Abstract
A 42-year-old woman presented with local recurrence and distant lung and liver metastases 7 years after resection of a primary intracranial haemangiopericytoma. Whole-body 18F-fluoro-2-deoxy-d-glucose (FDG)-positron emission tomography (PET)-CT scan showed no increased uptake in local recurrence or distant metastases except for a focus of increased FDG uptake within a hepatic metastasis. The hypermetabolic area correlated with an intratumoral hypoenhancing area on the CT scan. PET-CT scan may be useful to allow further understanding of the tumour.
Haemangiopericytoma is an uncommon tumour and accounts for less than 1% of all intracranial tumours. It caused much confusion because of its imaging similarities to meningioma and its uncertain cellular origin. Its rarity, non-specific imaging findings and lack of specific tumour markers pose diagnostic difficulties.
We report a case of intracranial haemangiopericytoma treated with surgical resection. Local recurrence and distant metastases in the lung and liver occurred seven years after complete resection of the primary intracranial tumour. 18F-fluoro-2-deoxy-d-glucose (FDG)-positron emission tomography–computed tomography (PET-CT) was performed for assessment of the extent of disease.
Case report
A 42-year-old woman presented with headache and unsteady gait for one month in September 2001. CT and MRI scans of the brain showed an 8 cm extra-axial homogeneous enhancing mass in the left posterior cranial fossa causing hydrocephalus. The initial diagnosis was left cerebellar tentorial meningioma. Angiogram showed hypervascular tumour and the tumour was supplied mainly by the tentorial branch of the left internal carotid artery and branches from the left vertebral artery and external carotid artery. Pre-operative embolisation with 150–250 μm polyvinyl alcohol particles and n-butyl cyanoacrylate was performed to reduce intra-operative bleeding.
Histology from an intra-operative frozen section and the whole surgical specimen diagnosed haemangiopericytoma. Randomly orientated plump cells and a rich network of reticulum fibres investing individual cells were found in the tumour. Characteristic staghorn vessels were identified. Immunohistochemistry staining of the tumour cells were focally positive for CD34, and the findings were compatible with meningeal haemangiopericytoma [1, 2]. After total resection of the tumour, the patient received post-operative cranial irradiation.
The patient underwent regular clinical follow-up. There was no sign of tumour recurrence until seven years after the operation, when she complained of a palpable epigastric mass with dull pain. Tumour markers including carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) were normal. Hepatitis B surface antigen was negative. Whole-body 18F-FDG-PET-CT was obtained for further evaluation. The CT part of the examination was performed with administration of intravenous contrast, including both arterial and portal venous phases of the liver.
CT showed seven well-defined arterial-phase enhancing lesions in both hepatic lobes with sizes ranging from 7 mm to 7 cm. All of them were eumetabolic to the uptake of the background liver parenchyma (maximal standardised uptake value (SUVmax) of 2.5) of except for the largest lesion in segment IVb. This lesion showed marked heterogeneous enhancement on arterial and later phases, with a 4 cm well-defined internal eccentric hypodense area (Figure 1). On PET scan, a small focus of elevated FDG uptake with SUVmax of 3.6 (Figure 2) was noted in the hypoenhancing centre, which is better demonstrated on the fused image (Figure 3).
Figure 1.
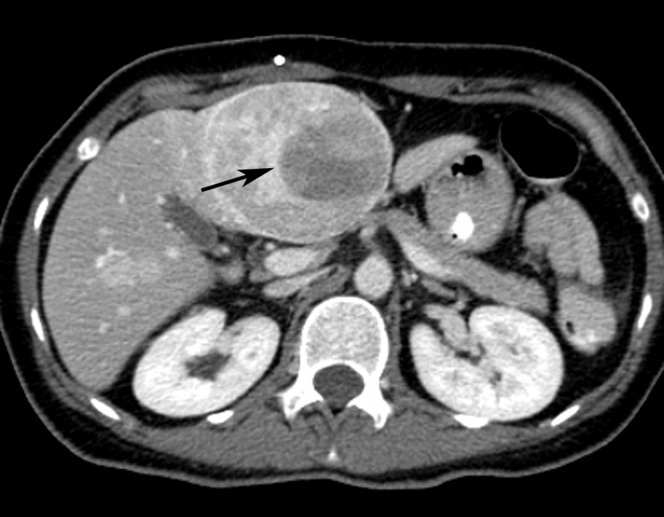
Axial contrast-enhanced CT image of the liver shows an enhancing mass with an internal hypoenhancing centre (arrow) in hepatic segment IVb. Subsequent biopsy of the mass confirms metastatic haemangiopericytoma.
Figure 2.
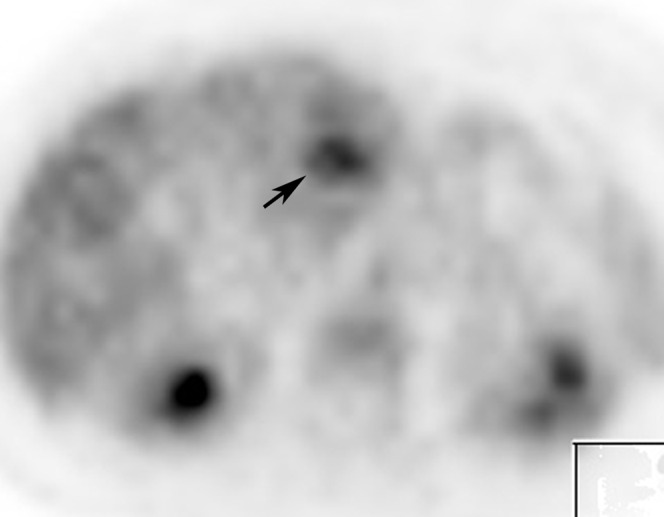
Axial 18F-fluoro-2-deoxy-d-glucose-positron emission tomography image of the abdomen shows similar tracer uptake in the lesion to adjacent liver parenchyma, except for a hypermetabolic focus (arrow) in the centre of the lesion.
Figure 3.
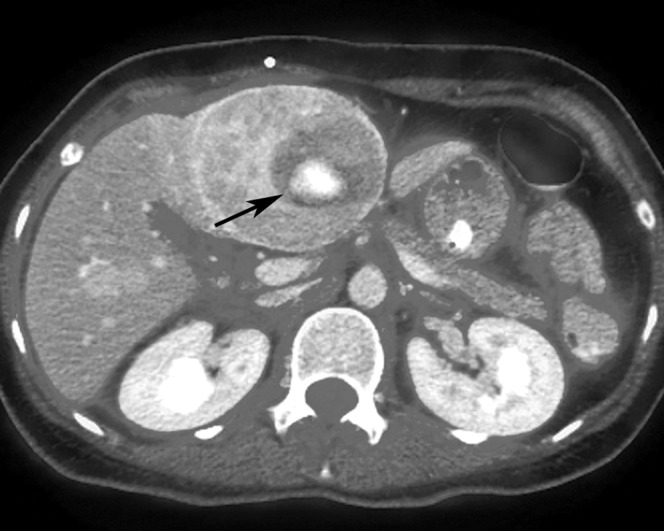
Axial positron emission tomography–CT fused image shows the hypermetabolic focus (arrow) is within the hypoenhancing centre of the lesion.
In addition, there were numerous metastatic nodules in both lungs with a maximal size of 1 cm. Local recurrence was also detected along the dura of the left tentorium cerebelli just beneath the craniotomy site (Figure 4). The lung and intracranial lesions showed no elevated FDG uptake.
Figure 4.
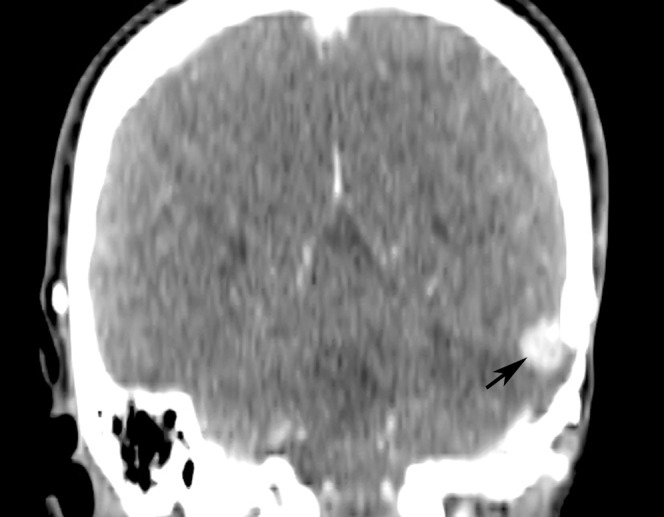
Reformatted coronal contrast-enhanced CT image of the brain shows an enhancing local recurrent tumour (arrow) along the left tentorium cerebelli beneath the craniotomy site.
Ultrasound-guided liver biopsy of the segment IVb lesion showed histological proof of metastatic haemangiopericytoma. Chemotherapy was arranged for the patient.
Discussion
Intracranial haemangiopericytoma is a rare tumour and it is difficult to make the correct pre-operative diagnosis based on clinical and imaging findings. Even on histological findings, there was much confusion about the origin of this tumour in the past. It was previously named “haemangiopericytic meningioma” because of its similar imaging findings to meningioma. Until 2000, the World Health Organization separated the tumour from meningioma owing to its distinctive histological features [3].
It is important to diagnose haemangiopericytoma as it tends to have local recurrence and its malignant type may lead to distant metastases. Complete surgical resection and adjuvant radiotherapy play an important role in the management of intracranial haemangiopericytoma [4]. However, complete resection may not be possible owing to its high vascularity and proximity to dural sinuses. Therefore, post-operative radiation therapy remains routine treatment even when complete resection is indicated during surgery. Because of the tumour's hypervascularity, neoadjuvant radiotherapy is also advocated to reduce intra-operative complications [5]. Distant metastasis tends to have an indolent growth. Liver and bone are common sites of metastasis and rare sites including the heart, pancreas and breast have been reported [6–8]. In our case, recurrence and distant metastases occurred 7 years after surgery.
Intracranial haemangiopericytoma shows very similar features to meningioma on both CT and MRI. It presents as a markedly enhancing extra-axial mass and could also show dural attachment and bone erosion, as in meninphygioma [9]. On angiography, it also shows marked vascularity similar to meningioma. Therefore, other imaging modalities and diagnostic methods are sought to increase the accuracy in reaching the diagnosis.
Previous PET studies on meningioma showed that the glucose metabolic rates of meningioma correlated with the tumour growth, and atypical meningioma had the highest glucose utilisation rate [10]. However, high-grade sacral meningioma metastasis showing only moderate metabolic activity on FDG-PET images has been reported [11]. Since haemangiopericytoma is even rarer than meningioma, the current literature on its PET findings is scarce. A previous case report found that haemangiopericytoma showed increased uptake of 11C-methionine and O-H2O but low uptake on 18F-FDG [12]. In our case, most of the recurrent haemangiopericytomas showed avid enhancement on CT but low FDG uptake on PET, indicative of low glucose utilisation. However, the dominant recurrent lesion in the liver demonstrated an area of hypoenhancement on CT that showed increased FDG uptake on PET. This kind of tumour imaging feature has not been reported previously. One case report on hepatic metastasis from haemangiopericytoma reported a central hypoenhancing area in the metastasis. However, the hypoenhancing area was necrotic and hypometabolic, whereas the enhancing portion of the metastasis was hypermetabolic [13]. We believe it is important to be aware of the variability in imaging features of haemangiopericytomas, although the underlying reason for these differences in imaging features is uncertain. We speculate that this variability in imaging features reflects the heterogeneity of histological or biological features of haemangiopericytomas and needs to be further explored.
FDG-PET may be helpful in differentiating necrotic tumours from hypoenhancing but viable tumours, both of which are hypoenhancing on CT but demonstrate different metabolic activities on PET, and thus potentially guide treatment decisions. However, further evaluation of 18F-FDG-PET-CT use and other molecular imaging techniques, and correlation of the imaging findings with histological features, would be helpful.
References
- 1.Pateau A, Zoltick P, Miettinen M. CD 34 immunoreactivity in nervous system tumors. Acta Neuropathol 1994;88:454–8 [DOI] [PubMed] [Google Scholar]
- 2.Perry A, Scheithauer BW, Nascimento AG. The immunophenotypic spectrum of meningeal hemangiopericytoma: a comparison with fibrous meningioma and solitary fibrous tumor of meninges. Am J Surg Pathol 1997;21:1354–60 [DOI] [PubMed] [Google Scholar]
- 3.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumours of the nervous system. J Neuropathol Exp Neurol 2002;61:215–29 [DOI] [PubMed] [Google Scholar]
- 4.Fountas K, Kapsalaki E, Kassam M, Feltes C, Dimopoulous V, Robinson J, et al. Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev 2006;29:145–53 [DOI] [PubMed] [Google Scholar]
- 5.Someya M, Sakata KL, Oouchi A, Nagakura H, Satoh M, Hareyama M. Four cases of meningeal hemangiopericytoma treated with surgery and radiotherapy. Jpn J Clin Oncol 2001;31:548–52 [DOI] [PubMed] [Google Scholar]
- 6.Spatola C, Privitera G. Recurrent intracranial hemangiopericytoma with extracranial and unusual multiple metastases: case report and review of the literature. Tumori 2003;90:265–8 [DOI] [PubMed] [Google Scholar]
- 7.Chakravarty BJ, Munn S, Lane MR. Hepatic metastases from a meningeal hemangiopericytoma. Aust N Z J Med 1991;21:884–5 [DOI] [PubMed] [Google Scholar]
- 8.Koyama H, Harada A, Nakao A, Nomany T. Intracranial hemangiopericytoma with metastasis to the pancreas. J Clin Gastroenterol 1997;25:706. [DOI] [PubMed] [Google Scholar]
- 9.Chiechi MV, Smirniotopoulos JG, Mena H. Intracranial hemangiopericytoma: MR and CT features. Am J Neuroradiol 1996;17:1365–71 [PMC free article] [PubMed] [Google Scholar]
- 10.Chiro GD, Hatazawa J, Katz DA, Rizzoli HV, De Micehele DJ. Glucose utilization by intracranial meningioma as an index of tumour aggressivity and probability of recurrence: a PET study. Radiology 1987;164:521–6 [DOI] [PubMed] [Google Scholar]
- 11.Ghodsian M, Obrzut SL, Hyde CC, Watts WJ, Schiepers C. Evaluation of metastatic meningioma with 2-deoxy-2-[18F]fluoro-D-glucose PET/CT. Clin Nucl Med 2005;30:717–20 [DOI] [PubMed] [Google Scholar]
- 12.Kracht LW, Bauer A, Herholz K, Terstegge K, Friese M, Schroder M, Heiss WD. Positron emission tomography in a case of intracranial hemangiopericytoma. J Comput Assist Tomogr 1999;23:365–8 [DOI] [PubMed] [Google Scholar]
- 13.Kim BW, Wang HJ, Jeong IH, Ahn SI, Kim MW. Metastatic liver cancer: a rare case. World J Gastroenterol 2005;11:4281–84 [DOI] [PMC free article] [PubMed] [Google Scholar]


