Abstract
A comparative analysis of low linear energy transfer (LET) γ-radiation-induced damage in the lymphatic tissue of a tropical seasonal breeder, Indian palm squirrel (Funambulus pennanti), during its reproductively active phase (RAP) and inactive phase (RIP) was performed with simultaneous investigation of the effects of long-term melatonin pre-treatment (100 μg/100 g body weight). A total of 120 squirrels (60 during RAP and 60 during RIP) were divided into 12 groups and sacrificed at 4, 24, 48, 72 and 168 h following 5 Gy γ-radiation exposure; control groups were excluded from exposure. Total leukocyte count and absolute lymphocyte count (ALC) and melatonin only of peripheral blood, stimulation index, thiobarbituric-acid-reactive substances (TBARS) level, superoxide dismutase (SOD) activity, and the apoptotic index of spleen as analysed by terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick-end labelling (TUNEL) noted at observed time-points were significantly reduced in melatonin pre-treated groups during RAP and RIP. Long-term melatonin pre-treatment mitigated radiation-induced alterations more prominently during RIP, as assessed by ALC, TBARS, SOD, TUNEL and caspase-3 activity, at some time-points. Our results demonstrate an inhibitory role of melatonin on caspase-3 activity in splenocytes during RAP and RIP following γ-radiation-induced caspase-mediated apoptosis. Hence, we propose that melatonin might preserve the viability of immune cells of a seasonal breeder against background radiation, which is constantly present in the environment.
The increasing number of personnel working with radiation, together with the development of nuclear weapons, stimulated an interest in the study of radiation effects on immunity. Radiation-induced injury due to free radicals and reactive oxygen intermediates (ROI) could impair specific immune responses. Besides ultraviolet (UV) radiation, a common environmental stressor that influences animals [1] and humans [2], X-rays and γ-rays are readily available from natural and artificial sources [3–5]. The earth and all living things are constantly bombarded by the background radiation present in soil, water, earth and vegetation [6, 7]; one of the major contributors to background radiation is γ-radiation [8, 9]. Funambulus pennanti (Indian tropical palm squirrel), a photoperiodic seasonal breeder that lives close to human populations, is exposed daily to solar radiation and UV as well as to background radiation. Immune functions of this squirrel are always under the effect of environmental and biotic factors, such as ambient temperature, food availability, shelter, rainfall, humidity and other stressful conditions, with hormonal variations in different seasons. As F. pennanti is sensitive to seasonal changes and stressors, it is a suitable model for studying high-dose γ-radiation effects on the immune function under seasonally recurrent stressors. Reports regarding the destructive effect of low linear energy transfer (LET) ionising radiation in the lymphatic tissues of mammalian species, such as laboratory mice and rats [10, 11], do exist, but studies in seasonal breeders are scarce. Previous studies in seasonal breeders involved only seasonal stress [12].
Melatonin plays an important role in the physiology of all mammalian systems, especially in seasonal breeders, as it adjusts the circadian and circa-annual rhythm of an organism with the environment [13–15]. F. pennanti exhibits seasonal variations in reproduction, survival and immune functions with photoperiod [16]. As a consequence, melatonin may coordinate reproductive, immunological and other physiological processes to cope successfully with energetic stressors during winter [17]. The annual reproductive cycle of F. pennanti has two crucial reproductive phases: the reproductively active phase (RAP), when melatonin levels are low and steroid levels are comparatively high in the circulation for reproductive success; and the reproductively inactive phase (RIP), when endogenous melatonin levels are higher than those of steroids for maximal immune responses against adverse environmental conditions [16, 18].
In addition to numerous important properties [19], melatonin has potential clinical applications [20]. The direct free radical-scavenging [21] and indirect antioxidant stimulatory [22, 23] properties of melatonin have been used in several studies for radioprotection. Recently, we have documented the effects of 2.06 Gy low-LET X-rays on F. pennanti during both RAP [24] and RIP [25] and investigated the protection afforded by melatonin. However, nothing is known about the effects of low-LET γ-radiation on tropical seasonal breeders during the two different phases (RAP and RIP) of the immune status within the body. Moreover, it is unknown whether melatonin can protect these breeders from radiation when they are under the influence of seasonally recurrent stressors of RAP and RIP.
X-rays and γ-rays are low-LET radiations that differ only in their origins. γ-Rays are essentially very energetic X-rays, similar in nature to X-rays but of shorter wavelength. The relative biological effectiveness (RBE) of X-rays and γ-rays is the same, but depends on the type of radiation, the energy of the particles and on the total dose administered. The amount of ionisation is directly correlated to the amount of energy deposited and tissue penetration [26, 27]. Owing to these properties, a 5 Gy dose of γ-rays might result in more dense ionisation of biomolecules than that caused by the previously used dose of 2.06 Gy X-rays [24, 25]; this could pose a challenge for melatonin in the modulation of γ-radiation effects. This is the first study aimed at comparing 5 Gy γ-radiation effects during RAP and RIP. We have also carried out a simultaneous investigation of long-term melatonin pre-treatment effectiveness against high-dose γ-radiation in the radiosensitive lymphatic tissue of F. pennanti.
Early research on radiation protection has unravelled the basic mechanisms and yielded a large number of radioprotectors; however, most of these compounds failed the transition from laboratory to clinic, owing to their acute toxicity and inability to differentiate between tumour and normal cells. Melatonin has emerged as a promising compound, both acting as a radioprotector and having an oncostatic role, but so far it has not been utilised for clinical radiotherapy. Our results will provide information on the health, survival and balancing capability of tropical semi-wild squirrels against background radiation, when they are already under the influence of other stressors and while coping with seasonal fluctuations during two varied reproductive phases. Not only are experiments with ionising radiation important from a radiation oncology point of view, but the results from this study should move us a step closer towards the clinical use of melatonin as a tool for preventing potential radiation-induced disorders.
Methods and materials
Animal care and maintenance
Experiments on animals were conducted in accordance with institutional practice and within the framework of the Revised Animals (Scientific Procedures) Act of 2002 of the Government of India on Animal Welfare. All adult male squirrels (F. pennanti), weighing 100 ± 120 g during RAP (May) and RIP (December), were obtained from local animal suppliers of Varanasi (latitude 25°18′ N; longitude 83°01′ E). Squirrels were kept in a well-ventilated animal room at room temperature (25 ± 2°C) and with a photoperiod of light and dark of 12 h:12 h (lights on from 6:00 am to 6:00 pm) during RAP and 10 h light:14 h dark cycle (lights on from 7:00 am to 5:00 pm) during RIP. Animals were fed soaked gram seed (Cicer arietinum), seasonal nuts and grains along with water ad libitum.
Treatments
Chemicals were purchased from Sigma-Aldrich Chemicals (St. Louis, MO). A stock solution of melatonin (10 mg ml−1) was prepared in ethanolic saline (0.9% NaCl and 0.01% absolute ethanol) and further diluted in saline to obtain 100 μg/100 g body weight of working concentration. The working concentration was the minimal effective dose selected after standardising with different doses (25, 50, 100, 250, 500 μg/100 g body weight) of melatonin. The injections were given subcutaneously (sc) in red light during evening hours (at 6:00 pm during RAP and at 5:00 pm during RIP).
Irradiation
The cobalt-60 teletherapy unit (Theratron, Atomic Canada Ltd, Canada) at Indian Railways Cancer Research Centre, Varanasi, India, was used for γ-radiation. The energy of administered 60Co γ-rays (LET) was 0.3 keV μ−1. A particular area (3 cm by 4 cm) of the upper left abdominal region of the squirrels was shaved and marked in order to ensure proper exposure and absorption of γ-rays onto the splenic region. The area below the γ-source where squirrels were to be exposed to irradiation was also marked and squirrels were immobilised by anaesthetising prior to irradiation to ensure reproducibility of the irradiated volume. The shaved and marked abdominal region was exposed with a dose rate of 1.12 Gy min−1 in a single fraction of γ-radiation. Skin-to-source distance was 80 cm to achieve a total absorbed dose of 5 Gy. Dose is the energy of radiation absorbed per given unit mass of the material. The energy absorbed would be nearly 0.0015 J.
Experimental design
After acclimatisation for 2 weeks to laboratory conditions during RAP, 60 male squirrels were divided into two sets of 30 squirrels each. Set 1 received 0.9% normal saline (NaCl) and set 2 received 100 μg melatonin/100 g body weight daily for 4 weeks (i.e. continuous 28 days' treatment). 12 h after treatment with the last dose, 25 saline-treated squirrels from set 1 and 25 melatonin-treated squirrels from set 2 were anaesthetised with 25 mg kg−1 thiopental sodium (sc) and exposed to 5 Gy of γ-radiation. Following exposure to γ-radiation, five squirrels from set 1 and five from set 2 were sacrificed at 4 h, 24 h, 48 h, 72 h and 168 h, and spleen and peripheral blood collected. The remaining five animals from each set were not irradiated and were used as a control group and a melatonin-treated group, respectively. A similar experiment, with divisions of another 60 animals into control, melatonin-treated, irradiated and melatonin-treated plus irradiated groups, was performed during RIP.
Total leukocyte count
Peripheral blood was collected directly from the heart under either anaesthesia using a heparinised leukocyte pipette. Samples were used to evaluate the total leukocyte count (TLC) (number mm−3) in Neubauer's counting chamber (Paul Marienfeld GmBH & Co. KG, Lauda-Königshofen, Germany) under a Nikon microscope (Nikon, Kawasaki, Japan).
Absolute lymphocyte count
A thin film of blood was prepared and stained with Leishman's stain, and lymphocyte number (number mm−3) was counted under an oil immersion lens of the microscope (Leitz MPV3, Wetzlar, Hessen, Germany).
Lymphocyte proliferation assay
To assess the cell-mediated immune response (i.e. blastogenic response to a mitogen), spleens were minced with sterile blades and passed through a sterile, stainless steel wire mesh. Splenic cells were suspended in RPMI-1640 medium and erythrocytes lysed by ice-cold 0.84% Tris-NH4Cl incubation for 10 min. The cell suspension was washed in culture medium before determining cell viability and cell count. Splenocyte suspensions in culture medium supplemented with 10% foetal bovine serum (FBS), 100 μg ml−1 streptomycin and 100 U ml−1 penicillin were seeded in 35 mm sterile culture plates at a density of 1 × 106 cells per plate with and without the T-cell mitogen concanavalin A (Con A, 10 μg ml−1). Plates were kept in a 37°C incubator with 5% CO2 atmosphere for 72 h. Cells were pulsed with 1 μCi of [3H]-thymidine (BARC, Mumbai, India) 18 h before the end of the incubation period. Cultures were harvested and transferred in scintillation tubes containing 5 ml of scintillation cocktail and counted in a beta liquid scintillation counter (Beckman, CA). The results were reported as the stimulation index (SI) (i.e. the ratio of cpm readings between mitogen-stimulated cells and non-stimulated cells).
Lipid peroxidation assay by TBARS level estimation
All spleens were excised and weighed for preparing 10% tissue homogenates in 20 mM Tris-HCl buffer (pH 7.4). To prevent new lipid peroxidation during homogenisation, butylated hydroxytoluene (BHT, 2.8 mM) was added to the samples. Homogenates were centrifuged at 3000 g for 15 min at 4°C. The supernatant was subjected to thiobarbituric acid (TBA) assay by reacting an aliquot with 8.1% sodium dodecyl sulphate (SDS), 20% cold acetic acid, 0.8% TBA and distilled water in a boiling water bath for 1 h to yield a chromogenic product. The reaction mixture was immediately cooled in running water and vigorously shaken with n-butanol and pyridine reagent (15:1); the sample was then centrifuged for 10 min at 1500 g to extract thiobarbituric acid-reactive substances (TBARS). The absorbance of the upper phase was read at 534 nm at 25°C [28]. The product concentration was expressed as TBARS level in nmol g−1 tissue weight using 1,1,3,3-tetraethoxy propane (TEP) as the source for the standard curve.
Superoxide dismutase activity assay
Superoxide dismutase (SOD) activity measurement was based on the ability of the enzyme to inhibit nitrite formation by superoxide radicals. Tissues were washed with 0.9% NaCl and 10% tissue homogenates were prepared in 150 mM phosphate-buffered saline (PBS, pH 7.4) and centrifuged for 45 min at 12 000 g at 4°C. To 0.5 ml of homogenate, 1.4 ml of reaction mixture (50 mM phosphate buffer, 20 mM l-methionine, 1% Triton X-100, 10 mM hydroxylamine hydrochloride, 50 mM EDTA) was added followed by pre-incubation at 37°C for 5 min. After adding 0.8 ml of riboflavin to all mixtures, including a control containing only buffer, samples were exposed to a 24 W fluorescent lamp fitted in an aluminium foil-coated wooden box. This exposure resulted in the photogeneration of superoxide anion upon illumination of riboflavin. After 10 min, 1 ml of freshly prepared Greiss reagent (1% sulphanilamide in 5% orthophosphoric acid, 0.1% N-1-naphthylethylenediamine dihydrochloride in distilled water) was added. The absorbance of diazo dye, formed as a function of nitrite concentration, was read at 543 nm at 25°C [29]. One unit (U) of SOD activity (defined as the amount of SOD inhibiting 50% nitrite formation under assay conditions) was expressed as SOD activity in U g−1 tissue weight.
DNA fragmentation detection by TUNEL assay
DNA fragmentation was detected using a terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick-end labelling (TUNEL) kit according to the manufacturer's instructions (R&D Systems, Inc. MN). Cells were fixed in 3.7% formaldehyde in PBS for 15 min at 25°C and then placed separately on clean glass slides and air-dried. The slides were treated with 0.05 ml proteinase K solution for 30 min, incubated with quenching solution for 3–4 min, immersed in 1×TdT labelling buffer for 5 min and incubated with 0.05 ml labelling reaction at 37°C for 1 h. The reaction was stopped by immersing slides in 1×TdT stop buffer. Slides were then incubated with 0.05 ml diluted (1:500) anti-bromodeoxyuridine (BrdU) at 37°C for 1 h, washed with PBS containing 0.05% Tween-20 and treated with 0.05 ml streptavidin–horseradish peroxidase solution for 10 min. TUNEL signals were visualised by immersing slides in diaminobenzidene (DAB) solution for 5 min. Slides were counter-stained in methyl green solution for 2 min and analysed for TUNEL-positive staining under a phase-contrast microscope at 400× magnification (Nikon, Tokyo, Japan). More than 300 cells per sample on a randomly selected area were counted to detect DNA fragmentation. Cells staining with brown fluorescence and showing cytoplasmic shrinkage and membrane blebbing features were as apoptotic cells and the number of such cells was expressed as the percentage TUNEL positivity of the total sample. Normal cells stained green, showing an intact membrane and no chromatin condensation (see the Results section).
Detection of caspase-3 activity of splenocytes
Splenic cell suspensions from both the RAP and RIP groups (control, melatonin-treated, 4 h irradiation and melatonin pre-treated plus 4 h irradiation) were prepared in ice-cold 1×PBS, and erythrocytes were lysed by incubating in 0.84% Tris-NH4Cl for 10 min at 4°C. After washing, cell pellets were collected by centrifugation at 500 g for 10 min at 4°C and the supernatant was gently removed. Cell pellets were lysed by the addition of 50 μl of cold lysis buffer (5 mM Tris, 20 mM EDTA, 0.5% Triton-X 100, pH 6.0) per 2 × 106 cells and incubated on ice for 10 min. Lysates were centrifuged at 10 000 g for 1 min at 4°C, and the supernatant was transferred to a fresh tube and processed for caspase-3 activity using a caspase-3 colorimetric assay kit, according to manufacturer's instructions (R&D Systems, Inc. MN). Each enzymatic reaction, carried out in a 96-well flat bottom microplate, required 50 μl cell lysate, 50 μl reaction buffer and 5 μl caspase-3 colorimetric substrate (DEVD-pNA). The plate was incubated at 37°C for 2 h with a substrate blank and sample blank. At the end of the incubation period, the absorbance of enzymatically released chromophore p-nitroanilide (pNA) was read at 405 nm in a microplate reader (Tecan, Spectra II-microelisa plate reader, Austria). Caspase-3 activity was determined by comparing the absorbance or optical density (OD) of pNA from apoptotic samples with the untreated control and expressed as fold increase in OD405/106 cells per ml.
Statistical analysis
Data were expressed as the mean±standard error of the mean (SEM) of at least five animals per group. Data comparisons were statistically analysed using ANOVA followed by Student's Newman–Keuls multiple range tests. Differences were considered to be statistically significant when p<0.05.
Results
Effect of γ-radiation and melatonin pre-treatment on total leukocyte count of peripheral blood
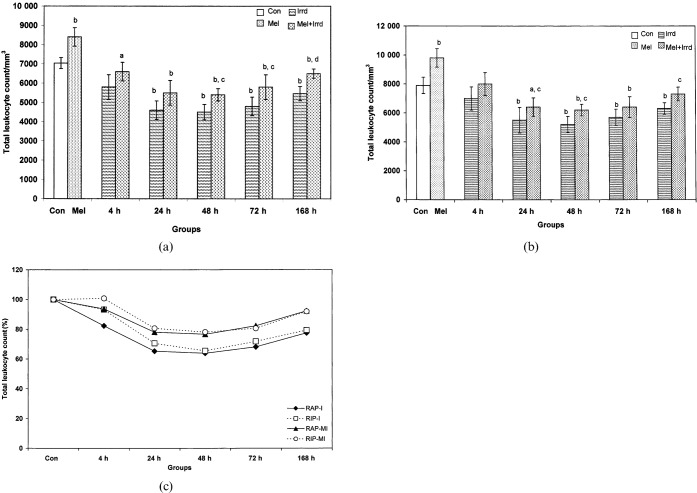
A significant decrease in peripheral TLC of irradiated groups during both RAP (Figure 1a) and RIP (Figure 1b) was noted, which was restored 168 h after exposure. There was a significant 1.2-fold decrease at 4 h (p<0.05), 1.53-fold at 24 h, 1.56-fold at 48 h, 1.46-fold at 72 h and 1.29-fold at 168 h (p<0.01) during RAP and a 1.43-fold decrease at 24 h, 1.52-fold at 48 h, 1.38-fold at 72 h and 1.25-fold at 168 h (p<0.01) during RIP, when compared with the corresponding controls. Melatonin pre-treatment significantly restored TLC by 1.2-fold at 48 h, 72 h (p<0.05) and 168 h (p<0.01) during RAP and by 1.19-fold at 48 h and 1.16-fold at 168 h (p<0.05) during RIP, when compared with corresponding irradiated groups.
Figure 1.
Effect of melatonin pre-treatment (100 μg/100 g body weight) on total leukocyte count (TLC) of Funambulus pennanti following exposure to 5 Gy γ-radiation. Animals were sacrificed after 4, 24, 48, 72 and 168 h during (a) the reproductively active phase (RAP, May) and (b) the reproductively inactive phase (RIP, December). (c) A comparison of TLC between RAP and RIP in irradiated (I) and melatonin-treated plus irradiated (MI) groups, in which controls of both phases were assumed to be 100%, was completed. Vertical bars represent mean±SEM (standard error of the mean), n _ 5 for each group. The statistical significance is indicated: a _ p<0.05 and b _ p<0.01 when compared with the control group; c _ p<0.05 and d _ p<0.01 when compared with the irradiated group. Con, control; Mel, melatonin only; Irrd, irradiation only; Mel+Irrd, melatonin treatment and irradiation.
Effect of γ-radiation and melatonin pre-treatment on absolute lymphocyte count of peripheral blood
We observed a highly significant drop (p<0.01) in the absolute lymphocyte count (ALC) of both irradiated RAP and RIP squirrels: 1.78- and 1.77-fold at 4 h, 2.04- and 1.88-fold at 24 h, 1.96- and 1.66-fold at 48 h, 1.79- and 1.25-fold at 72 h and 1.46- and 1.17-fold at 168 h, when compared with RAP and RIP controls, respectively. Melatonin pre-treatment significantly restored peripheral ALC by 1.54- and 1.54-fold at 4 h (p<0.01), 1.6- and 1.55-fold at 24 h (p<0.01), 1.6- and 1.5-fold at 48 h (p<0.01), 1.52- and 1.24-fold at 72 h (p<0.01), and 1.32-fold (p<0.05) and 1.29-fold (p<0.01) at 168 h, when compared with RAP and RIP irradiated groups, respectively (Figure 2a and b).
Figure 2.
Effect of melatonin pre-treatment (100 μg/100 g body weight) on absolute lymphocyte count (ALC) of Funambulus pennanti following exposure to 5 Gy γ-radiation. Animals were sacrificed after 4, 24, 48, 72 and 168 h during (a) the reproductively active phase (RAP) and (b) the reproductively inactive phase (RIP). (c) A comparison of ALC between RAP and RIP irradiated (I) and RAP and RIP melatonin-treated plus irradiated (MI) groups, in which controls of both phases were assumed to be 100%. Vertical bars represent the mean±SEM (standard error of the mean), n _ 5 for each group. The statistical significance is indicated: a _ p<0.05 and b _ p<0.01 when compared with the control group; c _ p<0.05 and d _ p<0.01 when compared with the irradiated group. Con, control; Mel, melatonin only; Irrd, irradiation only; Mel+Irrd, melatonin treatment and irradiation.
γ-Radiation-induced blastogenic responses of splenocytes
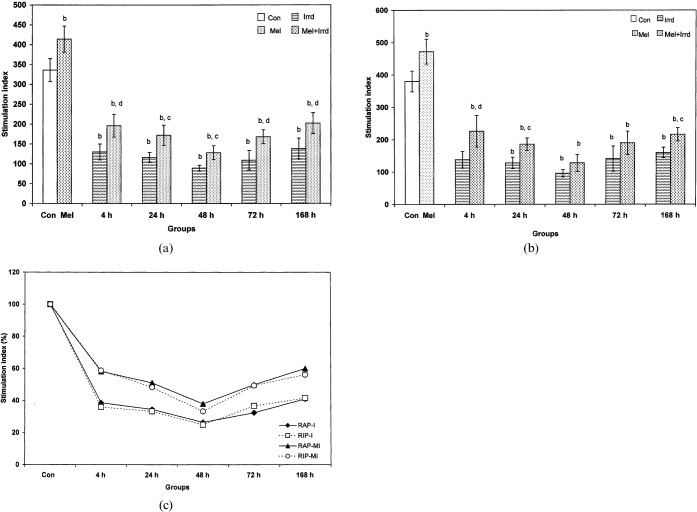
Significant radiation-induced inhibition of splenocyte proliferation (p<0.01) was noted at all time-points during RAP and RIP: 2.58-fold at 4 h, 2.89-fold at 24 h, 3.77-fold at 48 h, 3.08-fold at 72 h and 2.43-fold at 168 h (during RAP) and 2.75-fold at 4 h, 2.97-fold at 24 h, 3.96-fold at 48 h, 2.69-fold at 72 h and 2.37-fold at 168 h (during RIP), when compared with the corresponding controls. Melatonin pre-treatment could not induce splenocyte proliferation when compared with the control, but an increase was noted at all time-points (4 h, 72 h, 168 h, p<0.01; 24 h, 48 h, p<0.05) during RAP (Figure 3a) and at 4 h (p<0.01), 24 h and 168 h (p<0.05) during RIP (Figure 3b) when compared with the corresponding irradiated groups.
Figure 3.
Stimulation index (SI) of splenocytes against concanavalin A mitogenic challenge of Funambulus pennanti following exposure to 5 Gy γ-radiation. Animals were sacrificed after 4, 24, 48, 72 and 168 h during (a) the reproductively active phase (RAP) and (b) the reproductively inactive phase (RIP). (c) A comparison of SI between RAP and RIP irradiated (I) and RAP and RIP melatonin-treated plus irradiated (MI) groups, in which controls of both phases were assumed to be 100%. Vertical bars represent mean±SEM (standard error of the mean), n _ 5 for each group. The statistical significance is indicated: b _ p<0.01 when compared with the control group; c _ p<0.05 and d _ p<0.01 when compared with the irradiated group. Con, control; Mel, melatonin only; Irrd, irradiation only; Mel+Irrd, melatonin treatment and irradiation.
Melatonin pre-treatment ameliorated γ-radiation-induced oxidative injury in splenocytes
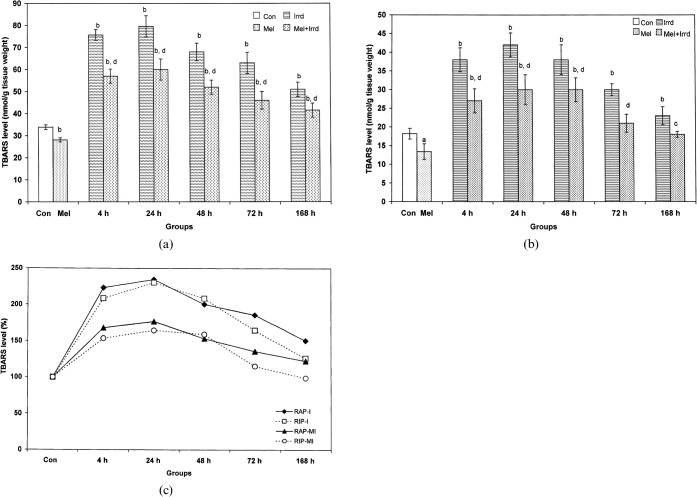
During RAP, 5 Gy γ-radiation significantly increased (p<0.01) the TBARS level of spleen at all time-points (2.24-fold at 4 h, 2.35-fold at 24 h, 2-fold at 48 h, 1.86-fold at 72 h, 1.5-fold at 168 h) when compared with controls. Melatonin pre-treatment significantly inhibited (p<0.01) the radiation-induced elevated TBARS level (1.33-fold at 4 h, 1.32-fold at 24 h, 1.3-fold at 48 h, 1.37-fold at 72 h, 1.23-fold at 168 h) when compared with the corresponding irradiated groups (Figure 4a). Like RAP, radiation significantly enhanced (p<0.01) the TBARS level during RIP (2.08-fold at 4 h and 48 h, 2.3-fold at 24 h, 1.65-fold at 72 h, 1.26-fold at 168 h) when compared with controls, whereas melatonin significantly reduced (p<0.05) the TBARS level (1.4-fold at 4 h and 24 h, 1.26-fold at 48 h, 1.43-fold at 72 h and 1.27-fold at 168 h) when compared with the corresponding irradiated groups (Figure 4b).
Figure 4.
Effect of melatonin pre-treatment (100 μg/100 g body weight) on thiobarbituric acid-reactive substances (TBARS) level in the spleen of Funambulus pennanti following exposure to 5 Gy γ-radiation. Animals were sacrificed after 4, 24, 48, 72 and 168 h during (a) the reproductively active phase (RAP) and (b) the reproductively inactive phase (RIP). (c) A comparison of the TBARS level between RAP and RIP irradiated (I) and RAP and RIP melatonin-treated plus irradiated (MI) groups, in which controls of both phases were assumed to be 100%. Vertical bars represent mean±SEM (standard error of the mean), n _ 5 for each group. The statistical significance is indicated: a _ p<0.05 and b _ p<0.01 when compared with the control group; d _ p<0.01 when compared with the irradiated group only. Con, control; Mel, melatonin only; Irrd, irradiation only; Mel+Irrd, melatonin treatment and irradiation.
γ-Radiation-induced effects on antioxidant activity and the role of melatonin as an antioxidant stimulator
Radiation significantly decreased (p<0.01) the total SOD activity of splenic tissue of reproductively active squirrels at all time-points (39.77-fold at 4 h, 22.5-fold at 24 h, 14.28-fold at 48 h, 8.78-fold at 72 h and 3.75-fold at 168 h) when compared with controls. Melatonin pre-treatment was unable to stimulate SOD activity at 4 h and 24 h, but we noted highly significant (p<0.01) increased activity from 48 h onwards (4.1-fold at 48 h, 3.96-fold at 72 h and 1.99-fold at 168 h) when compared with irradiated groups; however, the increase in activity of the melatonin pre-treated and irradiated groups was still very low (p<0.01) when compared with controls (Figure 5a). During RIP, SOD activity following γ-radiation remained unchanged except at 24 h and 48 h when a significant decrease (p<0.01) of 1.56-fold and 1.29-fold, respectively, compared with controls was noted. Melatonin pre-treatment significantly enhanced SOD activity by 1.33-fold at 4 h (p<0.05), 1.58-fold at 24 h (p<0.01) and 1.42-fold at 48 h (p<0.01) when compared with the corresponding irradiated groups (Figure 5b).
Figure 5.
Effect of melatonin pre-treatment (100 μg/100 g body weight) on superoxide dismutase (SOD) activity of the spleen of Funambulus pennanti following exposure to 5 Gy γ-radiation. Animals were sacrificed after 4, 24, 48, 72 and 168 h during (a) the reproductively active phase (RAP) and (b) the reproductively inactive phase (RIP). (c) A comparison of SOD activity between RAP and RIP irradiated (I) and RAP and RIP melatonin-treated plus irradiated (MI) groups, in which controls of both phases were assumed to be 100%. Vertical bars represent mean±SEM (standard error of the mean), n _ 5 for each group. The statistical significance is indicated: a _ p<0.05 and b _ p<0.01 when compared with the control group; c _ p<0.05 and d _ p<0.01 when compared with the irradiated group. Con, control; Mel, melatonin only; Irrd, irradiation only; Mel+Irrd, melatonin treatment and irradiation.
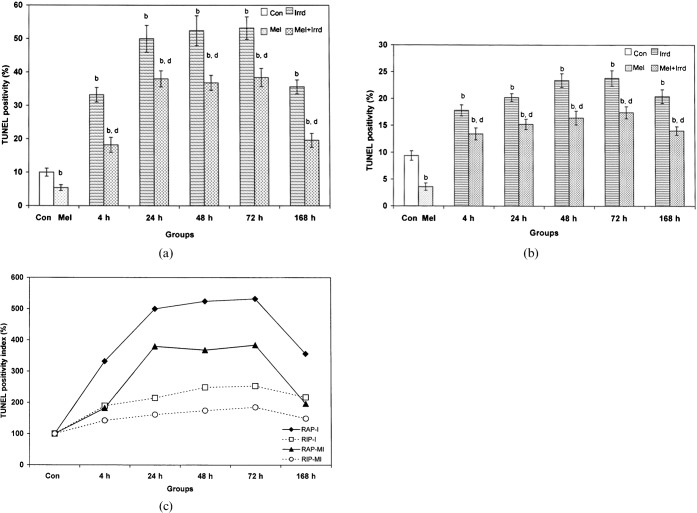
Melatonin pre-treatment restores γ-radiation-triggered apoptosis in splenocytes
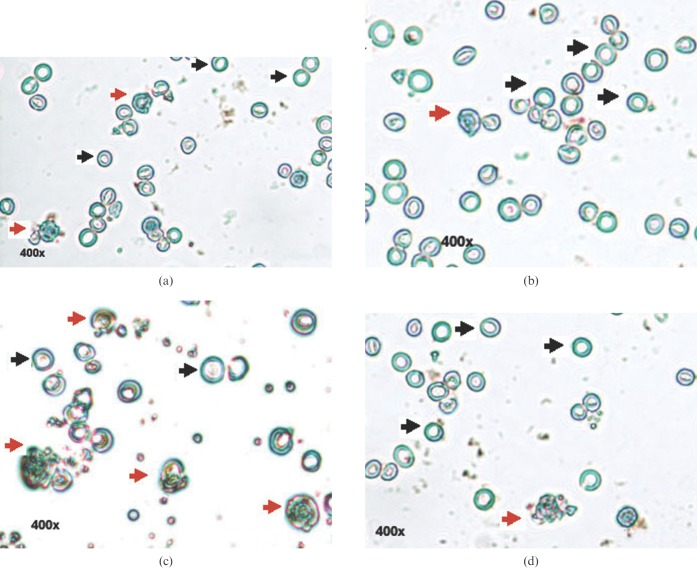
We observed TUNEL-positive staining (cells with brown fluorescence, indicated in the figures by black dotted arrows) in the nuclear region of cells of the irradiated groups with condensed chromatin and fragmented DNA (Figure 6c). The melatonin-pre-treated RAP and RIP groups showed a 1.85-fold and 2.6-fold decrease in TUNEL positivity, respectively, when compared with their corresponding controls (Figures 6a and b and 7a and b). The cells of the melatonin-pre-treated group were resistant to radiation, as indicated by the low index of TUNEL positivity (Figure 6d). 5 Gy γ-radiation significantly increased (p<0.01) the apoptotic index of splenocytes of RAP (Figure 7a) and RIP squirrels (Figure 7b) by, respectively, 3.32-fold and 1.89-fold at 4 h, 5-fold and 2.15-fold at 24 h, 5.24-fold and 2.49-fold at 48 h, 5.32-fold and 2.53-fold at 72 h, and 3.56-fold and 2.17-fold at 168 h when compared with their corresponding RAP and RIP controls. Melatonin pre-treatment significantly inhibited (p<0.01) TUNEL positivity and the apoptotic index in the RAP and RIP groups by, respectively, 1.82-fold and 1.33-fold at 4 h, 1.3-fold and 1.33-fold at 24 h, 1.42-fold and 1.43-fold at 48 h, 1.38-fold and 1.37-fold at 72 h, and 1.82-fold and 1.46-fold at 168 h when compared with the irradiated groups.
Figure 6.
DNA fragmentation detected using an apoptosis detection TUNEL kit. TUNEL-negative staining in (a) the control group and (b) the melatonin-treated group. (c) TUNEL-positive staining in the γ-irradiated group. (d) Melatonin decreased the number of TUNEL-positive cells. Cells visualised at 400× magnification: cells indicated with orange arrows are apoptotic and those indicated with black arrows are non-apoptotic.
Figure 7.
Inhibitory effect of melatonin pre-treatment on apoptosis or TUNEL positivity index of splenocytes of Funambulus pennanti following exposure to 5 Gy γ-radiation. Animals were sacrificed after 4, 24, 48, 72 and 168 h during (a) the reproductively active phase (RAP) and (b) the reproductively inactive phase (RIP). (c) A comparison of the TUNEL positivity index between RAP and RIP irradiated (I) and RAP and RIP melatonin-treated plus irradiated (MI) groups, in which controls of both phases were assumed to be 100%. Vertical bars represent mean±SEM (standard error of the mean), n _ 5 for each group. The statistical significance is indicated: b _ p<0.01 when compared with the control group; d _ p<0.01 when compared with the irradiated group. Con, control; Mel, melatonin only; Irrd, irradiation only; Mel+Irrd, melatonin treatment and irradiation.
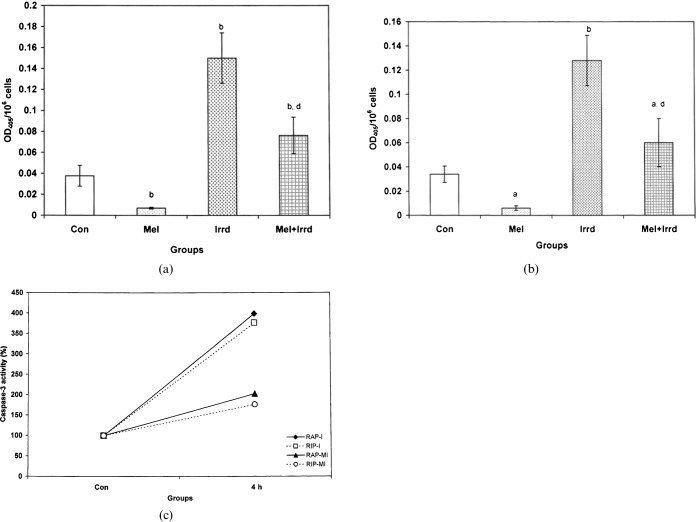
Effect of γ-radiation and melatonin on caspase-3 activity of splenocytes
There was a significant increase (p<0.01) in caspase-3 activity in the γ-irradiated groups: by 3.99-fold during RAP (Figure 8a) and by 3.76-fold during RIP (Figure 8b), when compared with controls. Melatonin-pre-treated groups showed resistance to γ-radiation with a significant decrease (p<0.01) of 2.03-fold and 1.76-fold in caspase-3 activity, when compared with the OD405 of the control, and a decrease of 1.97-fold and 2.13-fold when compared with irradiated groups during RAP and RIP.
Figure 8.
Effect of melatonin on 5 Gy γ-radiation-induced caspase-3 activity in the splenocytes of Funambulus pennanti. Animals were sacrificed after 4 h exposure during (a) the reproductively active phase (RAP) and (b) the reproductively inactive phase (RIP). (c) A comparison of caspase-3 activity between RAP and RIP irradiated (I) and RAP and RIP melatonin-treated plus irradiated (MI) groups, in which controls of both phases were assumed to be 100%. Vertical bars represent mean±SEM (standard error of the mean), n _ 5 for each group. The statistical significance is indicated: a _ p<0.05, b _ p<0.01 when compared with the control group; d _ p<0.01 when compared with the irradiated group. Con, control; Mel, melatonin only; Irrd, irradiation only; Mel+Irrd, melatonin treatment and irradiation.
Comparative analysis of radiation and melatonin effects between RAP and RIP squirrels
By assuming controls of all the parameters (TLC, ALC, SI, TBARS level, SOD activity, TUNEL positivity index, caspase-3 activity) to be 100%, a percentage of the mean of each parameter was calculated. On comparing splenocyte proliferation (SI) results of both RAP and RIP, we observed similar radiation-induced effects at all time-points during both phases (Figure 3c). However, saline-treated squirrels showed more radiation-induced damage during RAP than during RIP, as reflected in the TLC (4 h, 24 h), ALC (48 h, 72 h, 168 h), TBARS (4 h, 24 h, 72 h, 168 h), SOD (4 h, 24 h, 48 h, 72 h, 168 h), TUNEL poitivity (4 h, 24 h, 48 h, 72 h, 168 h) and caspase-3 results. Melatonin treatment significantly altered all the observed parameters in RAP and RIP melatonin-pre-treated plus irradiated groups, but the change followed a similar pattern to those observed in the irradiated only groups during both phases (Figures 1c, 2c, 3c, 5c, 7c and 8c). More attenuation of radiation-induced damage was noted during RIP with an increase in TLC (4 h), ALC (24 h onwards) and SOD activity (4 h onwards), and a decrease in TBARS level (4 h, 24 h, 72 h, 168 h), TUNEL positivity (4 h onwards) and caspase-3 activity. Marked differences in TLC at 4 h and 24 h (Figure 1c) and ALC from 48 h onwards (Figure 2c), TBARS level at 72 h and 168 h (Figure 4c), SOD activity at all time-points (Figure 5c), and TUNEL positivity index from 4 h onwards with a maximum at 24 h, 48 h and 72 h (Figure 7c) were noted between irradiated and melatonin-pre-treated plus irradiated groups during RAP and RIP.
Discussion
The immune status of F. pennanti varies with changes in the seasons. Immune status is at a maximum during RIP, as F. pennanti faces maximum challenges from nature during this period (November to January) when the tropical ambient temperature in North India is quite low (8 ± 2°C). Moreover, during this time, food grains and shelter are scarce and seasonal diseases emerge. This winter-bound stress increases free radical load and induced damage. During RAP, the immune status of these squirrels is comparatively low [16] for two reasons. First, there is a comparatively high level of circulatory gonadal steroids [18] and an energy trade-off towards maintaining high reproductive capacity in the body [30]. Second, melatonin synthesis and secretion are low under the long days–short night phase of summer (RAP), compared with the short day–long night phase of winter (RIP) [18]. To establish an interrelationship between melatonin and immune function following γ-radiation in tropical seasonal breeders (F. pennanti), we assessed the effect of long-term exogenous melatonin (100 μg/100 g body weight) during RAP and RIP when peripheral levels of melatonin are different. Long-term melatonin administration has been shown to influence the temporal circadian variations of biochemical variables in rats [31]. In addition, reports of long-term melatonin therapy in seasonal breeders [24, 25, 32, 33] supported our results of long-term melatonin pre-treatment efficacy against γ-radiation in F. pennanti.
The spleen, a secondary lymphoid organ situated high in the left abdominal cavity and populated with T- and B-lymphocytes, is a highly proliferative organ and thus is sensitive to the effects of γ-radiation. This is in agreement with our results for the DNA SI of splenic cells following T-cell mitogenic challenge. Changes in circulating leukocyte and lymphocyte counts following radiation depict the immune status of animals [34]. Thus, in the present study, the observed suppression of TLC, which was mainly the result of a decreased circulating population of lymphocytes, indicates a suppressed immune function of the animals. Furthermore, the SI of Con A-stimulated lymphocytes showed a direct correlation with ALC, implying a link between the amelioration of lymphocyte blastogenesis and the decrease in lymphocyte count. However, long-term exogenous melatonin counteracted radiation-induced suppression of the leukocyte and lymphocyte population (Figures 1a and b and 2a and b). Reports exist that suggest that the peak time of circulating lymphocytes corresponds closely with that of peak melatonin levels, and these levels correlate with a high immune status over the circadian cycle. Immunity levels were found to increase following melatonin treatment [35, 36].
Lipid damage and protein malfunctioning are critical events following exposure to low-LET radiation. The free radicals generated as a result of radiation interact with the polyunsaturated fatty acids of the lipid membrane to form lipid breakdown products, collectively known as TBARS. The levels of TBARS thus give a measure of lipid peroxidation following oxidative stress [37]. Biological specimens contain a mixture of TBARS, including lipid hydroperoxides and aldehydes such as malondialdehyde (MDA). TBARS like MDA form an adduct with cellular DNA and proteins, introducing cross-links in proteins and causing alterations in their biochemical properties [38]. 5 Gy γ-rays were damaging enough to generate significant levels of TBARS and reduce SOD activity in the spleen of active melatonin-pre-treated squirrels for up to 168 h. Melatonin protection was more effective during RIP (Figures 4c and 5c); this might be due to the additive effect of long-term treatment of melatonin during RIP, as endogenous melatonin is already high compared with that during RAP. Highly significant differences in the SOD activity of both RAP and RIP squirrels were seen following radiation, with a drastic prompt drop during RAP. This rapid drop indicates the production of more toxic superoxide radicals as a result of the indirect action of γ-radiation (i.e. water radiolysis in tissues and recombination of primary free radicals). Gradual rises of SOD activity in the melatonin pre-treated and irradiated RAP groups were observed from 48 h onwards compared with the irradiated group with no melatonin pre-treatment. This might be result of the production of radiation-induced reactive oxygen species (ROS) within the cellular system.
The enhanced TBARS level accompanied by a decrease in SOD activity during RAP is in agreement with the work of Dubner et al [39]. High SOD activity and overexpression increases the resistance of cells to radiation-induced oxidants [40]. The role of SOD lies in the elimination of toxic superoxide (O2−) radicals. Unbalanced conditions can deplete SOD activity and, hence, lead to an increased sensitivity of cells to γ-radiation. SOD concentrations other than the optimal lead to increased lipid peroxidation and, therefore, a decrease in cell viability [41]. SOD activation results in the formation of hydrogen peroxide (H2O2) [42], the accumulation of which can be more toxic for cells than O2− [43, 44]. This is because H2O2 causes the formation of the •OH radical, which is one of the most toxic oxygen molecules in vivo owing to its high reactivity, short life-time and limited diffusion rate [45]. The upregulated nuclear translocation of extracellular SOD under oxidative stress suggested a protective role of SOD against oxidative damage to genomic DNA [46]. Therefore, an increase in SOD activity during winter (RIP) in the control group highlighted the physiological importance of SOD in protecting against radicals generated because of winter-bound stresses. This protective effect of SOD might be explained by correlating its activity pattern with the seasonal variation in internal melatonin level of F. pennanti [16].
The effects of melatonin on the levels of a number of biochemical variables, on hormones and on oxidative and antioxidative status in mammals, and how they change in relation to their circadian patterns, have been reported [47, 48]. Besides being a circadian and seasonal biorhythm regulator, a wide spectrum of targets and effects of melatonin has evolved in a variety of tissues, such as the gut, cerebrospinal fluid, ovary, skin, bone marrow, bile fluid and lymphocytes, as well as in whole organisms [49–55]. Antioxidant enzyme activities have been shown to exhibit circadian rhythms corresponding to melatonin rhythmicity and total antioxidant status in chicken brain, liver and lung [56]. Daily fluctuations in SOD gene expression correspond to day–night changes in circulating melatonin levels in rats [57]. Melatonin is known to stimulate SOD under both basal and oxidative stress conditions [58] and it significantly upregulates free radical-scavenging systems in many body compartments [59, 60]. Melatonin forms an adduct with MDA [61], thus breaking the peroxidation chain reaction and further stopping its action towards protein alterations. The reduction in TBARS levels and elevation in SOD activities in melatonin-treated animals suggested a role of melatonin in scavenging free radicals generated as a result of γ-radiation.
Evidence of melatonin as an enhancer of antioxidant enzyme activity and expression with activation of its membrane receptors (MT1, MT2), via G inhibitory protein, supported our results [59, 62]. Melatonin is a highly electroreactive molecule that acts primarily as a powerful electron donor and detoxifies electrodeficient ROS. Through the use of a scavenging cascade, melatonin reduces the overall toxic environment in the cell [63].
Ionising radiation interacts with biological molecules to produce radiolytic products that have a major role in cell injury and, subsequently, induce apoptotic cell death through activation of a pro-apoptotic pathway. Two mechanisms of nuclear DNA damage that involve direct free radical attack on DNA and endonuclease-mediated DNA fragmentation via the caspase cascade have been reported [64]. Caspase activation, via receptor-mediated and mitochondrial pathways, is a common response to ionising radiation that actively initiates and executes apoptosis [65]. Caspase-3 initiates fragmentation by translocating from the cytoplasm to the nucleus, where it cleaves genomic DNA at internucleosomal regions, generating oligonucleosomal fragments [66]. Free 3′-OH ends were available for TdT-labelling in the apoptotic cells where TUNEL positivity was detected. Melatonin, in addition to the ability of scavenging radiation-induced •OH radicals, might also influence the stimulation of enzymes involved in the repair of lesions in cellular DNA [67]. Melatonin provides direct protection in the nucleus by reducing the extent of DNA damage through the generation of signal(s) that trigger the activation of DNA repair enzymes and promote expression of a set of genes that lead to de novo protein synthesis associated with DNA repair [68]. Our data show that melatonin treatment leads to markedly reduced TUNEL positivity of γ-radiated groups from 4 h onwards during both phases (Figure 7a and b). A reduced frequency of TUNEL signals (Figure 6d) in melatonin-pre treated plus irradiated groups indicated a positive role of melatonin towards DNA repair (i.e. melatonin may modulate endogenous DNA repair activity because of a lower incidence of double-strand breaks that is not related to antioxidative and anti-apoptotic effects).
Caspase-3 appears to play its role 3 h after radiation [69], leading to cellular apoptosis. In agreement with this finding, in the present study, in vitro caspase-3 activity peaked 4 h after radiation and the caspase-3-mediated pathway was involved in executing apoptosis in both the RAP and RIP irradiated groups. Melatonin appeared to have a protective action, suggesting that melatonin might reduce apoptosis through a caspase-3-mediated pathway by blocking caspase-3 activity. On correlating the results of the apoptotic splenocyte index and caspase-3 activity at 4 h, we found that DNA fragmentation was mostly caspase-3-dependent, as supported by the work of Inagaki-Ohara et al [70].
The results presented here suggest that the pattern of changes in TLC, SI, TBARS and caspase-3 activity of irradiated and melatonin-treated plus irradiated groups at different time-points were irrespective of the reproductive phase, whereas changes in ALC, SOD activity and TUNEL positivity were dependent on the reproductive phase. Melatonin pre-treatment significantly increased the basal count of lymphocytes and induced a prompt decrease in both the basal activity of SOD and the basal number of TUNEL-positive cells; however, the effects still followed a similar pattern to those of the untreated irradiated groups during both phases (Figures 2c, 5c and 7c). Immune function varies on a seasonal basis [71] and requires substantial energy [72]. During RAP, seasonal breeders channel energy for reproductive success; thus, steroidal levels are higher than during RIP. Steroids are known to suppress melatonin synthesis; thus, circulating melatonin is comparatively low during RAP when the days are long. By contrast, during the short day and long night phase (RIP), animals possess low steroid and high melatonin levels. As circulating steroid hormones (testosterone, oestradiol, corticosterone) mediate immunosuppression [32, 33], it follows that squirrels possess better immune function during RIP than during RAP. The enhanced immune function seen in RIP might result from the channelling of energy to strengthen the immune function in order to face the adverse challenges of energetically demanding winters. The stress of coping with energetically demanding conditions increases steroid levels to the extent that immune function is compromised [73], which further increases the risk of infection and death under conditions in which there are insufficient energy reserves to sustain immunity. Nelson et al [12] have reported seasonal changes in mammalian immune function, showing enhanced function during short day lengths. The maintenance of lymphoid tissue function and activity during RIP could result from the elevated internal melatonin levels produced in response to environmental stress. These high levels of melatonin might not only strengthen the immunity of F. pennanti to fight winter-bound stress, but could also provide protection against the free radicals generated by high doses of γ-radiation.
Comparing the effects of 5 Gy γ-radiation on lymphocytes with our previously reported results for 2.06 Gy X-rays, in terms of TBARS level and apoptosis percentage, we observed a similar pattern in both RAP and RIP squirrels; however, with γ-rays we noted 95% and 71% more lipid damage and 186% and 31% more apoptosis during RAP [24] and RIP [25], respectively. Also, following γ-radiation, squirrels pre-treated with melatonin (100 μg/100 g body weight) showed a nearly 88% and 38% increase in TBARS level and an 85% and 16% increase in apoptosis per cent during RAP and RIP, respectively, when compared with squirrels pre-treated with a comparatively lower dose of melatonin (25 μg/100 g body weight) following exposure to X-rays. Radiation effects in biological systems depend on the dose rate and are proportional to the absorbed dose, which is the average energy deposited inside a test volume [74, 75]. In the present study, a 5 Gy dose of γ-rays deposited approximately 2.5 times more energy than 2.06 Gy X-rays within the same irradiated volume (spleen), thus increasing the probability of tissue damage. In addition, the damage induced by the 5 Gy γ-rays was more difficult for cells to repair, owing to the increased exposure time (∼4.47 min) to the tissue, as compared with X-rays (1 min). This longer exposure might have increased the concentration of local radicals with increased radical attack, disturbing the internal balance of antioxidative enzymes and internal melatonin levels inside the cell. The results reported here support the statement that γ-rays induce more dense ionisation of biomolecules than X-rays [26, 27].
Any radiation dose is capable of causing cancer and a strong immune system is important for preventing mutations from developing into a cancer. Hence, pre-application of melatonin could be beneficial for cancer patients undergoing radiation treatment. Our results may partially explain various discrepancies observed by clinicians following radiotherapy and may provide a fundamental basis for using melatonin as a substrate to minimise the deleterious effects of ionising radiation used in clinical therapy. Our results documenting the role of melatonin in inhibiting caspase-3 activity in the radiation-induced caspase-3-dependent apoptotic pathway has prompted further investigations of the mechanism of melatonin action in the apoptotic signalling pathway at points other than the caspase cascade. Moreover, changes in protective endogenous cell physiological pathways caused by melatonin-induced gene expression, as well as the inhibition of damaging processes such as inflammation and mitochondrial metabolism that could be disrupted following radiation, need to be considered in future studies.
Acknowledgments
We express our gratitude to physicist H S Bhuiyan for dosimetry calculations and to R K Lad, R Sharma and A Paul of Indian Railways Cancer Research Centre, Varanasi, India, for excellent technical assistance during γ-radiation. We are thankful to Inter University Accelerator Centre-University Grants Commission, New Delhi, India, for financial support throughout the work. Special thanks to laboratory colleagues Dr S Rai, Dr R Yadav, Dr S Panshikar, R Ahmad, Kaushalendra and R Kharwar for help with the experiments.
References
- 1.Slominski A, Pawalek J. Animals under the sun: effects of ultraviolet radiation on mammalian skin. Clin Dermatol 1998;16:503–15 [DOI] [PubMed] [Google Scholar]
- 2.Cesarini JP. Impact of ultraviolet radiation on humans. Indoor Built Environ 2001;10:310–16 [Google Scholar]
- 3.Anjos RM, Veiga R, Carvalho C, Sanches N, Estellita L, Zanuto P, et al. Natural sources of radiation exposure and the teaching of radioecology. Phys Edu 2008;43:423–8 [Google Scholar]
- 4.Valinia A, Kinzer RL, Marshall FE. Measurement of the galactic x-ray/gamma-ray background radiation: contribution of discrete sources. Astrophys J 2000;534:277–82 [Google Scholar]
- 5.Ramachandran TV, Eappen KP, Mayya YS. Background radiation exposure levels: Indian scenario. Int Congr Ser 2005;1276:337–8 [Google Scholar]
- 6.Amutha R, Brahmanandhan GM, Malathi J, Khanna D, Selvasekarapandian S, Sarida R, et al. Study of background radiation from soil samples of Pollachi taluk, Tamilnadu, India. Int Congr Ser 2005;1276:331–2 [Google Scholar]
- 7.Ojovan MI, Lee WE. Background Radiation. In: Ojovan MI, Lee WE, editors. An introduction to nuclear waste immobilisation. US: Elsevier, 2005:53–60 [Google Scholar]
- 8.Sivintsev YV. Natural background radiation. Atomic Energy 1988;64:55–77 [Google Scholar]
- 9.Appleton JD. Radon in air and water. In: Selinus O, Alloway B J, Centeno JA, Finkelman RB, Fuge R, Lindh U, et al. Editors. Essentials of medical geology: impacts of the natural environment on public health. Amsterdam, Boston: Elsevier Academic Press, 2005:227–61 [Google Scholar]
- 10.Nunia V, Sancheti G, Goyal PK. Protection of Swiss albino mice against whole-body gamma irradiation by diltiazem. Br J Radiol 2007;80:77–84 [DOI] [PubMed] [Google Scholar]
- 11.Karnaukhova NA, Sergiyevich LA, Aksenova GY, Karnaukhov VN. Synthetic activity of rat blood lymphocytes under acute and continuous gamma-irradiation: fluorescent microspectral study. Radiat Environ Biophys 1999;38:49–56 [DOI] [PubMed] [Google Scholar]
- 12.Nelson RJ, Demas GE, Klein SL, Kriegsfeld LJ, editors Seasonal patterns of stress, immune function and disease. Cambridge University Press, UK: 2002. [Google Scholar]
- 13.Bechtold DA, Loudon ASI. Hypothalamic thyroid hormones: mediators of seasonal Physiology. Endocrinology 2007;148:3605–7 [DOI] [PubMed] [Google Scholar]
- 14.Skwarlo-Sonta KSS, Majewski P, Pawlak J, Markowska M. Photoeriodic VS. Non-photoperiodic animals: circadian and seasonal changes in immunity. In: Pandi-Perumal SR, Cardinali DP, Editors. Melatonin: from molecules to therapy. New York: Nova Science Publishers, 2007:247–60 [Google Scholar]
- 15.Singh SS, Haldar C. Peripheral melatonin modulates seasonal immunity and reproduction of Indian tropical male bird Perdicula asiatica. Comp Biochem Physiol, Part A 2007;146:446–50 [DOI] [PubMed] [Google Scholar]
- 16.Haldar C, Singh R, Guchhait P. Relationship between the annual rhythms in melatonin and immune system status in the tropical palm squirrel, Funambulus pennanti. Chronobiol Int 2001;18:61–9 [DOI] [PubMed] [Google Scholar]
- 17.Nelson RJ, Drazen DL. Melatonin mediates seasonal changes in immune function. Ann NY Acad Sci 2000;917:404–15 [DOI] [PubMed] [Google Scholar]
- 18.Haldar C, Sharma S, Singh SS. Reproductive phase dependent circadian variations of plasma melatonin, testosterone, thyroxine and corticosterone in Indian palm squirrel, Funambulus pennanti. Biol Rhythm Res 2006;37:1–10 [Google Scholar]
- 19.Tan DX, Manchester LC, Hardeland R, Lopez Burillo S, Mayo JC, Sainz RM, et al. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res 2003;34:75–8 [DOI] [PubMed] [Google Scholar]
- 20.Reiter RJ. Melatonin: clinical relevance. Best Pract Res Clin Endocrinol Metab 2003;17:273–85 [DOI] [PubMed] [Google Scholar]
- 21.Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocrine J 1993;1:57–60 [Google Scholar]
- 22.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress: a review. J Biomed Sci 2000;7:444–58 [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004;36:1–9 [DOI] [PubMed] [Google Scholar]
- 24.Sharma S, Haldar C. Melatonin prevents X-ray irradiation induced oxidative damage in peripheral blood and spleen of the seasonally breeding rodent, Funambulus pennanti during reproductively active phase. Int J Radiat Biol 2006;82:411–19 [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, Haldar C, Chaube SK. Effect of exogenous melatonin on X-ray induced cellular toxicity in lymphatic tissue of Indian tropical male squirrel, Funambulus pennanti. Int J Radiat Biol 2008;84:363–74 [DOI] [PubMed] [Google Scholar]
- 26.Psonka K, Gudowska-Nowak E, Brons S, Elsasser T, Heiss M, Taucher-Scholz Ionizing radiation-induced fragmentation of plasmid DNA – atomic force microscopy and biophysical modeling. Adv Space Res 2007;39:1043–9 [Google Scholar]
- 27.Holley WR, Chatterjee A. Clusters of DNA damage induced by ionizing radiation: formation of short DNA fragments. I. Theoretical modelling. Radiat Res 1996;145:188–99 [PubMed] [Google Scholar]
- 28.Ohkawa H, Ohishi N, Yagi K. Reaction of linoleic acid hydroperoxide with thiobarbituric acid. J Lipid Res 1978;19:1053–7 [PubMed] [Google Scholar]
- 29.Das K, Samanta L, Chainy GBN. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys 2000;37:201–4 [Google Scholar]
- 30.Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 2000;88:87–98 [Google Scholar]
- 31.Sankaran M, Subramanian P. Modulation of biochemical circadian rhythms during long-term melatonin treatment in rats. Singapore Med J 2006;47:42–7 [PubMed] [Google Scholar]
- 32.Haldar C, Rai S, Singh R. Melatonin blocks dexamethasone induced immunosuppression in a seasonally breeding rodent Indian palm squirrel, Funambulus pennanti. Steroids 2004;69:367–77 [DOI] [PubMed] [Google Scholar]
- 33.Singh SS, Haldar C. Melatonin prevents testosterone induced suppression of immune parameters and splenocyte proliferation in Indian tropical jungle bush quail, Perdicula asiatica. Gen Comp Endocrinol 2005;141:226–32 [DOI] [PubMed] [Google Scholar]
- 34.Ohkoshi H, Asukata N, Tajima N, Yamamoto K, Sasaki M, Mokari M, et al. The influence of transmeridian flight on human circulating lymphocytes. Aviat Space Environ Med 1991;62:14–18 [PubMed] [Google Scholar]
- 35.Levi F, Reinberg A, Canon C. Clinical immunology and allergy. In: Arendt J, Minors DS, Waterhouse JM, editors. Biological rhythms in clinical practice. London: Wright, 1989:99–135 [Google Scholar]
- 36.Litvinenko GI, Shurlygina AV, Shirinskii VS, Nepomnyashchikh VM, Shirinskii IV, Leonova MI, et al. Circadian variations in immune values and serum melatonin in asthmatics. Bull Exp Biol Med 2006;142:553–6 [DOI] [PubMed] [Google Scholar]
- 37.Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Meth Mol Biol 1998;108:101–6 [DOI] [PubMed] [Google Scholar]
- 38.Slatter DA, Bolton CH, Bailey AJ. The importance of lipid derived malondialdehyde in diabetes mellitus. Diabetologia 2000;43:550–7 [DOI] [PubMed] [Google Scholar]
- 39.Dubner D, Gisone P, Jaitovich I, Perez M. Free radicals production and estimation of oxidative stress related to gamma irradiation. Biol Trace Elem Res 1995;47:265–70 [DOI] [PubMed] [Google Scholar]
- 40.Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, et al. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol 2003;23:2362–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCord JM. The importance of oxidant-antioxidant balance. In: Montagneir L, Olivier R, Pasquier C, editors. Oxidative stress in cancer, AIDS, and neurodegenerative diseases. New York: Marcel Dekker, 1998:1–8 [Google Scholar]
- 42.Liochev SI, Fridovich I. The effects of superoxide dismutase on H2O2 formation. Free Radic Biol Med 2007;42:1465–9 [DOI] [PubMed] [Google Scholar]
- 43.Mao GD, Thomas PD, Lopaschuk GD, Poznansky MJ. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J Biol Chem 1993;268:416–20 [PubMed] [Google Scholar]
- 44.Offer T, Russo A, Samuni A. The pro-oxidative activity of SOD and nitroxide SOD mimics. FASEB J 2000;14:1215–23 [DOI] [PubMed] [Google Scholar]
- 45.Kieber DJ, Peake BM, Scully NM. Reactive oxygen species in aquatic ecosystems. In: Helbling EW, Zagarese H, editors. UV effects in aquatic organisms and ecosystems. UK: Royal Society of Chemistry, 2003:251–88 [Google Scholar]
- 46.Ookawara T, Eguchi H, Nishimura M, Kizaki T, Eiji Takayama , Saitoh D, et al. Effects of oxidative stress on the nuclear translocation of extracellular superoxide dismutase. Biochem Biophys Res Commun 2003;11:914–19 [DOI] [PubMed] [Google Scholar]
- 47.Esquifino AL, Castrillon PO, Bonacho MG, Vara E, Cardinali DP. Effect of melatonin treatment on 24-hour rhythms of serum ACTH, growth hormone, prolactin, thyrotropin, luteinizing hormone and insulin in rats injected with Freund's adjuvant. J Pineal Res 1999;27:15–23 [DOI] [PubMed] [Google Scholar]
- 48.Baydas G, Gursu MF, Yilmaz S, Canpolat S, Yasar A, Cikim G, Canatan H. Daily rhythm of glutathione peroxidase activity, lipid peroxidation and glutathione levels in tissues of pinealectomized rats. Neurosci Lett 2002;323:195–8 [DOI] [PubMed] [Google Scholar]
- 49.Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: nature's most versatile biological signal? FEBS J 2006;273:2813–38 [DOI] [PubMed] [Google Scholar]
- 50.Bubenik GA, Hacker RR, Brown GM, Bartos L. Melatonin concentrations in the luminal fluid, mucosa, and muscularis of the bovine and porcine gastrointestinal tract. J Pineal Res 1999;26:56–63 [DOI] [PubMed] [Google Scholar]
- 51.Skinner DC, Malpaux B. High melatonin concentrations in third ventricular cerebrospinal fluid are not due to Galen vein blood recirculating through the choroid plexus. Endocrinology 1999;140:4399–405 [DOI] [PubMed] [Google Scholar]
- 52.Itoh MT, Ishizuka B, Kuribayashi Y, Amemiya A, Sumi Y. Melatonin, its precursors, and synthesizing enzyme activities in the human ovary. Mol Hum Reprod 1999;5:402–8 [DOI] [PubMed] [Google Scholar]
- 53.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J 2006;20:1564–6 [DOI] [PubMed] [Google Scholar]
- 54.Tan DX, Manchester LC, Reiter RJ, Qi WB, Zhang M, Weintraub ST, et al. doi: 10.1016/s0304-4165(99)00125-7. Identification of highly elevated levels of melatonin in bone marrow: its origin and significance. Biochim Biophys Acta 1999;1472:206–14. [DOI] [PubMed] [Google Scholar]
- 55.Carrillo-Vico A, Calvo JR, Abreu P, Lardone PJ, García-Maurião S, Reiter RJ, et al. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J 2004;18:537–9 [DOI] [PubMed] [Google Scholar]
- 56.Albarran MT, Lopez-Burillo S, Pablos MI, Reiter RJ, Agapito MT. Endogenous rhythms of melatonin, total antioxidant status and superoxide dismutase activity in several tissues of chick and their inhibition by light. J Pineal Res 2001;30:227–33 [DOI] [PubMed] [Google Scholar]
- 57.Martin V, Sainz RM, Mayo JC, Antolin I, Herrera F, Rodriguez C. Daily rhythm of gene expression in rat superoxide dismutases. Endocr Res 2003;29:83–95 [DOI] [PubMed] [Google Scholar]
- 58.Oner-Iyidogan Y, Gurdol F, Oner P. The effects of acute melatonin and ethanol treatment on antioxidant enzyme activities in rat testes. Pharmacol Res 2001;44:89–93 [DOI] [PubMed] [Google Scholar]
- 59.Antolin I, Rodriguez C, Sainz RM, Mayo JC, Uria H, Kotler ML, et al. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J 1996;10:882–90 [DOI] [PubMed] [Google Scholar]
- 60.Kotler M, Rodriguez C, Sainz RM, Antolin I, Menendez-Pelaez A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res 1998;24:83–9 [DOI] [PubMed] [Google Scholar]
- 61.Li G, Li L, Yin D. A novel observation: melatonin's interaction with malondialdehyde. Neuroendocrinol Lett 2005;26:61–6 [PubMed] [Google Scholar]
- 62.Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci 2002;59:1706–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys 2001;34:237–56 [DOI] [PubMed] [Google Scholar]
- 64.Sun FY, Lin X, Mao LZ, Ge WH, Zhang LM, Huang YL, et al. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J Pineal Res 2002;33:48–56 [DOI] [PubMed] [Google Scholar]
- 65.Takahashi A, Earnshaw WC. ICE-related proteases in apoptosis. Curr Opin Genet Dev 1996;6:50–5 [DOI] [PubMed] [Google Scholar]
- 66.Kamada S, Kikkawa U, Tsujimoto Y, Hunter T. Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s). J Biol Chem 2005;280:857–60 [DOI] [PubMed] [Google Scholar]
- 67.Vijayalaxmi , Reiter RJ, Meltz ML, Herman TS. Melatonin: Possible mechanisms involved in its ‘radioprotective’ effect. Mutat Res 1998;404:187–9 [DOI] [PubMed] [Google Scholar]
- 68.Shirazi A, Ghobadi G, Ghazi-Khansari M. A radiobiological review on melatonin: a novel radioprotector. J Radiat Res 2007;48:263–72 [DOI] [PubMed] [Google Scholar]
- 69.Chong MJ, Murray MR, Gosink EC, Russell HRC, Srinivasan A, Kapsetaki M, et al. Atm and Bax cooperate in ionizing radiation induced apoptosis in the central nervous system. Proc Natl Acad Sci USA 2000;97:889–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inagaki-Ohara K, Takamura N, Yada S, Alnadjim Z, Liu E, Yu X, et al. Radiation-induced crypt intestinal epithelial cell apoptosis in vivo involves both caspase-3-dependent and independent pathways. Dig Dis Sci 2002;47:2823–30 [DOI] [PubMed] [Google Scholar]
- 71.Nelson RJ, Demas GE. Seasonal changes in immune function. Q Rev Biol 1996;71:511–48 [DOI] [PubMed] [Google Scholar]
- 72.Maier SE, Washout LR, Fleshner M. Psychoneuroimmunology: the interface between behavior, brain, and immunity. Am Psychol 1994;49:1004–17 [DOI] [PubMed] [Google Scholar]
- 73.Ader R, Cohen N. Psychoneuroimmunology: conditioning and stress. Annu Rev Psychol 1993;44:53–85 [DOI] [PubMed] [Google Scholar]
- 74.Kato F, Ootsuyama A, Nomoto S, Kondo S, Norimura T. Threshold effect for teratogenic risk of radiation depends on dose-rate and p53-dependent apoptosis. Int J Radiat Biol 2001;77:13–19 [DOI] [PubMed] [Google Scholar]
- 75.Attix FH, editor Introduction to radiological physics and radiation dosimetry. Berlin, Germany: Wiley-VCH, 1986 [Google Scholar]