Abstract
Objective
Assessment of the potential doses to the hands and eyes for interventional radiologists and cardiologists can be difficult. A review of studies of doses to interventional operators reported in the literature has been undertaken.
Methods
Distributions for staff dose to relevant parts of the body per unit dose–area product and for doses per procedure in cardiology have been analysed and mean, median and quartile values derived. The possibility of using these data to provide guidance for estimation of likely dose levels is considered.
Results
Dose indicator values that could be used to predict orders of magnitude of doses to the eye, thyroid and hands from interventional operator workloads have been derived, based on the third quartile values, from the distributions of dose results analysed.
Conclusion
Dose estimates made in this way could be employed in risk assessments when reviewing protection and monitoring requirements. Data on the protection provided by different shielding and technique factors have also been reviewed to provide information for risk assessments. Recommendations on the positions in which dosemeters are worn should also be included in risk assessments, as dose measurements from suboptimal dosemeter use can be misleading.
Interventional radiologists and cardiologists have the highest exposure to radiation of any staff working with medical X-ray techniques [1,2]. The continual improvements in technology and methodology enable more challenging clinical problems to be tackled, so the numbers of procedures being performed continues to rise [3]. Because of the potential for doses received by interventional operators to be high, it is important that they are monitored effectively. Doses to the trunk are recorded routinely, but it is often not easy to decide when it is appropriate to monitor other parts of the body that are more difficult to protect and have their own dose limits [2]. The tissues that may be exposed to higher doses are the head, particularly the eyes and thyroid, the hands and the legs. The eyes have their own dose limit of 150 mSv for a classified worker, and lens opacities have been reported among interventional radiology operators that are thought to be due to X-ray exposure [4,5]. The thyroid is known to be radiosensitive and makes a significant contribution to the effective dose (E) if it is not shielded [6-9]. The skin has a dose limit of 500 mSv, which is applied to the dose averaged over 1 cm2. Exposure of the hands is a matter of concern because of the need for the operator to be close to the X-ray field to carry out manipulations, and the possibility of higher doses from poor practice if the hands are exposed to the primary beam. The skin dose limit also applies to the legs and these need to be considered as they are usually closer to the region of scatter from the unattenuated X-ray beam with undercouch X-ray tubes [10].
Analysis of results from extremity monitoring for medical applications reported by seven European countries has shown that hand doses from routine monitoring are lower than those reported in dedicated studies published in the literature [11]. Several factors probably contribute to this difference. The most exposed workers may not be monitored, dosemeters may not be worn on the most exposed part of the hand and use of dosemeters may be erratic. A more systematic, evidence-based approach to dose monitoring would be beneficial in trying to alleviate these problems. It is impractical to monitor doses to all sites for every interventional operator. Practices should be reviewed in risk assessments to determine appropriate dosimetry arrangements, but before any dose measurements are carried out, the information available on potential doses is limited. Measurements of the distribution of air kerma made with ionisation chambers during simulated interventional procedures can be useful for establishing where dose rates are higher [2,8,10,12-15] and are helpful in assessing where staff should stand, but the information they provide relating to doses to different parts of the body, especially the hands, is limited. Many studies of the doses received by interventional operators have been carried out and information gained from these could be used in evaluating likely dose levels for application in risk assessments to establish the dose monitoring that should be undertaken.
Methods and materials
A review has been undertaken of studies reported in the literature for doses to interventional operators in radiology and cardiology applications over the last 20 years [16]. A literature search was carried out using the NHS Greater Glasgow and Clyde Literature Search Service based on MEDLINE and EMBASE in March 2009 for the original study, and this was followed up by a further search in January 2010. Indexes listing all papers published in the journals British Journal of Radiology, Radiation Protection Dosimetry and Health Physics over the period were examined, and all relevant papers in these journals reviewed. Additional papers referenced elsewhere were also obtained for review. Data on dose levels per procedure for the eyes, thyroid and extremities, and associated patient dose–area product (DAP) data have been collated. A requirement was set that dose levels for interventional operators were based on at least 20 procedures. The distributions of mean dose values from each study have been analysed.
Sufficient data were available for interventional cardiology to assess operator doses per procedure for the eye, thyroid and extremities. There would be significant variations between procedures, but most of these studies include a mix of the cardiology procedures performed routinely in each hospital. Where studies have reported results for diagnostic coronary angiography (CA) and percutaneous coronary interventions (PCIs), both data sets have been included in the data pool. For interventional radiology the amounts of radiation used vary substantially between different types of procedure, and individual radiologists specialise in different procedures, so an approach based on dose per procedure has little value.
Mean values for relationships between doses to the eyes, thyroid and extremities and the patient dose-related measurement DAP have been analysed for the interventional radiology and cardiology studies where this has been measured. Values that were quoted in the papers have been used where these were available. Where data on staff doses have been plotted against DAP or results quoted separately, ratios have been calculated from the values given. Doses to the hands have been measured with dosemeters worn on finger rings or a wrist band. For the legs the dosemeter positions, where specified, were on the foot or the anterior part of the lower leg. The reported values were used directly in the collation, with no adjustment for position, so these differences contribute to the uncertainty. Results have also been collated for the ratio of the dose to the eye and the thyroid in studies where both have been recorded. Where results for several groups of staff have been reported, because they used different techniques or equipment or were obtained in different hospitals, these have been included separately. Where results for individual radiologists or cardiologists in one department have been reported, the mean values for all the individuals have been used in order to avoid results being heavily weighted by a single department. Thyroid doses for two studies were excluded from the thyroid dose data set. In these reports the thyroid doses were less than half those in any other studies, and the ratios of the eye to thyroid doses were about two, compared with less than one for other centres, suggesting inappropriate placement of the thyroid dosemeters.
In the analysis of data per unit DAP, results are presented for all interventional procedures combined to allow a larger data set to be collated. This quantity allows adjustment for the amount of radiation used, which is one of the most significant factors determining staff doses, but does not take account of variations relating to the technique and position taken up by the operator. In two cases data sets were split, because there were obvious differences resulting from a single factor. Hand doses for percutaneous procedures, for which the operators’ hands were adjacent to the primary beam, and leg doses where no shield was used were both substantially higher than other doses in the same data set and results for these procedures were therefore analysed separately. Where sufficient cardiology data were available, results for dose per unit DAP were also derived. Mean, median and quartile values and ranges have been derived for the distributions. References used in the analysis for each aspect are listed in Table 1 [8,10,17-51]. Possible approaches that might be used to give indicative doses to the eyes and hands are considered.
Table 1. Studies used for interventional cardiology doses and doses per unit dose–area product (DAP).
| Tissue | Cardiology doses per procedure | Interventional doses per unit DAP |
| Eye | 8 (2), 17, 18 (3), 19 (2), 20 (2), 21 (3), 22 (2), 23 (2), 24, 25, 26, 27 (2), 28 | 18, 23 (5), 25, 26, 29 |
| Thyroid | 17, 20 (2), 22 (2), 23 (2), 25, 28, 30, 31 (4), 32 (2), 33 (2) | 23 (5), 30, 31 (4), 32 (2), 33 (2), 34 (3), 35 (3) |
| Hand: non-percutaneous procedures | 17, 19, 20, 22 (2), 24, 25, 27 (2), 28, 33 (2), 37, 38, 39 (2), 40, 41 (2), 42 | 22, 23 (5), 24, 25, 33 (2), 34 (3), 35, 36, 39 (2), 41 (5), 43, 44 |
| Hand percutaneous | 29, 35, 41, 44, 45 | |
| Leg: screen | 25, 27, 42, 46 (8), 47 (5), 48 (3) | 10, 24, 25, 42, 43, 47 (4), 48 (3) |
| Leg : no screen | 10, 23(5), 45, 46(2), 47 | |
| Ratio eye dose/thyroid dose | 17, 28, 20(2), 22(3), 23(5), 45(2), 49, 50, 51 |
Where several groups have been assessed in one study, separate results have been included for each and the number of groups taken from each reference is given in brackets. The groups may relate to ones using different protection practices or ones from different hospitals. References 47 and 48 contain data sets from hospitals in a number of different countries.
Studies of the protection provided by shielding devices have also been reviewed. These results have been derived in different ways. In some studies, a direct comparison of the doses received by the same staff groups using different techniques has been possible. Examples include dose measurements with and without leg protection made before and after a lead curtain was installed [52] and measurements of hand doses from cardiology procedures performed with radial and femoral access routes, where choice of technique was determined by a clinical decision [41]. Other results were derived from comparisons between different groups for whom shields were or were not available, or who employed different techniques. Other studies have been based on dose measurements made using anatomical phantoms to simulate exposure conditions during clinical procedures. The results of these investigations together with the methodology used are reported to give an indication of the influence of different factors prior to consideration of the dose values.
Results
Protection measures and dose levels
Data for distributions of mean doses per procedure for different parts of the body from the cardiology studies are given in Table 2. The ratios of maximum and minimum mean doses reported are between 60 and 100. There are many factors that contribute to variations in the amounts of radiation used which are difficult to quantify for the separate studies. These include the complexities of the procedures, the dose options on the X-ray equipment used, the size of the patient [53] and the technique and skill of the operator, but these will not be considered further here. Results from studies that have assessed the degrees of protection afforded by various factors are summarised in Table 3. Radiology and cardiology staff members working in fluoroscopy rooms wear wrap-around lead/rubber aprons to protect the trunk from scattered radiation. A separate thyroid collar incorporating 0.35 mm or 0.5 mm of lead is usually worn by the operator. Monte-Carlo simulation has indicated that collars of 0.5 mm or 0.35 mm lead equivalent worn in an ideal position have the potential to provide protection factors for the thyroid of 12 or 7, respectively [54], although practical simulations with surface-mounted thermoluminescent dosemeters (TLDs) for collars worn in positions more representative of actual practice suggest that the dose reduction is less (Table 3), but thyroid collars still represent an important element of the protection. Differences arise because the collars are worn more loosely for reasons of comfort. The doses to other parts of the body not protected by lead coats or collars depend on how close they are to the X-ray tube and patient, and the use made of local shielding. The degree of protection in practice depends on the availability of protective devices and the willingness of operators to use them (Table 3) [5,15,16,21,37,41,52,54-62].
Table 2. Data on the distributions of operator doses in microsieverts per procedure from cardiology studies reviewed.
| Organ | No. of data sets | Min. | First quartile | Median | Mean | Third quartile | Max. |
| Eye | 23 | 5 | 7.5 | 34 | 66 | 89 | 439 |
| Thyroid | 18 | 3.8 | 32 | 53 | 88 | 98 | 392 |
| Hand | 20 | 8 | 27 | 136 | 175 | 338 | 514 |
| Leg (protective screen) | 19 | 5 | 14 | 25 | 32 | 41 | 96 |
Table 3. Degrees of protection offered by different factors.
| Organ | Protection factor | Dose reduction factor | Study methodology | Reference |
| Head and thyroid | Face shield normal usage | 10 | Practical simulation | 55 |
| 14 | Practical simulation | 15 | ||
| Mobile floor shield | 4 | Measurements on staff | 21 | |
| Eyes | Lead glass spectacles | 30 | Practical simulation | 5 |
| Thyroid | Thyroid collar | 5–6 | Monte Carlo simulation | 54 |
| 4–6 | Practical simulation | 56 | ||
| 2–3 | Measurements on staff | 37 | ||
| Hands | Hand position | 3 | Practical simulation | 57 |
| Access route | 6 | Measurements on staff | 41 | |
| Protective glove | 1.1–1.8 | Practical simulation | 58–61 | |
| Legs | Lead/rubber skirt | 19 | Measurements on staff | 62 |
| 14 | Comparison of studies | Present | ||
| 16–20 | Practical simulation | 15 | ||
| 10 | Practical simulation | 55 | ||
| Ankle | Lead/rubber skirt | 3 | Measurements on staff | 52 |
The positions in which interventional operators stand relative to the X-ray beam are largely determined by the procedures performed. Cardiologists carrying out CA and PCI procedures need to stand closer to the area being imaged when introducing catheters via the radial artery route than when they use a femoral access route. As a result, operators will tend to receive higher doses for radial access procedures [21,41]. However, the radial route may have advantages for patient management that outweigh the higher dose to the operator. In interventional radiology, femoral access is used for the majority of procedures, but for percutaneous procedures such as biliary stent or drainage, the operator will need to stand closer to the region being imaged, so the scatter dose will be proportionately higher than for other procedures [41]. All these factors contribute to the variations in dose between different studies.
Monitoring doses to the body
The standard method used for monitoring doses to staff working with X-rays in the UK is to wear a single dosemeter under the lead/rubber apron to measure Hp(10), which is the individual equivalent dose for penetrating radiation assessed at a depth of 10 mm. This provides a measure of the exposure of sensitive organs in the trunk, which is equated to E, but does not take into account exposure of unprotected parts of the body, especially the head. The International Commission on Radiological Protection (ICRP) has recommended that a policy of wearing two dosemeters should be adopted for highly exposed staff undertaking interventional radiology procedures, but this is not a regulatory requirement and the practice is limited [2]. One dosemeter would normally be worn at chest height under the apron, and the other at the level of the neck outside the apron. Many different algorithms have been developed combining the doses recorded to provide an assessment of E [9,61,63-67]. The method developed by Niklason et al [64] is widely used and has different options depending upon whether or not a thyroid collar is worn.
Without a thyroid collar
where Hu is Hp(10) on the trunk under the apron and Hos is Hp(0.07) at the neck outside any protection worn,
and with a thyroid collar
Doses to the body per interventional procedure are generally between 0.2 μSv and 10 μSv per procedure for undercouch X-ray tube configurations [21,30-32,45,48], although doses as high as 19 μSv per procedure have been reported [22]. If an individual performs 500 procedures in a year, this would result in an annual dose between 0.1 and 2 mSv. From the limited data available, the quartile values for the dose per unit DAP were 0.1 and 0.6 μSv Gy−1 cm−2 [21,30-32,48,54,68,69].
Doses to the eye and thyroid
Studies suggest that the most important factors determining eye dose are the X-ray equipment and the use of eye shields (Table 3) [5,18,22,55], but other factors such as tube angulation, operator position and beam collimation also affect eye doses [5]. The wearing of lead glass spectacles or masks will provide effective protection, but there are issues of comfort and practicality, and the use of ceiling suspended shields is recommended as, if used properly, they will protect the whole head and neck from scatter. When employed optimally they should enable high-dose procedures to be performed without the annual accumulated eye doses approaching three-tenths of the dose limit. Data on the distribution of eye and thyroid doses from interventional cardiology studies are given in Table 2. A rule of thumb proposed for predicting eye dose levels for cardiac catheterisation procedures is 5 mSv per 100 procedures (50 μSv per procedure) [18]. The median eye dose from all the interventional cardiology and radiology studies reviewed was 34 μSv per procedure and the third quartile value was 89 μSv. The results reflect a skewed distribution with a number of relatively high values. The range of eye doses in interventional radiology is large and a mean dose of 403±300 μSv per procedure has been reported for complex transjugular intrahepatic portosystemic shunt (TIPS) procedures [49]. One factor contributing to the large range may be the positioning of the dosemeter. A recent study using sets of 10 TLDs positioned in a line across the foreheads of cardiologists showed that doses to the eyebrow ridge on the side nearest to the X-ray tube and intensifier were 3–5 times greater than those in the middle of the head [27]. Thus, dosemeters for the monitoring of eye doses should be positioned on the side of the brow ridge adjacent to the X-ray tube immediately above the eye, in order to give an indication of the maximum potential eye dose.
It is not always convenient to monitor eye doses, but the dose from an unprotected dosemeter at the neck can be used as an indicator for the dose to the eyes (Table 2). The equivalent dose to the crystalline lens of the eye should ideally be assessed at a depth of 3 mm using Hp(3), but the quantity more commonly recorded by personal dosemeters is Hp(0.07) and for diagnostic X-rays this should be within 10% of the actual value; results reported here are based on Hp(0.07). For a person standing with their body 0.5 m from the patient and image intensifier, the distances to the thyroid and the forehead would be about 0.7 m and 0.8 m, respectively, giving a ratio of 0.76 from a simple inverse square law. Analysis of data from studies in which both eye and thyroid doses have been recorded showed that results for the eye dose were usually between 0.4 and 0.9 of the thyroid dose, derived from Hp(0.07) measured outside the lead apron at the level of the neck (Table 4). In two studies reviewed, the measured thyroid dose was about half that of the eye dose [25,43], possibly owing to unusual placement of the thyroid dosemeter (e.g. on the side away from the X-ray tube), so these two studies were excluded from the analysis of the eye/thyroid dose ratio. Simulations using a phantom have shown that the tube orientation has a significant influence [50]. Monte-Carlo simulations suggest that the eye dose is about 0.75 times the dose at the neck [66]. Following a collation of the evidence available, the equation
Table 4. Data on the distributions for ratio of eye and thyroid dose, and for ratios of operator doses to dose–area product (DAP) (μGy Gy−1 cm−2) from studies reviewed.
| No. of data sets | Min. | First quartile | Median | Mean | Third quartile | Max. | |
| Dose ratio: eye/thyroid | 17 | 0.24 | 0.38 | 0.54 | 0.63 | 0.86 | 1.25 |
| Tissue dose/DAP μGy Gy−1 cm−2 All interventional procedures | |||||||
| Eye | 9 | 0.29 | 0.32 | 0.43 | 0.79 | 1.0 | 1.9 |
| Thyroid | 20 | 0.28 | 0.71 | 0.99 | 1.38 | 1.7 | 4.3 |
| Hand (percutaneous procedures) | 5 | 11 | 16 | 32 | 27 | 34 | 60 |
| Hand (non-percutaneous procedures femoral and internal jugular vein access) | 24 | 0.43 | 1.3 | 2.4 | 2.8 | 4.3 | 6.7 |
| Leg (no screen) | 8 | 2.6 | 5.0 | 6.2 | 6.7 | 9.0 | 10.6 |
| Leg (protective screen) | 14 | 0.21 | 0.28 | 0.45 | 0.85 | 1.6 | 2.3 |
| Cardiology procedures | |||||||
| Thyroid | 11 | 0.28 | 0.6 | 0.8 | 0.9 | 1.2 | 1.8 |
| Hand (femoral and radial access) | 10 | 0.43 | 1.1 | 1.4 | 2.5 | 2.9 | 9.0 |
| Leg (protective screen) | 10 | 0.21 | 0.26 | 0.34 | 0.65 | 1.2 | 1.9 |
is recommended for estimating the eye dose from a measurement with an unshielded neck dosemeter.
Links between doses to the eyes or the thyroid and the DAP have been investigated in a number of studies and data on the distributions are given in Table 4. In the two studies in which the ratio of the eye to the thyroid dose was low, the ratio of thyroid dose to DAP was also exceptionally low [16,25,43] and results from these studies were again excluded. Where a neck dosemeter is used, this should again be worn on the side adjacent to the X-ray tube.
Hand doses
The position of the operator has a significant influence on the magnitude of the dose to the hands and contributes to the range of doses from different studies (Table 2). It will sometimes be appropriate to monitor doses to the hands to assess whether good practice is being followed. As the hands of the interventionalist are often relatively close to the edge of the X-ray field, the dose will not decline as a simple inverse square law relationship, but when the hands are further from the beam they may be partly shielded by the patient’s body. Protection of the hands provided by screens is often limited, as the hands are below the level of ceiling-suspended screens, and although freestanding adjustable overtable screens can provide good protection, interventional operators often find them too intrusive, as space is limited, and they raise additional problems of sterility. Protective gloves are available, but are comparatively expensive and the protection offered is limited (Table 3) [58-61].
For a right-handed interventional operator the left hand is usually closer to the image receptor and X-ray tube, as the left hand maintains the position of the catheter while the right hand carries out the manipulations. Doses to the right hand were generally about half of those to the left and only results for the most exposed hand have been analysed. Doses for percutaneous interventional radiology procedures such as biliary drainage are highest because the hands need to be close to the area under investigation in order to manipulate catheters inserted directly into the liver [35,41,44]. This causes the range of hand dose per unit DAP to be larger than for the eye and thyroid and results for percutaneous and non-percutaneous procedures per unit DAP have therefore been analysed separately (Table 4). In procedures such as TIPS that are carried out via the internal jugular vein (IJV), doses to the hands may be high because the procedures are technically challenging and screening times are long. Mean doses per procedure to the hand reported in studies of TIPS procedures are between 500 and 600 μSv per procedure [41,49], but the doses per unit DAP for TIPS are similar to those for interventional procedures via the femoral access route, such as angioplasties and stents [41]. Ratios for percutaneous procedures for which the hand is positioned adjacent to the X-ray field are in the range 10–60 μSv Gy−1 cm−2, whereas ratios for procedures using the femoral and IJV access routes were in the range 0.4–7 μSv Gy−1 cm−2 [16]. The ratio of hand dose over DAP for a study of PCIs using a radial approach was 9 μSv Gy−1 cm−2 [41] and this has not been included in the combined data in Table 4. Results for doses per unit DAP for the hands, thyroid and legs for cardiology procedures (femoral and radial access) were 25–30% lower than those obtained when all the interventional procedure data were combined (Table 4). When only results for femoral access cardiology procedures were considered, then the hand doses were 43% lower than the combined result. Since radial access may be used and leads to a higher hand dose for some cardiology procedures, a combined approach to evaluating doses for cardiology procedures is considered to be justified.
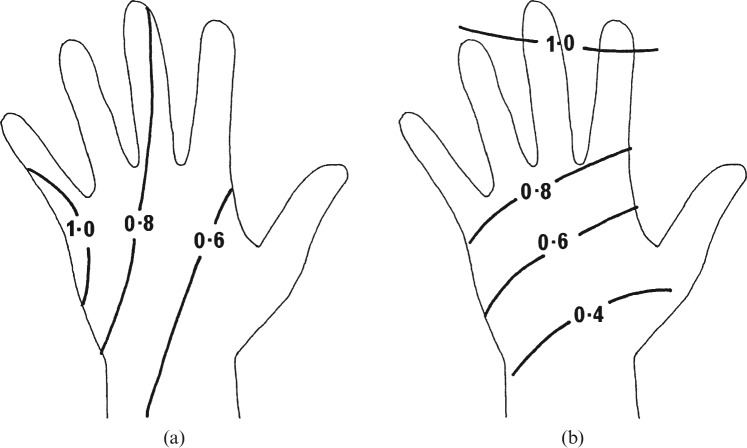
The ICRP recommendations and UK national legislation require an assessment to be made of the dose to the most exposed area of skin and that the dose limit is applied to the dose averaged over an area of 1 cm2 [2,70,71]. The magnitude of the dose to the most highly exposed part of the hand is determined primarily by the distance from the X-ray beam, but the part of the hand receiving the highest dose is affected by the orientation of the hands relative to the scatter field. Most procedures involve several different hand positions, with one where the hand is side-on to the X-ray beam often being predominant as a twisting action is used to manipulate the catheter. The side of the hand receives the highest exposure when the catheter is being twisted [41]. A sketch of the dose distribution that might be expected across the hand for procedures using the femoral access approach is shown in Figure 1a. Since the side of the hand receives a higher dose, it is more appropriate to wear the dosemeter on either the little finger or the ring finger. A dosemeter worn on the index finger may underestimate the maximum dose to an area of skin on the hand by 10–30%, and one on the wrist may underestimate the dose by a factor of three. Comparison of results from routine wrist and finger monitoring in individual countries suggests that the doses recorded by wrist monitors are between two and seven times lower [11]. Two sets of results from one cardiology study using radial access were derived from wrist monitors [33]. The reported values for the ratio hand dose per unit DAP were in the range of 1.3–1.9 μGy Gy−1 cm−2 for PCI and CA, so ratios relating to the maximum skin dose from this study are likely to be in the range of 4–6 μGy Gy−1 cm−2. For percutaneous procedures, the hand movement is predominantly a prodding action combined with pushing and retracting the catheter, and the tips of the index and middle fingers may receive slightly higher doses than the base (Figure 1b). However, these procedures would seldom make up the majority of a radiologist’s workload, so a ring dosemeter worn on the little finger of the hand nearest to the X-ray tube is likely to be the most appropriate method for monitoring the hands of all interventional operators.
Figure 1.
Diagrammatic representations of the distributions of dose across the left hand based on data from thermoluminescent dosemeter studies for (a) interventional procedures using femoral access and (b) percutaneous procedures relative to the dose recorded at the ring position on the little finger taken from [16]. In both cases the left hand is assumed to be closest to the X-ray tube and image intensifier. Reproduced with permission from Oxford University Press.
There are logistical difficulties in ensuring that hand dosemeters are sterile and put on at the correct time during the preparation for a procedure, as well as in achieving the compliance of staff in wearing them. Ring dosemeters that are watertight may be sterilised. Ginjaume et al [44] reported that the variation in dose results caused by the sterilisation process for dosemeters consisting of a TLD chip in a ring-shaped PVC holder was less than 3%, but this is not ideal. If interventionalists scrub their hands before the procedure with the dosemeter in place and put surgical gloves over the dosemeter, sterility should not be an issue in minimally invasive surgery. However, scrubbing up with a dosemeter already in place may not be consistent with some local procedures for surgical cleanliness.
Leg doses
Most interventional procedures are carried out on undercouch units. Since the primary beam is scattered downwards from the base of the couch, the legs can receive significant doses. Doses to the legs for a radiologist or cardiologist using a femoral access route can be several times those to the hands and could approach the dose limit, if there is no lead/rubber shield to offer protection [10]. Lead/rubber drapes should be attached to the side of the couch to provide the operator with protection and should be included in specifications for interventional units. The better type of screen is one that is integral with the X-ray couch. For units on which procedures such as TIPS are performed, where the operator is positioned near the head, the need to provide protection near the head end of the couch must be considered. The doses to the legs should not present a protection issue if appropriate shields are in place (Table 2). Data on doses to all parts of the leg have been included in the pool for Table 2, but those in the upper half of the distribution were all measured near the foot. Where doses to different parts of the leg have been measured, the doses to the ankle and foot are double those to the knee [10,27], as the couch may on occasions be raised too high for the drapes to fully protect the feet.
When there is no protection for the legs, the doses are strongly related to the DAP [10] (Table 4). There are substantial variations in dose per unit DAP where there is a screen in place, with a factor of six between the first and third quartile values, which is double that for other tissues (Table 4). This is likely to result from the position on the leg where the dose is measured. Lead/rubber drapes attached to the side of the couch provide effective protection for the majority of the leg, but may not protect the feet and ankles all of the time, especially for tall operators. Comparisons of the median values for the distributions of leg dose per unit DAP with and without a shield suggest that shields reduce leg doses by factor of 14. However, measurements made just above the ankle on the same group of radiologists before and after lead drapes were installed only showed a reduction by a factor of three [52], and this probably reflects the level of protection afforded to the lower leg.
Discussion
The number of interventional procedures is steadily increasing and it is important that appropriate protection measures are in place for interventional operators and that their doses are monitored to check whether protection devices are deployed effectively. There are significant variations in the doses recorded in different studies owing primarily to differences in the X-ray equipment and the techniques used, but also to practices in implementation of protection measures. Doses to the eyes depend on the availability and use of ceiling suspended and other shields. Doses to the hands are influenced by the proximity to the X-ray beam, which varies with the type of procedure, and the legs can receive significant doses if protective drapes attached to the X-ray couch are not available. Information on the degree of protection offered by different techniques is given in Table 3.
The question of what dose monitoring is appropriate for an interventional facility is not straightforward. A dosemeter should be worn to record body dose, but decisions on whether a second dosemeter should be worn at the neck and whether the hands should be monitored are left to the judgement of the employer with advice from the local radiation protection expert and the operator. Ensuring compliance with dose-monitoring requirements can be difficult. Overuse of dosemeters can result in erratic use. The wearing of dosemeters at incorrect positions can give doses several times lower than the dose to the area of maximum exposure. Both these approaches will give reassuringly low doses, but could hide possible high exposure levels. The recommended strategy is therefore to prepare risk assessments with the aim of identifying staff members who need to be monitored and then put effort into having systems in place to ensure that the monitoring is done effectively. The first requirement for the risk assessment is to decide on the dose levels at which monitoring should be carried out. These should be linked to the dose limits and the likelihood of dose levels reaching three-tenths of those limits when worker classification is required. They should also take account of the variability in the doses received, such as whether single high exposures could occur from occasional complex or difficult cases. Suggested monitoring arrangements for different parts of the body linked to dose levels, together with protection requirements, have been developed from earlier proposals [16,72] and are set out in Table 5.
Table 5. Proposed dose levels for guidance on implementation of protection and dose monitoring.
| Tissue | Annual dose (mSv) | Monthly dose (mSv) | Protection/dose monitoring |
| Eyesa | 10–20 | 1–2 | Initial monitoring to establish dose levels |
| Eyesa | 20–36 | 2–3 | Regular monitoring should be considered |
| Eyesa | >36 (45)b | >3 | Regular monitoring is required |
| Thyroid | >7 | >0.6 | Thyroid collar recommended |
| Hands | 30–60 | 2.5–5 | Initial monitoring to establish dose levels |
| Hands | 60–120 | 5–10 | Regular monitoring should be considered |
| Hands | >120 (150)b | >10 | Regular monitoring is required |
| Legs | >50 | >4 | Lead/rubber shield is recommended |
aMonitoring may be through second dosemeter on collar.
bThree-tenths of dose limit in brackets.
However, data on staff dose levels will not be available unless monitoring has already been performed. Indicative dose levels could be derived from studies undertaken in hospitals around the world, but the substantial variations in the amounts of radiation involved in different procedures must be recognised. The adoption of indicators linked to patient dose is perhaps the best that can be achieved. The DAP provides an ideal patient dose quantity to use for this, since it gives a measure of the total radiation emitted during a procedure and so is linked closely to the amount of scatter to which operators will be exposed [73,74]. Data on the distribution of operator doses per unit DAP for different parts of the body are set out in Table 4. The mean, median, third quartile or maximum values could all provide possible options for predicting operator doses. If the mean or median values of the distributions were used, then 50% would have higher doses and some doses could be significantly greater. Alternatively, use of the maximum dose would ensure that the worst case was being addressed, but would be unrealistically conservative and would rely on the validity of the result from a single study. The third quartile reflects the distribution of results, and errs on the side of safety in that it should overestimate the dose in 75% of hospitals, so it probably provides the best option on which to base any indicators. Ratios of the third and first quartile values for the tissue dose per unit DAP show that 50% of results are within ranges of two to three for the eye, thyroid and hands (Table 4). Dose indicators based on rounded values of the third quartile results are proposed in Table 6. Use of similar values for indicators in interventional cardiology and radiology is recommended for simplicity. The dose per unit DAP ratios for hand doses were 43% lower for cardiology procedures performed using femoral access, but ratios for the maximum skin dose per unit DAP for the radial approach were in the range 4–9 μGy Gy−1 cm−2. Use of a single value based on the result for all procedures is considered the best compromise. A separate indicator is proposed relating to hand doses for percutaneous procedures based on the five sets of data reported. These could be applied to the DAP workload to estimate potential doses to the hands and eyes. The uncertainties are large, but over 50% of the mean results analysed would lie within ±50% of the values derived using the proposed indicators. The doses used in the analysis were mean values from individual hospitals. There will be greater variations among individuals. Nevertheless, the doses could be employed in risk assessments for determining initial control measures and for planning dose-monitoring strategies based on the dose levels in Table 5. Particular attention will be required for radiologists performing percutaneous procedures, since the hand dose levels are larger, and the number of results available in the literature was limited. However, despite the larger uncertainty, doses are unlikely to be more than double the indicator value.
Table 6. Indicators for deriving dose estimates for use in risk assessments.
| Organ | Dose/DAP (μGy Gy−1 cm−2) | DAP per montha (Gy cm2) | Dose per cardiology procedure (μGy) | No. of cardiology procedures per montha |
| Eye | 1 | 2000 | 80 | 25 |
| Thyroidb | 1.5 (0.2) | 100 | ||
| Hand (percutaneous procedures) | 40 | 120 | ||
| Hand (femoral access) | 5 | 1000 | 300 | 16 |
| Legb | 10 (2) | (40) | (100) |
DAP, dose–area product.
aWorkload for which dose monitoring should be considered. It is likely to be required for an individual with a workload double this value.
bDoses in brackets relate to where protection is being used.
More data on operator doses are available for cardiology procedures, so another approach that could be applied for interventional cardiology is to use indicative doses per procedure if DAP data are not available. The dose of 50 μSv per procedure for the eye dose [18] has already been mentioned. Dose values for cardiology based on the third quartile data from Table 2 are included in Table 6, together with the numbers of procedures being performed per month above which dose monitoring should be considered. However, it is recommended that dose values per unit DAP based on data in Table 6 should be used wherever possible. Although this contains data from a wider range of procedures, the link to DAP is an important factor. These values can provide guidance as to the need for dose monitoring, but they can never be a substitute if this is indicated by the risk assessment, because of the variations that occur between individuals. Levels of monthly workload at which monitoring might be required derived from the values are also given in Table 6.
Regular monitoring of doses to the legs is generally impractical, but since protection can be provided more readily, use of leg shields to maintain doses as low as reasonably practicable (ALARP) is a better option. If the DAP workload of an individual exceeds 400 Gy cm2 per month, then their leg dose may be in the region of 50 mGy per year at the level of the dose limit for a member of the public using coefficients in Table 6 [70,71]. Leg shields should be in place for workloads of this size in order to follow the ALARP principle. Protection of the thyroid is easier to accomplish through wearing of a collar, and since the thyroid is radiosensitive, it is prudent to apply more stringent criteria. It is suggested that protection of the thyroid should also be recommended for workloads over 400 Gy cm2, which would correspond to a thyroid dose of 7 mGy per year that would contribute 0.3 mSv to the effective dose [70], which is equal to the dose constraint applied to a member of the public in implementation of protection measures.
The results in Table 6 indicate that the part of the body for which additional monitoring is most likely to be required is the hand. The need will depend on the access route used for the procedures undertaken, but dose assessment may be necessary for cardiologists performing 15–30 cardiology procedures per month. If eye doses could be a problem, then dosemeters might be worn either on a head band or at the neck on the side of the body nearest to the X-ray tube and intensifier. A neck dosemeter can be used for an assessment of eye dose using Equation (3) and would also provide a second dosemeter that could be used in calculation of E from Equation (1). A significant factor resulting in the lower doses found in routine dose monitoring than in studies reported in the literature [11] is likely to be the attention paid to the positions at which dosemeters are worn. This can give a dose to the eye lower by a factor of 3 to 5 [27]. Therefore, it is important to ensure that dosemeters are worn at appropriate positions when it is decided that monitoring is required. Dosemeters should be worn towards the side of the head or neck, or on the hand adjacent to the X-ray tube if a meaningful result is to be obtained. If the lower extremity is monitored, then this should be done using a dosemeter attached to the ankle or foot of the leg nearest to the X-ray tube.
In order to keep doses as low as practicable, protective devices must be used effectively. Studies made over many years have demonstrated that training of the operator in radiation protection methods is important [74]. It should be borne in mind that interventional operators may receive slightly higher doses per procedure during their training, as the periods of fluoroscopy for manipulating catheters may be longer and they may need to stand in a position where they have an unobstructed view of the procedure at other times [68,69]. Low dose monitoring results will usually demonstrate that good protection measures are in place, but results must be treated with some circumspection as low results may also reflect erratic use of dosemeters by some clinicians.
Conclusion
Dose levels are suggested at which monitoring of doses to the eyes and hands should be carried out for interventional operators. Indicators that could be used for estimating potential doses to the eyes, thyroid and hands from workload data have been proposed based on third quartile values from distributions of doses reported in the literature. Indicators for dose per unit DAP are given to cover all interventional procedures, and average doses per procedure for cardiology. These can be used to assess potential doses based on workload for application in risk assessments to decide dose-monitoring strategies. Risk assessments should include recommendations on positions in which dosemeters should be worn and close attention should be paid to ensuring staff compliance if monitoring regimes are to provide realistic assessments of staff doses.
References
- 1.Kim KP, Miller DL, Balter S, Kleinerman RA, Linet MS, Kwon D, et al. Occupational radiation doses to operators performing cardiac catheterization procedures. Health Phys 2008;94:211–27 [DOI] [PubMed] [Google Scholar]
- 2.International Commission on Radiological Protection. doi: 10.1016/S0146-6453(01)00004-5. Avoidance of radiation injuries from medical interventional procedures. ICRP Publication 85. Annals of the ICRP 30(2) 2000. [DOI] [PubMed] [Google Scholar]
- 3.Togni M, Balmer F, Piffner D, Meier W, Zeiher AM, Meier B. Percutaneous coronary interventions in Europe 1992–2001. Eur Heart J 2004;25:1208–13 [DOI] [PubMed] [Google Scholar]
- 4.Vaño E, González L, Beneytez F, Moreno F. Lens injuries induced by occupational exposure in non-optimized interventional radiology laboratories. Br J Radiol 1998;71:728–33 [DOI] [PubMed] [Google Scholar]
- 5.Vano E, Gonzalez L, Fernandez JM. Eye lens exposure to radiation in interventional suites: caution is warranted. Radiology 2008;248:945–93 [DOI] [PubMed] [Google Scholar]
- 6.Niklason LT, Marx MV, Chan H-P. Interventional radiologists: occupational radiation doses and risks. Radiology 1993;187:729–33 [DOI] [PubMed] [Google Scholar]
- 7.Mateya CF, Claycamp HG. Phantom-derived estimation of effective dose equivalent from X-rays with and without a lead apron. Health Phys 1997;72:842–7 [DOI] [PubMed] [Google Scholar]
- 8.Theocharopoulos N, Damilakis J, Perisinakis K, Manios E, Vardas P, Gourtsoyiannis N. Occupational exposure in the electrophysiology laboratory quantifying and minimising radiation burden. Br J Radiol 2006;79:644–51 [DOI] [PubMed] [Google Scholar]
- 9.Siiskonen T, Tapiovaara M, Kosunen A, Lehtinen M, Vartiainen E. Monte Carlo simulations of occupational radiation doses in interventional radiology. Br J Radiol 2007;80:460–8 [DOI] [PubMed] [Google Scholar]
- 10.Donadille L, Carinou E, Ginjaume M, Jankowski J, Rimpler A, Sans Merce M, et al. An overview of the use of extremity dosemeters in some European countries for medical applications. Rad Prot Dosim 2008;131:62–6 [DOI] [PubMed] [Google Scholar]
- 11.Whitby M, Martin CJ. Radiation doses to the legs of radiologists performing interventional procedures: are they a cause for concern? Br J Radiol 2003;76:321–7 [DOI] [PubMed] [Google Scholar]
- 12.Marshall NW, Faulkner K. The dependence of the scattered radiation dose to personnel on technique factors in diagnostic radiology. Br J Radiol 1993;65:44–9 [DOI] [PubMed] [Google Scholar]
- 13.Marx DL, Balter S. The distribution of stray radiation patterns in a cardiac catheterisation laboratory. Health Phys 1995;68:s84 [Google Scholar]
- 14.Kuon E, Schmitt M, Dahm JB. Significant reduction of radiation exposure to operator and staff during cardiac interventions by analysis of radiation leakage and improved lead shielding. Am J Cardiol 2002;89:44–9 [DOI] [PubMed] [Google Scholar]
- 15.Morrish OWE, Goldstone KE. An investigation into patient and staff doses from X-ray angiography during coronary interventional procedures. Br J Radiol 2008;81:35–45 [DOI] [PubMed] [Google Scholar]
- 16.Martin CJ. A review of radiology staff doses and dose monitoring requirements. Rad Prot Dosim 2009;136:140–57 [DOI] [PubMed] [Google Scholar]
- 17.Janssen RJJN, Hadders RH, Henkelman MS, Bosll AJJ. Exposure to operating staff during cardiac catheterisation measured by thermoluminescence dosimetry. Rad Prot Dosim 1992;43:175–7 [Google Scholar]
- 18.Pratt TA, Shaw AJ. Factors affecting the radiation dose to the lens of the eye during cardiac catheterization procedures. Br J Radiol 1993;66:346–50 [DOI] [PubMed] [Google Scholar]
- 19.Grant SCD, Faragher EB, Hufton AP, Bennett DH. Use of a remotely controlled mechanical pump for coronary arteriography: a study of radiation exposure and quality implications. Br Heart J 1993;70:479–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steffenino G, Rossettti V, Ribichini F, Dellavalle A, Garbarino M, Cerati R, et al. Staff dose reduction during coronary angiography using low framing speed. Br J Radiol 1996;69:860–4 [DOI] [PubMed] [Google Scholar]
- 21.Mann JT, 3rd, Cubeddu G, Arrowood M. Operator radiation exposure in PTCA: comparison of radial and femoral approaches. J Invasive Cardiol 1996;8:D22–D25 [PubMed] [Google Scholar]
- 22.Vaño E, González L, Guibelalde E, Fernández JM, Ten JI. Radiation exposure to medical staff in interventional and cardiac radiology. Br J Radiol 1998;71:954–60 [DOI] [PubMed] [Google Scholar]
- 23.Kicken PJH, Kemerink GJ, van Engelshoven JMA. Dosimetry of occupationally exposed persons in diagnostic and interventional arteriography. Part I: Assessment of entrance doses. Rad Prot Dosim 1999;82:93–103 [Google Scholar]
- 24.Efstathopoulos EP, Makrygiannis SS, Kottou S, Karvouni E, Giazitzoglou E, Korovesis S, et al. Medical personnel and patient dosimetry during coronary angiography and intervention. Phys Med Biol 2003;48:3059–68 [DOI] [PubMed] [Google Scholar]
- 25.Efstathopoulos EP, Katritsis DG, Kottou S, Kalivas N, Tzanalaridou E, Giazitzoglou E, et al. Patient and staff radiation dosimetry during cardiac electrophysiology studies and catheter ablation procedures: a comprehensive analysis. Europace 2006;8:443–8 [DOI] [PubMed] [Google Scholar]
- 26.Lie ØØ, Paulsen GU, Wøhni T. Assessment of effective dose and dose to the lens of the eye for interventional cardiologist. Rad Prot Dosim 2008;132:313–18 [DOI] [PubMed] [Google Scholar]
- 27.Jankowski J. Methods of radiation exposure estimation of patient and medical staff during some cardiology procedures. 5th Internatonal Workshop on Individual Monitoring of Ionising Radiation. Orai, Japan, (in press) [Google Scholar]
- 28.Li LB, Kai M, Takano K, Ikeda S, Matsuura M, Kusama T. Occupational exposure in pediatric cardiac catheterization. Health Phys 1995;69:261–4 [DOI] [PubMed] [Google Scholar]
- 29.Vehmas T. Radiation exposure during standard and complex interventional procedures. Br J Radiol 1997;70:296–8 [DOI] [PubMed] [Google Scholar]
- 30.Zorzetto M, Bernadi G, Morocutti G, Fontanelli A. Radiation exposure to patients and operators during diagnostic catheterisation and coronary angioplasty. Cathet Cardiovasc Diagn 1997;40:348–51 [DOI] [PubMed] [Google Scholar]
- 31.Delichas M, Psarrakos K, Molyvda-Athanassopoulou E, Giannoglou G, Sioundas A, Hatziioannou K, et al. Radiation exposure to cardiologists performing interventional cardiology procedures. Eur J Radiol 2003;48:268–73 [DOI] [PubMed] [Google Scholar]
- 32.Tspaki V, Kottou S, Vano E, Komppa T, Padopvani R, Dowling A, et al. Occupational dose constraints in interventional cardiology procedures: the DIMOND approach. Phys Med Biol 2004;49:997–1005 [DOI] [PubMed] [Google Scholar]
- 33.Goni H, Papadopoulou D, Yakoumakis E, Stratigis N, Benos J, Siriopoulos V, et al. Investigation of occupational radiation exposure during interventional cardiac catheterisations performed via radial artery. Rad Prot Dosim 2005;117:107–10 [DOI] [PubMed] [Google Scholar]
- 34.Fisher H, Przetak C, Teubert G, Ewen K, Mödder U. Die Strahlenexposition des radiologen bei angiographien: Dosismessungen ausserhalb der bleischürze. Fortschr Röntgenstr 1995;162:152–6 [DOI] [PubMed] [Google Scholar]
- 35.Williams JR. The interdependence of staff and patient doses in interventional radiology. Br J Radiol 1997;70:498–503 [DOI] [PubMed] [Google Scholar]
- 36.Stranden E, Seske T, Widmark A. Indicators for the finger doses in interventional radiology. Rad Prot Dosim 2007;124:164–6 [DOI] [PubMed] [Google Scholar]
- 37.McParland BJ, Nosil J, Burry B. A survey of the radiation exposures received by the staff at two cardiac catheterization laboratories. Br J Radiol 1990;63:885–8 [DOI] [PubMed] [Google Scholar]
- 38.Wu JR, Huang TY, Wu DK, Hsu PC, Weng PS. Radiation exposure of pediatric patients and physicians during cardiac catheterization and balloon pulmonary valvuloplasty. Am J Cardiol 1991;68:221–5 [DOI] [PubMed] [Google Scholar]
- 39.Padovani R, Novario R, Bernardi G. Optimisation in coronary angiography and percutaneous transluminal coronary angioplasty. Radiat Prot Dosim 1998;80:303–6 [Google Scholar]
- 40.Chong NS, Yin WH, Chan P, Cheng MC, Ko HL, Jeng SC, et al. Evaluation of absorbed radiation dose to working staff during cardiac catheterization procedures. Chinese Med J 2000;63:816–21 [PubMed] [Google Scholar]
- 41.Whitby M, Martin CJ. A study of the distribution of dose across the hands of interventional radiologists and cardiologists. Br J Radiol 2005;78:219–29 [DOI] [PubMed] [Google Scholar]
- 42.Tsapaki V, Patsilinakos S, Voudris V, Magginas A, Pavlidis S, Maounis T, et al. Level of patient and operator dose in the largest cardiac centre in Greece. Rad Prot Dosim 2008;129:71–3 [DOI] [PubMed] [Google Scholar]
- 43.Bor D, Çekirge S, Türkay T, Turan O, Gülay M, Önal E, et al. Patient and staff doses in interventional neuroradiology. Rad Prot Dosim 2005;117:62–8 [DOI] [PubMed] [Google Scholar]
- 44.Ginjaume M, Pérez S, Ortega X. Improvements in extremity dose assessment for ionising radiation medical applications. Rad Prot Dosim 2007;125:28–32 [DOI] [PubMed] [Google Scholar]
- 45.Olgar T, Bor D, Berkemen G, Yazar T. Patient and staff doses for complex x-ray examinations. J Radiol Prot 2009;29:393–407 [DOI] [PubMed] [Google Scholar]
- 46.Tspaki V, Kottou S, Vano E, Komppa T, Padovani R, Dowling A, et al. Occupational dose constraints in interventional cardiology: the DIMOND approach. Phys Med Biol 2004;49:997–1005 [DOI] [PubMed] [Google Scholar]
- 47.Tspaki V, Kottou S, Vano E, Parviainen T, Padovani R, Dowling A, et al. Correlation of patient and staff doses in interventional cardiology. Rad Prot Dosim 2005;117:26–9 [DOI] [PubMed] [Google Scholar]
- 48.Trianni A, Padovani R, Foti C, Cragnolini G, Toh H, Bernadi G., et al Dose to cardiologists in haemodynamic and electrophysiology cardiac interventional procedures. Rad Prot Dosim 2005;117:111–15 [DOI] [PubMed] [Google Scholar]
- 49.Hidajat N, Wust P, Kreuschner M, Felix R, Schröder Radiation risks for the radiologist performing transjugular intrahepatic portosystemic shunt. Br J Radiol 2006;79:483–6 [DOI] [PubMed] [Google Scholar]
- 50.Sturchio G, Schueler B, Hindal M, Landsworth R, Magnuson D. Lens dose equivalent assessment of an interventional radiologist. Health Phys 2009:97(Suppl 1):TAM-B.6, S39 [Google Scholar]
- 51.Safak M, Olgar T, Bor D, Berkmen G, Gogus C. Radiation doses of patients and urologists during percutaneous nephrolithotomy. J Radiol Prot 2009;29:409–15 [DOI] [PubMed] [Google Scholar]
- 52.Shortt CP, Al-Hashimi H, Malone L, Lee MJ. Staff radiation doses to the lower extremities in interventional radiology. Cardiovasc Intervent Radiol 2007;30:1206–9 [DOI] [PubMed] [Google Scholar]
- 53.Vano E, Gonzalez L, Fernandez JM, Prieto C, Guibelalde E. influence of patient thickness and operation modes on occupational and patient radiation doses in interventional cardiology. Rad Prot Dosim 2006;118:325–30 [DOI] [PubMed] [Google Scholar]
- 54.Kicken PJH, Kemerink GJ, Schultz FW, Zoetelief J, Broerse JJ, van Engelshoven JMA. Dosimetry of occupationally exposed persons in diagnostic and interventional arteriography. Part II: Assessment of effective dose. Rad Prot Dosim 1999;82:105–14 [Google Scholar]
- 55.Mesbahi A, Mehnati P, Keshtkar A, Aslanabadi N. Comparison of radiation dose to patients and staff for two interventional cardiology units: a phantom study. Rad Prot Dosim 2008;131:399–403 [DOI] [PubMed] [Google Scholar]
- 56.Marshall NW, Faulkner K, Clarke P. An investigation into the effect of protective devices on the dose to radiosensitive organs in the head and neck. Br J Radiol 1992;65:799–802 [DOI] [PubMed] [Google Scholar]
- 57.Felmlee JP, McGough PF, Morin RL, Classic L. Hand dose measurements in interventional radiology. Health Phys 1991;60:265–7 [DOI] [PubMed] [Google Scholar]
- 58.Wagner LK, Mulhern OR. Radiation-attenuating surgical gloves: Effects of scatter and secondary electron production. Radiology 1996;200:45–8 [DOI] [PubMed] [Google Scholar]
- 59.Nickoloff EL, Khandji A, Dutta A. Radiation doses during CT fluoroscopy. Health Phys 2000;79:675–81 [DOI] [PubMed] [Google Scholar]
- 60.Rampersau YR, Foley KT, Shen AC, Williams S, Solomito M. Radiation exposure during the fluoroscopically assisted pedicle screw insertion. Spine 2000;25:2637–45 [DOI] [PubMed] [Google Scholar]
- 61.Neeman Z, Dromi SA, Sarin S. CT Fluoroscopy shielding: decreases in scattered radiation for the patient and operator. J Vasc Interv Radiol 2006;17:1999–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wyart P, Dumant D, Gourdier M, Nassar F, Bouthillon JC, Chestier Y. Contribution of self-surveillance of the personnel by electronic radiation dosimeters in invasive cardiology. Arch Mal Coaeur Vaiss 1997;90:233–8 [PubMed] [Google Scholar]
- 63.Järvinen H, Buls N, Clerinx P, Jansen J, Miljanić S, Nikodemová D, et al. Overview of double dosimetry procedures for the determination of the effective dose to the interventional radiology staff. Rad. Prot Dosim 2008;129:333–9 [DOI] [PubMed] [Google Scholar]
- 64.Niklason LT, Marx MV, Chan H-P. The estimation of occupational effective dose in diagnostic radiology with two dosimeters. Health Phys 1994;67:611–15 [DOI] [PubMed] [Google Scholar]
- 65.Rosentstein M, Webster EW. Effective dose to personnel wearing protective aprons during fluoroscopy and interventional radiology. Health Phys 1994;67:88–9 [PubMed] [Google Scholar]
- 66.Clerinx P, Buls N, Bosmans H, de Mey J. Double-dosimetry algorithm for workers in interventional radiology. Rad Prot Dosim 2008;129:321–7 [DOI] [PubMed] [Google Scholar]
- 67.Padovani R, Rodella CA. Staff dosimetry in interventional cardiology. Radiat Prot Dosim 2001;94:99–103 [DOI] [PubMed] [Google Scholar]
- 68.Renaud L. A 5-y follow-up of the radiation exposure to in-room personnel during cardiac catheterization. Health Phys 1992;62:10–15 [DOI] [PubMed] [Google Scholar]
- 69.Watson LE, Riggs MW, Bourland PD. Radiation exposure during cardiology fellowship training. Health Phys 1997;73:690–3 [DOI] [PubMed] [Google Scholar]
- 70.International Commission on Radiological Protection. doi: 10.1016/j.icrp.2007.10.003. The 2007 recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Annals of the ICRP 37(2–4), 2007. [DOI] [PubMed] [Google Scholar]
- 71.Ionising Radiations Regulations (Statutory Instrument 1999 No. 3232) London UK: HMSO, 1999. [Google Scholar]
- 72.Martin CJ, Whitby M. Application of ALARP to extremity doses for hospital workers. J Radiol Prot 2003;23:405–21 [DOI] [PubMed] [Google Scholar]
- 73.Williams JR. Scatter dose estimation based on dose-area product and the specification of radiation barriers. Br J Radiol 1996;69:1032–7 [DOI] [PubMed] [Google Scholar]
- 74.Vano E, Gonzalez L, Fernandez JM, Alfonso F, Macaya C. Occupational radiation doses in interventional cardiology: a 15-year follow-up. Br J Radiol 2006;79:383–8 [DOI] [PubMed] [Google Scholar]