Abstract
A 54-year-old man presented for radiology with pain and a feeling of fullness in the upper abdomen and an epigastric mass. Ultrasound revealed a large cystic mass with internal echoes, lying posterior and inferior to left lobe of the liver. The gallbladder was thick-walled and contracted, and contained a calculus and echogenic sludge. A cystic structure that produced swirling flow signals on colour Doppler was demonstrated within the gallbladder. The CT scan showed a thickened gallbladder with adjacent inflammation and a 2-cm pseudo-aneurysm in its wall. High-density material was present in the gallbladder lumen, in the extra-hepatic bile ducts and around the gastrohepatic ligament. A thick haemorrhagic pus, from which Escherichia coli was cultured, was drained from the gastrohepatic collection. An elective coeliac angiogram demonstrated a solitary pseudo-aneurysm of the medial branch of the cystic artery. Selective catheterisation of this artery with a micro-catheter enabled complete exclusion of the pseudo-aneurysm by a single micro-coil. Histological examination of the gallbladder, which was ultimately removed at open cholecystectomy, demonstrated xanthogranulomatous cholecystitis.
Cystic artery pseudo-aneurysm (CAP) is a very rare entity [1]; only 14 cases have been described in the literature, and its precise incidence is not known. Patients usually present with haemobilia, rarely with upper gastrointestinal bleeding and haemoperitoneum.
Most patients have a history of acute cholecystitis or have undergone a cholecystectomy. There is only one previously reported case in which a cystic artery aneurysm was associated with xanthogranulomatous cholecystitis (XGC). This patient presented with obstructive jaundice, later developed haemobilia and was diagnosed on CT as having CAP [2–4].
Here, we report a second patient with XGC and CAP, who presented with gastrohepatic ligament haematoma and haemobilia. We have not encountered this particular combination of pathologies in the published English language literature, and, to the best of our knowledge, this is the first report of this presentation. The CAP was successfully treated with coil embolisation followed by elective open cholecystectomy. Another distinct feature of this case is the imaging presentation of XGC, which does not match that described in previously published reports.
Case report
A 54-year-old man was referred for an ultrasound of the upper abdomen with a complaint of epigastric pain. The patient was under treatment for a superficial transitional cell carcinoma (TCC) of the urinary bladder and had undergone a transurethral resection of the lesion two months prior to presentation. one month after tumour removal, he developed vague abdominal pain. On the initial consultation, no definite clinical diagnosis could be made and the patient was symptomatically treated with analgesics. On follow-up, an epigastric mass was found on clinical examination.
This led to the referral for an ultrasound scan, which revealed a large cystic mass with internal echoes posterior and inferior to the left lobe of the liver. The gallbladder had thickened walls (17.5 mm) and was contracted, containing a calculus and echogenic sludge. In addition, a rounded cystic structure was present within the lumen of the gallbladder. This structure demonstrated swirling flow signals on colour Doppler evaluation. There was no pericholecystic fluid or ascites. The appearances on the ultrasound and colour Doppler scans were considered to be consistent with a pseudo-aneurysm.
CT scans of the abdomen were carried out with intravenous contrast in the arterial and venous phases because we already knew that there was a pseudo-aneurysm in the lumen of the gallbladder. These scans confirmed the thick-walled gallbladder with adjacent inflammation and a 2-cm pseudo-aneurysm in the gallbladder wall (Figure 1). The CT scans also showed high-density material in the lumen of the gallbladder and in extra-hepatic bile ducts but no intra- or extrahepatic biliary dilation. A high-density collection was present in the gastrohepatic ligament. Right-sided pleural effusion was also seen.
Figure 1.
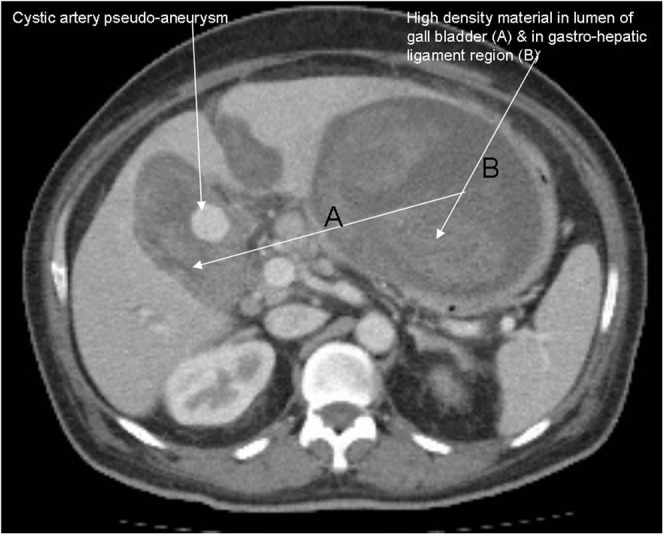
Contrast-enhanced CT scan of the abdomen revealed an enhancing rounded pseudo-aneurysm of the cystic artery, together with high-density material in the lumen of the gallbladder and in the region of the gastrohepatic ligament.
Following the CT scan, the patient was admitted to hospital. On admission, his vitals were stable and he was afebrile. His physical examination was unremarkable except for the presence of the tender epigastric mass. His haemoglobin was 11 g l−1, white cell count was 19 × 109 l−1 with 86.7% neutrophils and platelets were 289& × 109 l−1. Prothrombin time (PT) was 14.2 s with a control of 11 s, activated partial thromboplastin time (aPTT) was 32.5 s with a control of 30 s and blood coagulation measured at an International normalized ratio of 1.35.
A 10-French drainage catheter was placed in the gastrohepatic collection under image guidance. Thick haemorrhagic pus was aspirated and sent for culture and sensitivity tests. Escherichia coli colonies were subsequently isolated on the cultures. Approximately 400 ml of haemorrhagic pus was drained after placement of the catheter. Repeat haemoglobin was 7.6 g l−1. The patient was transfused two units of whole blood.
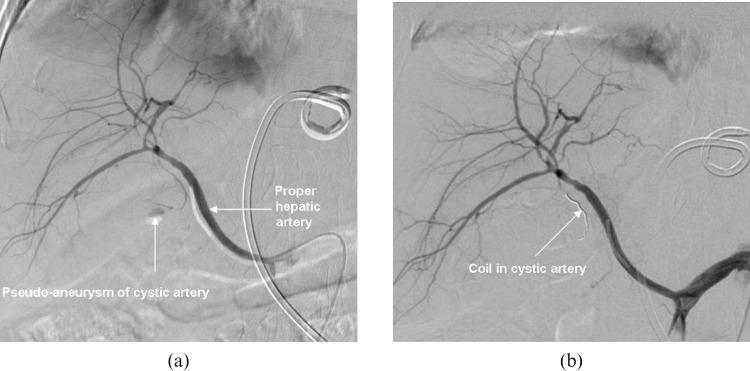
The next day, an elective coeliac angiogram was performed that demonstrated a solitary pseudo-aneurysm arising from the medial branch of the cystic artery, which is a branch of a replaced right hepatic artery (Figure 2). Selective catheterisation of the medial branch of the cystic artery was performed with a micro-catheter, followed by the deployment of a micro-coil in the artery. Complete exclusion of the pseudo-aneurysm was achieved.
Figure 2.
Digital subtraction angiography (DSA) images of the hepatic artery. (a) There is a pseudo-aneurysm in the medial branch of the cystic artery. (b) The artery supplying the pseudo-aneurysm is embolised with a coil. (Note: a drainage catheter can be seen in gastrohepatic ligament region.)
On a follow-up CT scan the gallbladder remained abnormal, with wall thickening and pericholecystic inflammatory changes, but the pseudo-aneurysm was not opacified. The gastrohepatic ligament collection was reduced in size. The drainage catheter was readjusted under ultrasound guidance and this resulted in almost complete resolution of this collection, as demonstrated on a repeat ultrasound.
As the patient was stable, he was discharged with a plan for an interval open cholecystectomy, which was performed within a month. The histopathology report on the gallbladder specimen showed acute and chronic inflammatory cell infiltrate along with foamy histiocytes; focal areas showed foreign body giant cell reaction, granulation tissue and areas of haemorrhage. No malignancy was seen. The histopathological findings are consistent with XGC.
Discussion
Kazuaki et al [2] described the first case of CAP with XGC in 2008. We have been able to find 14 reports of CAP in the published literature, and most of these cases were associated with cholecystitis or laproscopic cholecystectomy.
In our case, the patient had been suffering from epigastric pain for a month and his diagnosis was possibly delayed because of his previous procedure for TCC of the bladder. Sonographically, the patient had cholecystitis with calculus and sludge in the lumen of the gallbladder. The xanthogranulomatous nature of the underlying pathology was only revealed by the histopathological assessment.
XGC is characterised histologically by multiple yellow-brown intramural nodular formations, severe proliferative fibrosis and foamy macrophages. Although the exact causation is not certain, it is generally agreed that rupture and intramural extravasation of the inspissated bile and mucin from the occlusion of Rokitansky–Aschoff sinuses is the main cause for the development of XGC. The other possibile cause is gallstones with bile stasis and a chronic inflammatory reaction that provokes degeneration and necrosis of the gallbladder wall with subsequent intramural abscess formation. The intramural abscesses are subsequently replaced by xanthogranuloma with foamy histiocytic foreign body giant cells [5]. Perivascular inflammation causes thrombosis of vasa vasorum with damage to adventitia. These vessels are prone to rupture with the formation of pseudo-aneurysms [3, 5].
The most common presentation of CAP is with haemobilia and rarely with upper gastrointestinal bleeding or haemoperitoneum. In our case, the patient presented with vague abdominal pain and a mass in the epigastric region, which on subsequent imaging was revealed as a large collection in the gastrohepatic ligament. Haemobilia is caused by erosion of the artery by inflammation and stones. When the artery is weakened and an aneurysm is formed, it can erode into the biliary system, sometimes with the involvement of adjacent structures, resulting in upper gastrointestinal bleeding or haemoperitoneum. Surgery is the mainstay of treatment for XGC, but the primary treatment for CAP is endovascular embolisation, which has good results [6].
Conclusion
Cystic artery aneurysm associated with XGC is an extremely rare phenomenon. These pseudo-aneurysms may be safely treated with transcatheter techniques.
References
- 1.Atsuyuki M, Takao K, Satomi S, Keiya A, Toru M, Noriji N, et al. Pseudoaneurysm of the cystic artery with hemobilia treated by arterial embolization and elective cholecystectomy. J Hepatobiliary Pancreat Surg 2002;9:755–8 [DOI] [PubMed] [Google Scholar]
- 2.Kazuaki S, Yoshihiro S, Minoru E, Tomoo K. Pseudoaneurysm of the cystic artery associated with xanthogranulomatous cholecystitis. Dig Surg 2008;25:8–9 [DOI] [PubMed] [Google Scholar]
- 3.Saluja SS, Ray S, Gulati MS, Pal S, Sahni P, Chattopadhyay TK. Acute cholecystitis with massive upper gastrointestinal bleed: a case report and review of the literature. BMC Gastroenterol 2007;26:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghoz A, Kheir E, Kotru A, Halazun K, Kessel D, Patel JJ, et al. Hemoperitoneum secondary to rupture of cystic artery pseudoaneurysm. Hepatobiliary Pancreat Dis Int 2007;6:321–3 [PubMed] [Google Scholar]
- 5.Chun KA, Ha HK, Yu ES, Shinn KS, Kim KW, Lee DH, et al. Xanthogranulomatous cholecystitis: CT features with emphasis on differentiation from gallbladder carcinoma. Radiology 1997;203:93. [DOI] [PubMed] [Google Scholar]
- 6.Andreas G, Johannes G, Elmar MM. Endovascular treatment of visceral artery aneurysms. J Endovasc Ther 2002;9:38–47 [DOI] [PubMed] [Google Scholar]