasic hepatocytes is evident. Some of them show hypertrophic nuclei (HE 40×). (e) Case 4, upper abdominal ultrasound scan on the right hepatic lobe (3.5 MHz convex probe). Rare round focal lesions, isoechoic with a hyperechoic rim, with a larger diameter of 13 mm, are visible (arrows). (f) Case 4, reticulin staining clearly reveals both the nodular arrangement of hepatocytes and the absence of fibrosis between the nodules (40×).
Case report 3
A 65-year-old female with coeliac disease was being treated with a gluten-free diet. Abdominal ultrasound was performed to investigate the presence of thrombocytopenia (platelet count: 74 000 mm−3). Apart from this, all liver function tests were within the normal range. The ultrasound scan revealed massive splenomegaly and multiple round lesions in the liver. The lesions measured 10 mm in diameter and were isoechoic with thin hyperechoic rims (Figure 2c). CEUS showed no evidence of focal liver lesions. An ultrasound-guided fine-needle biopsy diagnosed NRH (Figure 2d).
Case report 4
A 12-year-old female presented with an episode of upper gastrointestinal bleeding. 9 years earlier she had been treated with vincristine for a nephroblastoma. Apart from thrombocytopenia (platelet count: 85 000 mm−3) and a mild increase in the serum alkaline phosphatase levels, liver function tests were within normal limits. An abdominal ultrasound scan showed round focal lesions (13 mm in diameter) in both liver lobes. The lesions appeared isoechoic with thin hyperechoic rims (Figure 2e), and they were associated with portal vasodilation and splenomegaly. A contrast-enhanced abdominal CT scan revealed signs of portal hypertension but no evidence of focal liver lesions. The diagnosis of NRH was made once again on the basis of ultrasound-guided fine-needle biopsy findings (Figure 2f).
Discussion
The term “nodular regenerative hyperplasia” was coined by Steiner in 1959 [9], although the condition it refers to was first described in 1953 by Ramstrom [10], who reported what he called “miliary hepatocellular adenomatosis” in a patient with Felty's syndrome. Similar histopathological findings were later reported by other authors under a wide variety of names, including “liver adenomatosis” [5], “multiple adenomas” [11], “nodular transformation” [12], “non-cirrhotic nodular transformation” [13], “partial nodular transformation” [14], “non-cirrhotic nodulation” [15], and “non-cirrhotic portal hypertension” [16]. Finally, in 1995 the decision was made to use the term NRH to refer to all of these conditions [17,18]. In a histopathological study of liver biopsies and autopsy specimens obtained from 107 patients with non-cirrhotic portal hypertension, 14% were diagnosed as NRH, 23% as “incomplete septal cirrhosis,” 2% as “partial nodular transformation,” and the remaining 62% as “idiopathic portal hypertension.” The authors noted that the pathological features of these four conditions are similar and suggested that they may represent different stages of a single disorder [19].
As its various names suggest, the pathological hallmark of NRH is the presence of small adenoma-like nodules composed of hyperplastic hepatocytes. They usually range in diameter from 1 to 3 mm. Nodules measuring over 15 mm have also been described, but they appear to be composed of smaller nodules [1,18,20]. The hepatocytes within the nodules are arranged in plates more than one cell thick, a clue that is best revealed by reticulin staining. Fibrosis is minimal or absent between one nodule and another, and this element distinguishes NRH from cirrhosis. Sometimes this condition may be very difficult to distinguish from adenomas as both are composed of hyperplastic hepatocytes but for the fact that NRH is a multifocal process and adenomas tend to be solitary or rarely several lesions.
NRH is considered an uncommon disease. Its prevalence in general autopsy series ranges from 0.6% to 2.6% [1,20], but higher figures have been reported in some patients. In fact, NRH has been increasingly linked to haematological diseases, including myeloproliferative disorders (essential thrombocythaemia, polycythaemia vera, chronic myelogenous leukaemia and myeloid metaplasia) [20-23], lymphoproliferative diseases (multiple myeloma, non-Hodgkin's lymphoma, chronic lymphocytic leukaemia and Hodgkin's disease) [21], spherocytosis [21] and sickle cell disease [23]. It has also been seen in patients with immunological diseases, such as Felty's syndrome [24], rheumatoid vasculitis [25], polyarteritis nodosa [26] and hypogammaglobulinaemia [27], and with a variety of other conditions (neoplastic disease [28], diabetes [29], coeliac disease [30] and renal or bone marrow transplantation [31]). Finally, it has been associated with the use of diverse drugs (chemotherapy agents [23,32], azathioprine [33] and antiretroviral agents [34]) and with exposure to toxic agents.
The most widely accepted view is that NRH is the result of obliterative vascular disease. Depending on the nature of the underlying systemic illness, the primary endothelial damage can affect the portal radicles or branches of the hepatic artery. In the latter case, portal vessel occlusion is caused by the extension of the damage from an adjacent arterial branch. In either case, the portal occlusion produces acinar atrophy, and this loss is thought to lead to a compensatory regenerative-hyperplastic response in nearby areas that have been unaffected by the portal vessel obliteration [20,35].
The disease is characterised by a long subclinical course. It is often diagnosed only after long-standing portal hypertension has produced dramatic complications (e.g. bleeding oesophageal varices). Portal hypertension occurs in up to 50% of cases [21], so that NRH represents a major cause of non-cirrhotic portal hypertension in the western world.
The fact that NRH occurs mostly in patients affected with predisposing disease should facilitate early diagnosis, but thus far no reliable method has been developed for this purpose. CT and MRI are not suitable in this setting [2-4], and they are also fairly expensive. Ultrasound has the advantages of being widely available, relatively easy to perform and less costly than other imaging methods. In the mid-1990s [7], our group described two cases of NRH presenting with a fairly non-specific ultrasound pattern consisting of multiple confluent hyperechoic liver lesions. Later-generation scanners and transducers allow higher-resolution imaging, and this has undoubtedly improved our ability to identify NRH lesions. The peculiar finding of small, round isoechoic lesions with a thin hyperechoic rim found in the first patient of our series resembles the ring-shaped coral-reef configuration known as an atoll (Figure 3). It was later encountered in three other patients and immediately raised the suspicion of NRH. All three were suffering from conditions known to be associated with NRH, and the diagnosis was subsequently confirmed by liver biopsy. The ultrasound diagnosis thus meant we avoided additional imaging studies.
Figure 3.

The focal lesions of nodular regenerative hyperplasia resemble the ring-shaped coral island known as an atoll (aerial view of Ari atoll, Maldives).
Regenerative-hyperplasic nodules are usually smaller than 3 mm, but diameters of up to 15 mm have been reported. The lesions identified on ultrasound in our patients appear to represent only the larger nodules. It is important to emphasise that neither CEUS nor contrast-enhanced CT proved useful in detecting the focal lesions of NRH in the four cases described above. This is consistent with the experience of other authors [4], and it probably reflects not only the small size of the nodules, but also their arterial and portal vascularisation patterns, which are similar to those of the normal liver parenchyma.
Although intranodular haemorrhage is more typical of adenomas, such an event has been occasionally described in NRH [2,3,21]. Imaging features of intralesional haemorrhage consist, on CT, of hyperdense areas within hypodense nodules, while, on MRI, foci of high signal on T1 may be present internally to isointense nodules.
Conclusion
The presence on ultrasound of the atoll-like pattern described in this report should raise the suspicion of NRH if the patient has one of the diseases known to be associated with this condition.
Acknowledgments
The authors wish to thank Ms Maria Antonietta Gabriele for her invaluable technical assistance and Ms Marian Everett Kent for her editorial assistance.
References
- 1.Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology 1990;11:787–97 [DOI] [PubMed] [Google Scholar]
- 2.Dachman AH, Ros PR, Goodman ZD, Olmsted WW, Ishak KG. Nodular regenerative hyperplasia of the liver: clinical and radiological observations. AJR 1987;148:717–22 [DOI] [PubMed] [Google Scholar]
- 3.Siegelman ES, Outwater EK, Furth EE, Rubin R. MR imaging of hepatic nodular regenerative hyperplasia. J Magn Reson Imaging 1995;5:730–2 [DOI] [PubMed] [Google Scholar]
- 4.Ames JT, Federle MP, Chopra K. Distinguishing clinical and imaging features of nodular regenerative hyperplasia and large regenerative nodules of the liver. Clin Radiol 2009;64:1190–5 [DOI] [PubMed] [Google Scholar]
- 5.Flejou JF, Barge J, Menu Y, Degott C, Bismuth H, Potet F, et al. Liver adenomatosis. An entity distinct form liver adenoma? Gastroenterology 1985;89:1132–8 [PubMed] [Google Scholar]
- 6.Pelletier G, Roche A, Boccaccio F, Patriarche C, Ink O, Fabre M, et al. The imaging of nodular regenerative hyperplasia of the liver. Study of nine cases. Gastroenterol Clin Biol 1988;12:687–90 [PubMed] [Google Scholar]
- 7.Grattagliano A, Rapaccini GL, Caturelli E, Vecchio FM, Carughi S, Gomes V, et al. Nodular regenerative hyperplasia of the liver: ultrasonographic appearance and echo-guided bioptic diagnosis. Ital J Gastroenterol 1994;26:349–53 [PubMed] [Google Scholar]
- 8.Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology 2006:7–14 [DOI] [PubMed] [Google Scholar]
- 9.Steiner PE. Nodular regenerative hyperplasia of the liver. Am J Pathol 1959;35:943–53 [PMC free article] [PubMed] [Google Scholar]
- 10.Ranstrom S. Miliary hepatocellular adenomatosis. Acta Path Microbiol Scand 1953;33:225–9 [PubMed] [Google Scholar]
- 11.Brophy CM, Bock JF, West AB, McKhann CF. Liver cell adenoma: diagnosis and treatment of a rare hepatic neoplastic process. Am J Gastroenterol 1989;84:429–32 [PubMed] [Google Scholar]
- 12.Lui AFK, Hiratzka LF, Hirose FM. Multiple adenomas of the liver. Cancer 1980;45:1001–4 [DOI] [PubMed] [Google Scholar]
- 13.Connoly CE, O'Brien MJ. Nodular transformation of the liver: report of a case. Human Pathol 1977;8:350–2 [DOI] [PubMed] [Google Scholar]
- 14.Shedlofsky S, Koehler RE, DeSchryver-Kecskemeti K, Alpers DH. Noncirrhotic nodular transformation of the liver with portal hypertension: clinical, angiographic and pathological correlation. Gastroenterology 1980;79:938–43 [PubMed] [Google Scholar]
- 15.Classen M, Elster K, Pesch HJ, Demling L. Portal hypertension caused by partial nodular transformation of the liver. Gut 1970;11:245–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JC. Noncirrhotic nodulation of the liver. Arch Pathol Lab Med 1978;102:398–401 [PubMed] [Google Scholar]
- 17.Reisman T, Levi JU, Zeppa R, Clark R, Morton R, Schiff ER. Non cirrhotic portal hypertension in Felty's syndrome. Am J Dig Dis 1977;22:145–8 [DOI] [PubMed] [Google Scholar]
- 18.Terminology of nodular hepatocellular lesions International Working Party. Hepatology 1995;22:983–93 [DOI] [PubMed] [Google Scholar]
- 19.Nakanuma Y, Hoso M, Sasaki M, Terada T, Katayanagi K, Nonomura A, et al. Histopathology of the liver in non-cirrhotic portal hypertension of unknown aetiology. Histopathology 1996;28:195–204 [DOI] [PubMed] [Google Scholar]
- 20.Wanless IR, Godwin TA, Allen F, Feder A. Nodular regenerative hyperplasia of the liver in hematologic disorders: a possible response to obliterative portal venopathy. A morphometric study of nine cases with an hypothesis on the pathogenesis. Medicine 1980;59:367–9 [PubMed] [Google Scholar]
- 21.Stromeyer FW, Ishak KG. Nodular transformation (nodular “regenerative” hyperplasia) of the liver. Human Pathol 1981;12:60–71 [DOI] [PubMed] [Google Scholar]
- 22.Rosen A, Iseri O, Fishbein G, Knodell R. Nodular regenerative hyperplasia of the liver: a cause of ascites and hepatomegaly after chemotherapy for leukemia. Am J Gastroenterol 1991;86:86–8 [PubMed] [Google Scholar]
- 23.Al-Mukhaizeem KA, Rosenberg A, Sherker AH. Nodular regenerative hyperplasia of the liver: an under-recognized cause of portal hypertension in hematological disorders. Am J Hematol 2004;75:225–30 [DOI] [PubMed] [Google Scholar]
- 24.Choen M, Ginsburg W, Allen G. Nodular regenerative hyperplasia of the liver and bleeding oesophageal varices in Felty's syndrome: a clinicopathologic case report and literature review. J Rheumatol 1982;8:716–18 [PubMed] [Google Scholar]
- 25.Reynolds WJ, Wanless IR. Nodular regenerative hyperplasia of the liver in a patient with rheumatoid vasculitis: a morphometric study suggesting a role for hepatic arteritis in the pathogenesis. J Rheumathol 1984;11:838–42 [PubMed] [Google Scholar]
- 26.Goritsas CP, Repanti M, Papadaki E, Lazarou N, Andonopoulos AP. Intrahepatic bile duct injury and nodular regenerative hyperplasia of the liver in a patient with polyarteritis nodosa. J Hepatol 1997;26:727–30 [DOI] [PubMed] [Google Scholar]
- 27.Malamut G, Ziol M, Suarez F, Beaugrand M, Viallard JF, Lascaux AS, et al. Nodular regenerative hyperplasia: the main liver disease in patients with primary hypogammaglobulinemia and hepatic abnormalities. J Hepatol 2008;48:74–82 [DOI] [PubMed] [Google Scholar]
- 28.Al-Hamoudi WK, Pasieka JL, Urbanski SJ, Lee SS. Hepatic nodular regenerative hyperplasia in a patient with advanced carcinoid tumour. Eur J Gastroenterol Hepatol 2009;21:1083–5 [DOI] [PubMed] [Google Scholar]
- 29.Thung SN, Gerber MA, Bodenheimer HC. Nodular regenerative hyperplasia of the liver in a patient with diabetes mellitus. Cancer 1982;49:543–6 [DOI] [PubMed] [Google Scholar]
- 30.Austin A, Campbell E, Lane P, Elias E. Nodular regenerative hyperplasia of the liver and celiac disease: potential role of IgA anticardiolipin antibody. Gut 2004;53:1032–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snover DC, Wieisdorf S, Bloomer J, McGlave P, Weisdorf D. Nodular regenerative hyperplasia of the liver following bone marrow transplantation. Hepatology 1989;9:443–8 [DOI] [PubMed] [Google Scholar]
- 32.Hubert C, Sempoux C, Horsmans Y, Rahier J, Humblet Y, Machiels JP, et al. Nodular regenerative hyperplasia: a deleterious consequence of chemotherapy for colorectal liver metastases? Liver Int 2007;27:938–43 [DOI] [PubMed] [Google Scholar]
- 33.Breen DP, Marinaki AM, Arenas M, Hayes PC. Pharmacogenetic association with adverse drug reactions to azathioprine immunosuppressive therapy following liver transplantation. Liver Transpl 2005;11:826–33 [DOI] [PubMed] [Google Scholar]
- 34.Saifee S, Joelson D, Braude J, Shrestha R, Johnson M, Sellers M, et al. Noncirrhotic portal hypertension in patients with human immunodeficiency virus-1 infection. Clin Gastroenterol Hepatol 2008;6:1167–9 [DOI] [PubMed] [Google Scholar]
- 35.Kondo F. Benign nodular hepatocellular lesions caused by abnormal hepatic circulation: etiological analysis and introduction of a new concept. J Gastroenterol Hepatol 2001;16:1319–28 [DOI] [PubMed] [Google Scholar]