Abstract
The purpose of our investigation was to determine the anatomical variations in the coeliac trunk–hepatic arterial system and the renal arteries in patients who underwent multidetector CT (MDCT) angiography of the abdominal aorta for various reasons. A total of 100 patients were analysed retrospectively. The coeliac trunk, hepatic arterial system and renal arteries were analysed individually and anatomical variations were recorded. Statistical analysis of the relationship between hepatocoeliac variations and renal artery variations was performed using a χ2 test. There was a coeliac trunk trifurcation in 89% and bifurcation in 8% of the cases. Coeliac trunk was absent in 1%, a hepatosplenomesenteric trunk was seen in 1% and a splenomesenteric trunk was present in 1%. Hepatic artery variation was present in 48% of patients. Coeliac trunk and/or hepatic arterial variation was present in 23 (39.7%) of the 58 patients with normal renal arteries, and in 27 (64.3%) of the 42 patients with accessory renal arteries. There was a statistically significant correlation between renal artery variations and coeliac trunk–hepatic arterial system variations (p = 0.015). MDCT angiography permits a correct and detailed evaluation of hepatic and renal vascular anatomy. The prevalence of variations in the coeliac trunk and/or hepatic arteries is increased in people with accessory renal arteries. For that reason, when undertaking angiographic examinations directed towards any single organ, the possibility of variations in the vascular structure of other organs should be kept in mind.
Anatomical variations of the hepatic arteries and coeliac trunk are of considerable importance in liver transplants, laparoscopic surgery, radiological abdominal interventions and penetrating injuries to the abdomen [1]. The frequency of inadvertent or iatrogenic hepatic vascular injury rises in the event of aberrant anatomy and variations [2]. Arterial vascularisation of the gastrointestinal system is provided by anterior branches at three different levels of the abdominal aorta (the coeliac trunk and the superior and inferior mesenteric arteries). Differences arising during several developmental stages in the embryonal process lead to a range of variations in these vascular structures.
Renal artery variations are not uncommon either and give rise to several problems that are encountered by clinicians. Kidneys with a large number of renal arteries are reported to have a higher rate of transplantation failure than those with a single renal artery [3, 4]. The risk represented by these vascular variations is not, however, limited to renal transplantations and to the surgical treatment of renovascular hypertension.
Digital subtraction angiography (DSA) is regarded as the gold standard in the evaluation of vascular structures, although its invasive nature significantly limits its role. In recent years, the introduction of multidetector CT (MDCT) and its ability to image vascular structures of small diameter have led to a significant reduction in the utilisation of invasive DSA examinations.
The aim of this study is to examine the anatomical variations that occur in the coeliac trunk–hepatic arterial system and renal arteries and their prevalence. We looked at these vascular systems in patients who underwent multidetector CT angiography of the abdominal aorta for various reasons.
Methods and materials
Patients and imaging technique
All patients who underwent CT angiography of the abdominal aorta and its branches for various reasons in Gulhane Military Medical school, Ankara Hospital from January 2006 through December 2006 were investigated retrospectively. 100 patients were evaluated in the study (61 males and 39 females). The mean age was 49 years (age range: 10–85 years). The reasons for MDCT angiography are presented in Table 1.
Table 1. Reasons for multidetector CT angiography examination in this series.
| Indication | Number of cases |
| Aortic aneurysm | 27 |
| Renovascular hypertension | 21 |
| Liver transplantation donor | 10 |
| Renal transplantation donor | 14 |
| Acute mesenteric ischaemia | 5 |
| Investigation of vascular invasion in pancreatic and hepatic malignancies | 23 |
| Total | 100 |
MDCT angiography examinations were performed using a 16 detector scanner (MX 8000 IDT Multislice CT System, Philips Medical Systems, Best, the Netherlands). The area from the lower thoracic spine to the symphysis pubis level, with the patient in a supine position, was adopted as the field of view. During examination, an 18–20 gauge angiocath needle inserted into patients' antecubital vein was used to inject 120 ml of non-ionic iodinated contrast medium using the bolus tracking technique (rather than a predetermined delay time) with an automatic injector at a rate of 4 ml s−1 (CT 9000 ADV, Digital Injection System, CT Multipack 200 ml syringe; Mallinckrodt Company, New Mexico, USA).
The axial images obtained were transferred to a workstation for analysis. Three-dimensional volume-rendering technique (3D VRT), maximum intensity projection (MIP) and multiplanar reconstruction (MPR) images were used for evaluation.
Vascular system analysis
The raw data axial images obtained by MDCT angiography as well as the post-processed 3D VRT, MIP and MPR images were evaluated by two radiologists in consensus. The anatomies of the coeliac trunk, hepatic arterial system and renal vascular structures were analysed individually and anatomical variations recorded. Anatomical variations of the coeliac trunk were described according to Uflacker's system (Table 2) [5]. Anatomical variations of the hepatic arterial system were defined according to Michels's [6] 1966 internationally recognised classification and Hiatt's [7] 1994 modification of that system (Table 3).
Table 2. Coeliac trunk variations: Uflacker's classification [5].
| Coeliac trunk variation | Uflacker's classification |
| Classic coeliac trunk | Type I |
| Hepatosplenic trunk | Type II |
| Hepatogastric trunk | Type III |
| Hepatosplenicmesenteric trunk | Type IV |
| Gastrosplenic trunk | Type V |
| Coeliac-mesenteric trunk | Type VI |
| Coeliac-colic trunk | Type VII |
| No coeliac trunk | Type VIII |
Table 3. Hepatic artery variations: Michels's and Hiatt's classifications [6, 7].
| Hepatic artery variation | Michels classification | Hiatt classification |
| Normal anatomy | Type I | Type I |
| Replaced left hepatic artery originating from the left gastric artery | Type II | Type II |
| Replaced right hepatic artery originating from the superior mesenteric artery | Type III | Type III |
| Co-existence of Type II and III | Type IV | Type IV |
| Accessory left hepatic artery originating from the left gastric artery | Type V | Type II |
| Accessory right hepatic artery originating from the superior mesenteric artery | Type VI | Type III |
| Accessory left hepatic artery originating from the left gastric artery and accessory right hepatic artery originating from the superior mesenteric artery | Type VII | Type IV |
| Accessory left hepatic artery originating from the left gastric artery and replaced right hepatic artery originating from the superior mesenteric artery | Type VIII | Type IV |
| Common hepatic artery originating from the superior mesenteric artery | Type IX | Type V |
| Right and left hepatic arteries originating from the left gastric artery | Type X | NOD |
| Common hepatic artery directly originating from the aorta | NOD | Type VI |
NOD, “not otherwise described” in the literature.
The existence of any artery other than a single hilar artery in each kidney was accepted as an anatomical variation. Anatomical variations of renal arteries, whether they are unilateral or bilateral, their numbers and origins were all determined in this study.
The possibility of a correlation between coeliac trunk and/or hepatic artery variations and renal artery variations was analysed using the χ2 test, with p <0.05 regarded as statistically significant.
Results
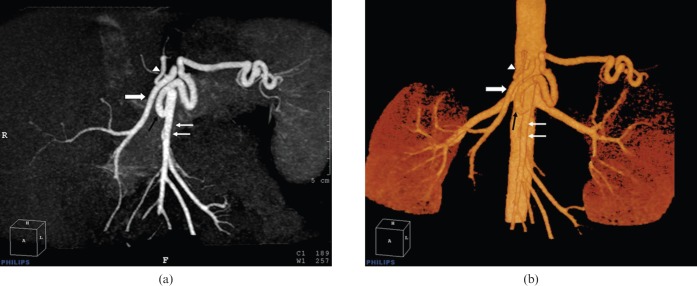
Both coeliac trunk and hepatic arteries had a normal anatomy in 50 of the 100 patients (50%); either coeliac trunk or hepatic artery variation was present in the remaining half (Tables 4 and 5). A normal coeliac trunk formed from the left gastric, splenic and common hepatic arteries was present in 89% of patients. Gastrosplenic trunk (Type V) was the most prevalent variation (4%), followed by hepatosplenic trunk (Type II) (3%). In one patient, the splenic artery and superior mesenteric artery originated from a common trunk while the common hepatic artery and left gastric artery originated from a separate common trunk. This variation has not been described by any classification, and is defined here as “splenomesenteric trunk” (Figure 1).
Table 4. Coeliac trunk variations found in this study.
| Anatomical structure | Uflacker type | Number of cases |
| Normal anatomy | I | 89 |
| Hepatosplenic trunk | II | 3 |
| Hepatogastric trunk | III | 1 |
| Hepatosplenomesenteric trunk | IV | 1 |
| Gastrosplenic trunk | V | 4 |
| No coeliac trunk | VIII | 1 |
| Splenomesenteric trunk | NOD | 1 |
| Total | 100 | |
NOD, “not otherwise described” in the literature.
Table 5. Hepatic artery variations found in this study.
| Anatomical structure | Type | Number of cases |
| Normal anatomy | M-I | 52 |
| H-I | ||
| Replaced left hepatic artery | M-II | 11 |
| H-II | ||
| Replaced right hepatic artery | M-III | 17 |
| H-III | ||
| Replaced right and left hepatic artery | M-IV | 1 |
| H-IV | ||
| Accessory left hepatic artery | M-V | 10 |
| H-II | ||
| Accessory right hepatic artery | M-VI | 1 |
| H-III | ||
| Accessory right hepatic artery | M-VII | 1 |
| H-IV | ||
| Accessory left and replaced right hepatic artery | M-VIII | 1 |
| H-IV | ||
| Common hepatic artery originating from the superior mesenteric artery | M-IX | 2 |
| H-V | ||
| Common hepatic artery originating from the aorta | M-NOD | 1 |
| H-VI | ||
| Right hepatic artery originating from the middle colic artery | NOD | 1 |
| Right hepatic artery originating from directly from the aorta | NOD | 1 |
| Left hepatic artery originating from the common hepatic artery | NOD | 1 |
| Total | 100 | |
H, Hiatt classification; M, Michels classification; NOD, “not otherwise described” in the literature.
Figure 1.
Splenomesenteric trunk: the superior mesenteric artery (thin white arrows) and splenic artery (black arrow) originate from a common trunk as seen in (a) coronal MIP and (b) volume-rendered images. The common hepatic artery (thick white arrow) and left gastric artery (white arrowhead) originate from a separate trunk.
The hepatic arteries had a normal anatomy (Type I) in 52% of patients. The most common hepatic artery variations in our series were replaced right hepatic artery (Type III) (17%), replaced left hepatic artery (Type II) (11%) and accessory left hepatic artery (Type V) (10%) according to Michels's classification. On the basis of Hiatt's classification, on the other hand, the most common hepatic artery variation was accessory or replaced left hepatic artery (Type II) (22%).
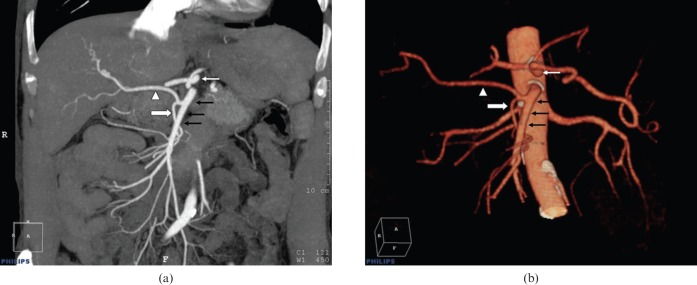
3 of the 100 patients in our series had unclassified hepatic artery variations. One of these was a right hepatic artery that originated from the middle colic artery (Figure 2); one was a right hepatic artery that originated directly from the aorta (Figure 3); and the third was a left hepatic artery originating from the common hepatic artery (Figure 4). Liver segment IV arteries, originating from the right hepatic artery, were determined in 35 of the 100 cases.
Figure 2.
Right hepatic artery (white arrowhead) originating from the middle colic artery (thick white arrow) as seen in (a) coronal MIP and (b) volume-rendered images. Black arrows point out the superior mesenteric artery and the thin white arrow indicates the coeliac trunk.
Figure 3.
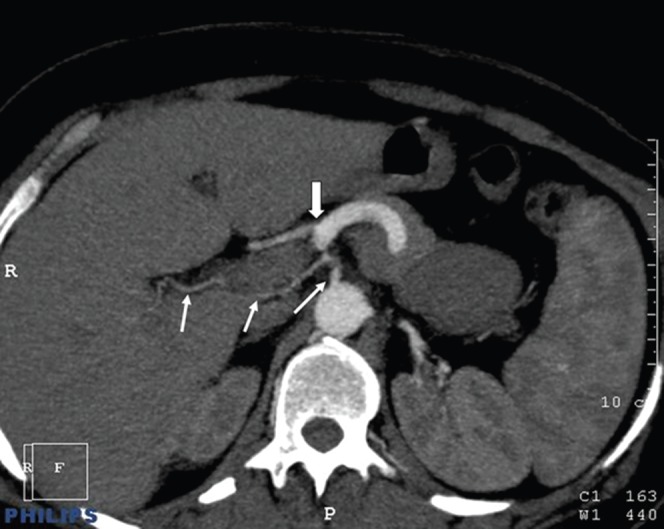
Right hepatic artery originating from the abdominal aorta. Axial MIP images demonstrate that the right hepatic artery (thin white arrows) originates directly from the aorta. The thick white arrow indicates the coeliac trunk.
Figure 4.
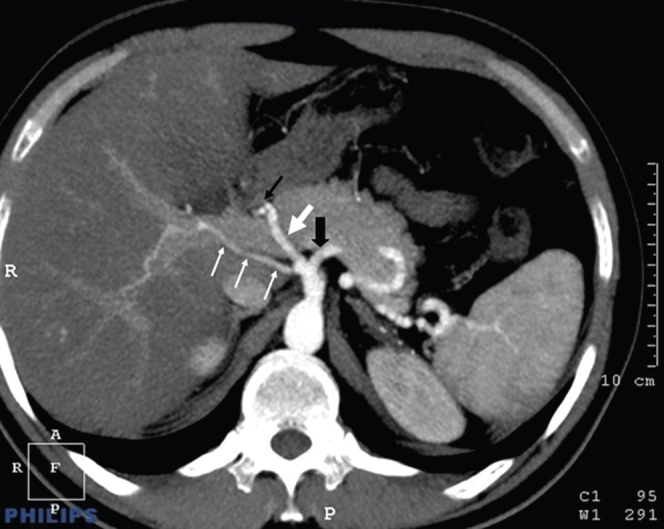
Right hepatic artery originating from the coeliac trunk. The left hepatic artery (thin black arrow) takes its origin from the common hepatic artery (thick white arrow), but the right hepatic artery (thin white arrows) originates directly from the coeliac trunk. The thick black arrow indicates the splenic artery.
Investigation of the patients' renal arteries revealed 1 renal artery in each kidney in 58 of the 100 patients, and more than 1 renal artery in 42 patients (Table 6, Figure 5). All of the accessory renal arteries originated from the abdominal aorta. The prevalence of one or more accessory renal arteries was higher on the right than on the left (18% vs 15%). The only anomaly among the cases with accessory renal arteries was a horseshoe kidney; in this case, four renal arteries led from the abdominal aorta to each side of the anomalous kidney.
Table 6. Renal artery variations found in this study.
| Variation | Number of cases |
| Normal anatomy | 58 |
| Two renal arteries on the right | 18 |
| Two renal arteries on the left | 15 |
| Three renal arteries on the left | 2 |
| Two renal arteries on each side | 4* |
| Two renal arteries on the right and three renal arteries on the left | 3 |
| Total | 100 |
*One with horseshoe kidney.
Figure 5.
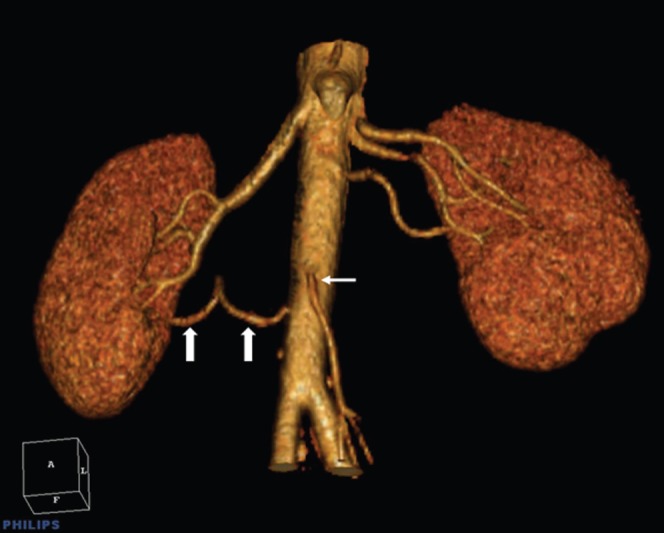
Accessory renal arteries. This volume-rendered image shows two renal arteries on the right and three on the left. The accessory renal artery supplying the inferior pole of the right kidney (thick white arrows) has an aortic origin even lower than that of the inferior mesenteric artery (thin white arrow).
23 (39.7%) of the 58 patients with no renal artery variation had coeliac trunk and/or hepatic arteries with variant anatomy, whereas 27 (64.3%) of the 42 patients with renal artery variation also had coeliac trunk and/or hepatic artery variation. The relation between coeliac trunk and/or hepatic artery variations and renal artery variations was significant (χ2 test; p = 0.015).
Discussion
DSA is regarded as the gold standard in the evaluation of vascular structures. Nevertheless, some variations can be overlooked when this technology is used in cases where description of the hepatic arterial system is difficult, where the catheter tip is far from where it should be in the coeliac trunk during selective angiographic processes, or when a high-quality angiograph cannot be obtained [8, 9]. MDCT angiography allows most of the body to be scanned helically in just one breath hold, and thus provides high-contrast resolution without any artefacts. Together with axial images, 3D images provide a very good anatomical orientation. Hence, detailed information regarding vascular structures, organs and their relations with one another can be obtained.
Michels's classification of the hepatic arterial system described 10 variant subtypes [6]. The accessory and replaced hepatic arterial systems were described separately within this classification. Under Hiatt's classification, no such distinction was made because it was angiographically difficult to distinguish between accessory and replaced hepatic arterial structures, hence six subtypes were described [7]. We used both classifications in our study. A normal hepatic arterial system has been reported in 51–80% of cases in most studies conducted using DSA [10, 11]. In our study, this ratio was about 52%. In the literature, the most frequently encountered variation is Type III, present in between 6% and 15.5% of all cases [12, 13]. The second most frequent variation, Type II, was reported in 2.5–10% of all cases [13, 14]. In our study too, Types III and II were the most commonly seen hepatic arterial variations. The prevalences of these variations in our series are slightly higher than those often reported, but are similar to those of Erbay et al [15]. Types VII, VIII and X have only been reported very rarely. In our study, Types VII and VIII were determined with a rate of 1%, and no type X was encountered.
Liver segment IV is of critical importance in transplant surgery. For that reason, it is important to know the origin of its blood supply. With the fast MDCT technology, better and artefact-free images can be obtained following bolus contrast administration. In this way, hepatic arterial supply, especially the fine-calibre artery of segment IV, can be displayed very clearly. In an MDCT study conducted by Kamel et al, the segment IV artery was reported to originate from the right hepatic artery in 62.5% of cases [11]. Erbay et al [15] reported this incidence as 9% [15]. In our study, the segment IV artery originated from the right hepatic artery in 35% of cases.
Rare anomalies that are not consistent with any type described under Michels's and Hiatt's classifications can also be seen. In their study, Koops et al [14] and Abdullah et al [16] reported frequencies of 1.8% and 1.4%, respectively, for such rare and unclassified anomalies (or variations) of abdominal aortic branches. Our study revealed a frequency of 3% (in 3 of 100 patients) for these unclassified abnormalities. A replaced right hepatic artery that originates from the aorta was also reported by Koops et al [14], but the other two unclassified variations that we describe here have not been reported beforehand to the best of our knowledge. The existence of such unique variations shows that the embryological development of the abdominal aortic branches may be influenced by many factors and is a complex process.
Normal coeliac trunks exhibiting trifurcation have been reported with a frequency of 72–90% in the normal population [17, 18]. One of the largest series, studied by Vandamme and Bonte [19], had a frequency of 86% for normal coeliac trifurcation, which is very close to the frequency in our series (89%). Coeliac trunk bifurcation as an anatomical variation has been reported at a rate of about 12% in the literature, and was present in 8% of cases in our series. Bergman et al [17] published a metaanalysis of “no coeliac trunk reports” in the literature and calculated the average absence rate of coeliac trunk as 0.4%. In our study, coeliac trunk was absent in 1 of the 100 cases.
Splenomesenteric trunk is a very rare variation with an occurrence rate of below 1% [20]. Oran et al [21] have reported a case in which the splenic artery formed a common trunk with the superior mesenteric artery, and the common hepatic artery formed a separate trunk with the left gastric artery [21]. Similarly, two separate trunks, a splenomesenteric trunk and a hepatogastric trunk, not described under any classification, were present in one patient in our study.
The kidneys, which are located inside the pelvis during the embryological period, obtain their blood supply from blood vessels in that region. Initially, the renal arteries take their origin from the common iliac arteries. As the kidneys ascend, blood supply is obtained from the distal end of the aorta. When they ascend further, the kidneys receive blood from new aortic branches. As the kidneys reach their final destination, they are fed by true permanent renal arteries from the abdominal aorta and the previous caudal renal feeders undergo involution [22]. The wide variations observed in the kidneys' blood supply are the result of these changes in the organs' blood supplies during embryological and early foetal life [22]. Accessory kidney arteries sometimes enter the kidney directly from the superior or inferior renal poles. An accessory artery in the inferior renal pole crosses the ureter obliquely from its anterior aspect, and may lead to hydronephrosis by compressing the ureter. It is important to be aware that accessory renal arteries are not juvenile arteries whose function has been superseded, and thus ligation of an accessory renal artery ends up with ischaemic damage to the supplied portion of the kidney. Therefore, a knowledge of variations in renal vasculature is of crucial importance for interventions involving the kidney arteries or abdominal aorta. In our study, the renal artery and its variations were demonstrated very well by MDCT angiography; the relationship with the pelvic collecting system and other anatomical structures was revealed very clearly. The prevalence of abdominal aortic aneurysm among the patients in this study was 27%. MDCT angiography clearly demonstrated the relationship between the renal vascular supply (including its variations) and the aortic aneurysms in these patients, thereby facilitating the determination of treatment options.
Satyapal et al [23] reported that the prevalence of multiple renal arteries in the literature was between 9% and 76%, and this vascular variation could be accompanied by other renal anomalies. Although the incidence of multiple renal arteries was 42% in our study, a renal anomaly was present in only one patient who had a horseshoe kidney with four renal arteries supplying each side.
In our study, there was no anatomical variation pertaining to a common origin of the visceral artery branches and the lateral branches of the aorta. However, whereas coeliac trunk and/or hepatic artery variation was present in 23 (39.7%) of the 58 patients with no renal artery variation, 27 (64.3%) of the 42 patients with renal artery variation had an associated coeliac and/or hepatic variation. Hence, there was a statistically significant correlation between patients with renal artery variation and patients with variation in the coeliac trunk and/or hepatic arterial system. The embryological development of the kidneys and the visceral organs or intestines are regarded as different and independent of each other. However, anomalies in the migration of both the liver and the kidneys can occasionally establish a link between the visceral branches of the aorta and lateral branches. Otherwise, it would be difficult to explain how renal vascular variations emerging during the developmental migration of the kidneys trigger the formation of coeliac trunk and/or hepatic arterial system variations, or vice versa. Examination of the literature shows that no such correlation has to date been reported. The results in our study, however, clearly show that there is an association between these two independent vascular variations. We consider that further investigation of this relationship with studies comprising larger populations would be useful.
In conclusion, MDCT angiography permits an accurate and detailed analysis of hepatic and renal vascular anatomy. Angiographic information required to determine treatment options and to prevent complications arising from vascular variations during surgery or intervention can easily be obtained using MDCT. The likelihood of coeliac trunk and/or hepatic arterial variation rises in those individuals with more than one renal artery. Therefore, if variations are discovered during the angiographic examination of one organ, it must be kept in mind that there may be variations in the vascular structures of the other organs, and a more detailed investigation must be performed.
References
- 1.Munshi IA, Fusco D, Tashjian D, Kirkwood JR, Polga J, Wait RB. Occlusion of an aberrant right hepatic artery, originating from the superior mesenteric artery, secondary to blunt trauma. J Trauma 2000;48:325–6 [DOI] [PubMed] [Google Scholar]
- 2.Rela M, McCall JL, Karani J, Heaton ND. Accessory right hepatic artery arising from the left. Transplantation 1998;66:792–4 [DOI] [PubMed] [Google Scholar]
- 3.Fox M, Yalin R. Renal transplantation with multiple arteries. Br J Urol 1979;51:333–6 [DOI] [PubMed] [Google Scholar]
- 4.Sampaio FJB, Passos MARF. Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat 1992;14:113–17 [DOI] [PubMed] [Google Scholar]
- 5.Uflacker R. Atlas of vascular anatomy: an angiographic approach. Baltimore: Williams & Wilkins, 1997 [Google Scholar]
- 6.Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966;112:337–47 [DOI] [PubMed] [Google Scholar]
- 7.Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 1994;220:50–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fasel JHD, Muster M, Gailloud P, Mentha G, Terrier F. Duplicated hepatic artery: radiologic and surgical implications. Acta Anat 1996;157:164–8 [DOI] [PubMed] [Google Scholar]
- 9.Weiglein AH. Variations and topography of the arteries in the lesser omentum in humans. Clin Anat 1996;9:143–50 [DOI] [PubMed] [Google Scholar]
- 10.Chen CY, Lee RC, Tseng HS, Chiang JH, Hwang JI, Teng MM. Normal and variant anatomy of the hepatic arteries: angiographic experience. Chin Med J 1998;61:17–23 [PubMed] [Google Scholar]
- 11.Kamel IR, Kruskal JB, Pomfret EA, Keogan MT, Warmbrand G, Raptopoulos V. Impact of multidetector CT on donor selection and surgical planning before living adult right lobe liver transplantation. AJR Am J Roentgenol 2001;176:193–200 [DOI] [PubMed] [Google Scholar]
- 12.Daly JM, Kemeny N, Oderman P, Botet J. Long-term hepatic arterial infusion chemotherapy. Arch Surg 1984;119:936–41 [DOI] [PubMed] [Google Scholar]
- 13.De Santis M, Ariosi P, Calo GF, Romagnoli R. Anatomia vascolare arteriosa epatica e sue varianti. Radiol Med 2000;100:145–51 [PubMed] [Google Scholar]
- 14.Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G. Anatomic variations of the hepatic arteries in 604 selective coeliac and superior mesenteric angiographies. Surg Radiol Anat 2004;26:239–44 [DOI] [PubMed] [Google Scholar]
- 15.Erbay N, Raptopoulos V, Pomfret EA, Kamel IR, Kruskal JB. Living donor liver transplantation in adults: vascular variants important in surgical planning for donors and recipients. AJR Am J Roentgenol 2003;181:109–14 [DOI] [PubMed] [Google Scholar]
- 16.Abdullah SS, Mabrut JY, Garbit V, De LaRoche E, Olagne E, Rode A, et al. Anatomical variations of the hepatic artery: study of 932 cases in liver transplantation. Surg Radiol Anat 2006;28:468–73 [DOI] [PubMed] [Google Scholar]
- 17.Bergman RA, Afifi AK, Miyauchi R. http://www.anatomyatlases.org/AnatomicVariants/Cardiovascular/Text/Arteries/coeliacTrunks.html. [Google Scholar]
- 18.Matoba M, Tonami H, Kuginuki M, Yokota H, Takashima S, Yamamoto I. Comparison of high-resolution contrast-enhanced 3D MRA with digital subtraction angiography in the evaluation of hepatic arterial anatomy. Clin Radiol 2003;58:463–8 [DOI] [PubMed] [Google Scholar]
- 19.Vandamme JP, Bonte J. The branches of the coeliac trunk. Acta Anat 1985;122:110–14 [DOI] [PubMed] [Google Scholar]
- 20.Michels NA. Blood supply of upper abdominal organs. Philadelphia: JB Lippincott, 1955:134–95. [Google Scholar]
- 21.Oran I, Yesildag A, Memis A. Aortic origin of right hepatic artery and superior mesenteric origin of splenic artery: two rare variations demonstrated angiographically. Surg Radiol Anat 2001;23:349–52 [DOI] [PubMed] [Google Scholar]
- 22.Moore KL. The developing human: clinically oriented embryology. Philadelphia: WB Saunders Company, 1973 [Google Scholar]
- 23.Satyapal KS, Haffejee AA, Singh B, Ramsaroop L, Robbs JV, Kalideen JM. Additional renal arteries: incidence and morphometry. Surg Radiol Anat 2001;23:33–8 [DOI] [PubMed] [Google Scholar]