Abstract
Whole brain radiotherapy (WBRT) is the standard non-surgical treatment for brain metastatic disease, but rarely eradicates bulky metastases from most common cancers. Recent literature has demonstrated the safety and efficacy of delivering very high focal doses of radiation (by radiosurgical techniques) to the gross tumour volume of bulky brain metastases, thereby obtaining more certain local control than is achieved by WBRT. In this paper we report a study of 11 patients with bulky brain metastases in whom an intensity-modulated radiation therapy (IMRT) facility has been used to concomitantly boost the gross tumour volume of bulky brain metastatic disease (to 40 Gy) during a standard 30 Gy in 10 fractions WBRT schedule. No acute or subacute morbidity was encountered, and good early control data were noted. We discuss the perceived advantages of such a technique.
Brain metastases are a common problem in adults with cancer, affecting up to 40% of all patients [1, 2]. Partly a result of improved systemic control of cancer, and partly owing to improved imaging (notably MRI), the diagnosis of brain metastases has increased [3]. Established brain metastases shorten survival and detract from quality of life. Neurocognitive impairment is common at the time of diagnosis of central nervous system metastatic disease [4].
Many patients who develop brain metastases will eventually die of progressive intracranial disease. Among selected patients with good performance status, controlled extracranial disease, favourable prognostic features and with a solitary brain metastasis, there are data from randomised clinical studies demonstrating that surgical excision followed by whole brain radiotherapy (WBRT) offers prolonged survival [5, 6]. Stereotactic radiosurgery (SRS), using technologies such as Gamma Knife (Elekta, Linkoping, Sweden), may be able to replace surgery in certain circumstances — delivering obliteratively high single doses to discrete (single) metastases. SRS is well suited to treating small numbers of brain metastases and achieves good local control of intracranial metastases [7, 8]. The doses that can be delivered to individual metastases by Gamma Knife SRS (up to 25 Gy on the margin of MRI-mapped lesions) offer a greater degree of certainty of local control than those from routine WBRT dose prescription. The Radiation Therapy Oncology Group (RTOG) 9508 trial randomised 333 patients with one to three cerebral metastases to standard WBRT either with or without a stereotactic radiation boost and demonstrated an improved quality and quantity of life in those receiving the stereotactic boost [9]. This level 1 evidence thereby demonstrated that packing a higher radiation dose into bulky cerebral metastases is beneficial in addition to standard WBRT.
Many patients are initially deemed unsuitable for neurosurgery or, later, SRS boost, owing to poor performance status, active extracranial disease or multiple brain metastases. In these patients, corticosteroids and WBRT are standard treatment. However, there is a subset of these patients receiving WBRT who have better prognostic factors from the systemic disease viewpoint, and who survive long enough to later die from uncontrolled brain metastatic disease. Benefit from brain radiation therapy was greater for the recursive partitioning analysis (RPA) Class I patients (age <65 years, Karnofsky performance status 70% or higher, with a controlled primary tumour and no extracranial metastases) in the RTOG database [10]. Individual metastases of some bulk are less likely to be sterilised by WBRT dosage (vide supra). It is these patients who will later be considered for Gamma Knife SRS for viable residual dose. In RTOG 9508, these patients were those with one to three brain metastases. It is our policy, in such better prognosis patients with systemic disease control, to re-scan 6–8 weeks after WBRT and deliver a Gamma Knife SRS boost to remaining viable metastases (gadolinium-enhancing on MRI or 18F-fluorodeoxyglucose–positron emission tomography [FDG-PET] positive).
In this article we explore the use of concomitant boost treatment by intensity-modulated radiation therapy (IMRT) during routine WBRT with 30 Gy in 10 daily fractions over two weeks, in an attempt to improve overall local control without resort to Gamma Knife SRS. The perceived advantage of such a technique is to increase the radiation dose to the bulky metastases (which has to be the benefit attributed to the SRS boost in the foregoing discussion) without prolonging a course of standard treatment and without recourse to any subsequent planned radiation therapy (“Phase II” or SRS) — with its extra labour intensity and expense.
Methods and results
Eleven patients diagnosed with brain metastatic disease of considerable size (25–80 mm in maximum diameter) on high-quality gadolinium-enhanced MRI, and with no more than four metastases, were studied. None had received prior radiotherapy to the brain, and the primary sites were as follows: bronchus (5), breast (4), colon (1) and kidney (1). Patients with more than four brain metastases or with smaller lesions were not selected.
All 11 patients were treated with WBRT on a TomoTherapy machine (TomoTherapy Hi-Art Systems, TomoTherapy Inc., Madison, WI). Helical tomotherapy combines on-board megavoltage imaging with inverse-planned IMRT delivery. Each patient was immobilised using a thermoplastic head shell (MEDTEC, Inc., Orange City, IA), which departmental audit has demonstrated to be associated with a set-up uncertainty of less than 3 mm in all directions. The whole brain clinical target volume (CTV) was contoured by visual comparison with a contrast-enhanced CT brain scan, and a margin of 3 mm was added to give the whole brain planned target volume (PTV). The brain metastasis gross tumour volume (GTV) was delineated on the contrast-enhanced planning CT scan with visual comparison to the T1 weighted axial diagnostic gadolinium-enhanced MRI scan, with no added margin (i.e. PTV _ GTV). A plan was created to deliver a dose of 30 Gy in 10 daily fractions over 14–16 days to the whole brain PTV, while simultaneously delivering a dose of 40 Gy in 10 fractions to the metastasis (Figures 1,2).
Figure 1.
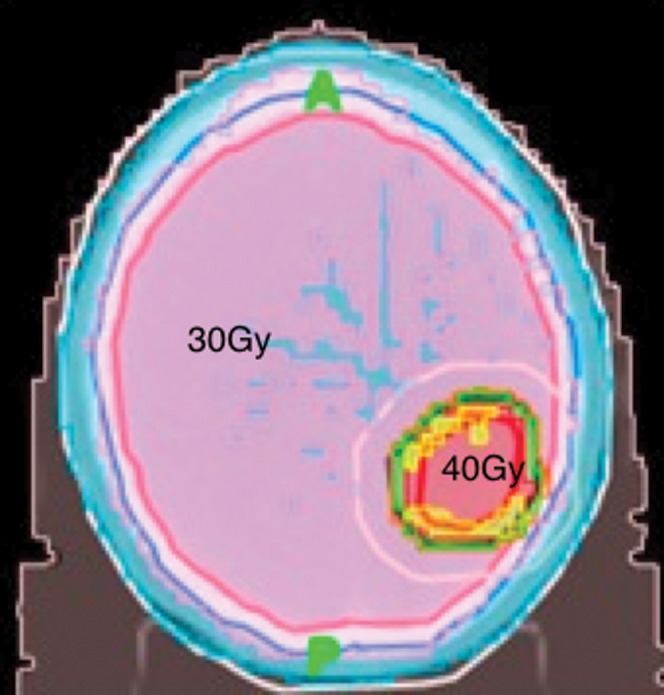
Axial planning CT scan depicting the radiation isodosimetry that delivered 10 × 3 Gy to the whole brain, while concomitantly boosting a bulky single metastasis with 10 × 4 Gy.
Figure 2.
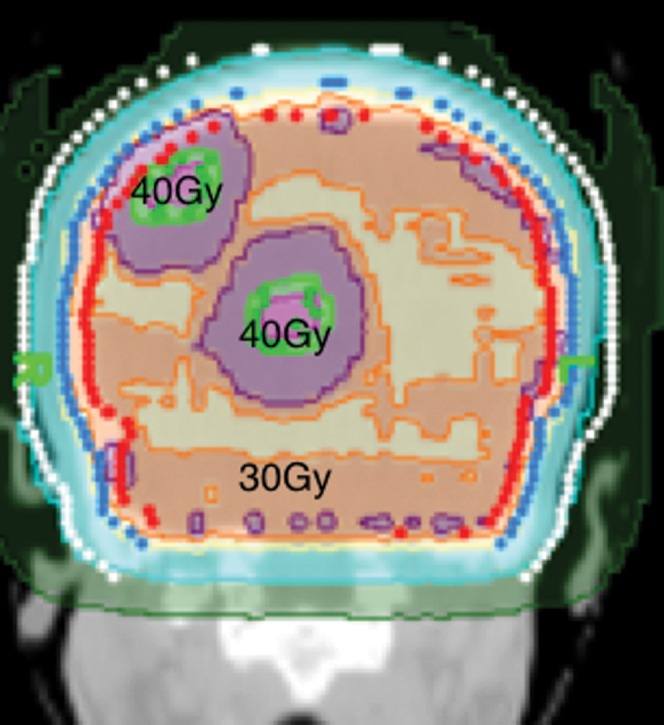
Coronal planning CT scan depicting the radiation isodosimetry of whole brain fractionated radiotherapy to 30 Gy while two bulky metastases concomitantly received 40 Gy to the gross tumour volume.
Although it is difficult to compare the biological equivalent dose (BED) of 40 Gy in 10 fractions to that of a single fraction radiosurgical dosage, it is nevertheless clear that the BED of this prescription (50–72 Gy assuming an α/β ratio for brain metastases in the range of 5–15 Gy) is more than that of 30 Gy in 10 fractions (36–48 Gy) [11]. Calculation of BED for normal brain tissue at the boost site is not relevant because of the fast-falling dose gradient at the margin of the boost PTV. However, it is worth noting that a whole brain dose of 40 Gy in 10 daily fractions would be perceived to be neurotoxic (with a BED for normal brain tissue of 75 Gy, assuming an α/β ratio of 2 Gy).
Our protocol for clinical follow-up included assessment of neurological symptoms and signs, and MRI scans were performed at 1 month and at 3–6 months. Quality of life measures were not prospectively assessed in this study.
There were no acute or subacute complications, such as may attend SRS [12]. Patients with larger lesions were prophylactically treated with corticosteroids (dexamethasone) during therapy. MRI brain scans were performed at 1 month and 3–6 months after therapy. All tumours (including dramatic shrinkage of the 80 mm colonic metastasis) showed response on the 1 month scan.
Median follow-up was 4 months. Four of the 11 patients died of systemic disease 6–9 months after receiving radiotherapy. The remaining patients are alive, with no evidence of progression of the treated brain disease or local recurrence at 2–9 months after radiotherapy. There have been no brain complications to date. No patient has required prolonged steroid therapy.
Discussion
There are a variety of treatment options for selected patients with brain metastatic disease. Surgical excision of single accessible brain metastases has been a standard treatment option in patients with good performance status and controlled extracranial disease and remains optimal when the lesions are superficial and with mass effect. Two randomised studies have demonstrated a significant survival benefit of surgery followed by WBRT compared with surgery alone among these patients [5, 6]. SRS has an established role for treating selected patients with single or multiple brain metastases, with local tumour control rates at 1 year of around 80% and with median survival of 6–12 months [13, 14]. SRS may replace conventional surgery for single brain metastases without major mass effect. However, by its focal nature, SRS does not prevent relapse at other sites within the brain, which is very common in many cancers (e.g. bronchus/breast). The RTOG 9508 randomised trial demonstrated that the addition of SRS to WBRT significantly increased median survival from 4.9 to 6.9 months for patients with one to three brain metastases and a Karnofsky performance status of 70% or more [9]. SRS has demonstrated the safety of delivering very high focal radiation doses to the GTV of brain metastases. Marginal doses to MRI-mapped GTV of metastases of 18–25 Gy are routine SRS prescriptions for single treatments of lesions up to 3 cm in diameter.
WBRT with concomitant corticosteroids has been established as the therapeutic mainstay for most patients with brain metastases, many of whom are unfit or unsuitable for surgical resection or who have brain metastases that are too large or numerous to be treated by SRS. An editorial in the International Journal of Radiation Biology and Physics in 1999 stated: “for over two decades, the standard radiotherapeutic management of patients with multiple brain metastases has been 30 Gy in 10 fractions of WBRT”. But, the editorial later welcomes new thoughts on improving radiotherapeutic control of brain metastases [15]. Rates of complete responses (CRs) and partial responses (PRs) to WBRT in patients in RTOG randomised clinical trials have been reported as approximately 60% [9]. However, it has been known for some time that WBRT has a substantial local failure rate, particularly with regard to pre-hoc established metastases. Kondziolka et al [8] demonstrated in a randomised trial that, in patients with two to four brain metastases, WBRT was associated with a 100% local failure rate at one year, compared with WBRT followed by Gamma Knife SRS, which had a significantly lower local failure rate of 8% at one year. The median time to local failure was six months for WBRT alone and 36 months for WBRT plus SRS. However, SRS by Gamma Knife has major cost implications (circa £10 000 per patient treated) and, bearing in mind that such therapy is palliative, must therefore be under scrutiny for rationing. If its routine use could be obviated, this would be an advantage; up to one-third of a Gamma Knife unit's workload in the USA is the treatment of metastases. The question arises as to whether, with modern IMRT technology, higher dose concomitant dose boosts to bulky metastases could provide the advantages of SRS during WBRT and obviate the need for the extra procedure.
Simple conventional planning techniques are generally preferred for delivering palliative radiotherapy, in order to shorten the planning process and to minimise use of resources. However, a number of studies have begun to explore the use of high-technology palliative radiotherapy in situations like this, in an attempt to improve on some of the deficiencies of conventional treatment. Samant et al [16] have recently described their use of image-guided IMRT employing a TomoTherapy unit for online rapid palliative radiotherapy planning and treatment delivery in small numbers of patients, most of whom complained of painful bone metastases in the spine or pelvis [17]. They report that it is possible to scan patients using megavoltage CT acquisition, delineate target volumes, plan IMRT treatment, verify treatment and deliver the first radiotherapy fraction within a 1 h appointment [17]. They compare this to 3 h and 3.5 h experiences for patients undergoing conventional simulation and CT simulation, respectively. They also report improved dose homogeneity and sparing of adjacent normal tissues with image-guided IMRT plans, compared with conventional techniques [16].
In this paper, we have reported our first 11 patients – all of whom had bulky brain metastases (up to four in number) and systemic disease that carried with it a perceived prognosis for life of greater than three months – treated with integrated boost during WBRT. By using IMRT technology to deliver a concomitant boost (4 Gy per fraction) during a standard 10 × 3 Gy fractionated WBRT course, we found no adverse effects and good early data on local control. Indeed, we have been surprised by the lack of acute or subacute complications over standard WBRT. Clearly, such a study needs to be expanded, but the prediction is that there will be a better brain control of metastatic disease and less need for recourse for later SRS, both being of benefit to the patient and reducing the overall costs of treatment.
In summary, taking the subject of improving the control of brain metastatic disease as our research project, utilising IMRT technology and assimilating the better local control data for individual brain metastases from SRS, we have developed an integrated concomitant boost technique for bulky brain metastases that can be performed during WBRT. We predict that this approach will have the advantages of the SRS boost demonstrated in the RTOG 9508 trial, but at the same cost as WBRT only (plus IMRT costs).
References
- 1.Soffietti R, Rudè R, Mutani R. Management of brain metastases. J Neurol 2002;249:1357–69 [DOI] [PubMed] [Google Scholar]
- 2.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol 2005;75:5–14 [DOI] [PubMed] [Google Scholar]
- 3.Soffietti R, Rudè R, Trevisan E. Brain metastases: current management and new developments. Curr Opin Oncol 2008;20:676–84 [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Wefel JS, Maor MH, Hassenbusch SJ, 3rd, Mahajan A, Lang FF, et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery 2007;60:277–83 [DOI] [PubMed] [Google Scholar]
- 5.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494–500 [DOI] [PubMed] [Google Scholar]
- 6.Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoeksra FH, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 1993;33:583–90 [DOI] [PubMed] [Google Scholar]
- 7.Mehta MP, Tsao MN, Whelan TJ, Morris DE, Hayman JA, Flickinger JC, et al. The American Society of Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 2005;63:37–46 [DOI] [PubMed] [Google Scholar]
- 8.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999;45:427–34 [DOI] [PubMed] [Google Scholar]
- 9.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665–72 [DOI] [PubMed] [Google Scholar]
- 10.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trails. Int J Radiat Oncol Biol Phys 1997;37:745–51 [DOI] [PubMed] [Google Scholar]
- 11.Jones B, Dale RG, Deehan C, Hopkins KI, Morgan DAL. The role of biological effective dose in clinical oncology. Clin Oncol (R Coll Radiol) 2001;13:71–81 [DOI] [PubMed] [Google Scholar]
- 12.Plowman PN. Stereotactic radiosurgery. VIII. The classification of postradiation reactions. Br J Neurosurg 1999;13:256–64 [DOI] [PubMed] [Google Scholar]
- 13.Flinckinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys 1994;28:797–802 [DOI] [PubMed] [Google Scholar]
- 14.Shiau CY, Sneed PK, Shu HK, Lamborn KR, McDermott MW, Chang S, et al. Radiosurgery for brain metastases: relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Phys 1997;37:375–83 [DOI] [PubMed] [Google Scholar]
- 15.Shaw EG. Radiotherapeutic management of multiple brain metastases: “3000 in 10” whole brain radiation is no longer a “no brainer”. Int J Radiat Oncol Biol Phys 1999;45:253–4 [DOI] [PubMed] [Google Scholar]
- 16.Samant R, Gerig L, Montgomery L, Macrae R, Fox G, Nyiri B, et al. High-technology palliative radiotherapy using image-guided intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2008;20:718–20 [DOI] [PubMed] [Google Scholar]
- 17.MacPherson M, Montgomery L, Fox G, Carty K, Gerig L, MacRae R, et al. On-line rapid palliation using helical tomotherapy: a prospective feasibility study. Radiother Oncol 2008;87:116–18 [DOI] [PubMed] [Google Scholar]


