Abstract
The aim of this study was to identify the effect of the installation of Premium View post-processing software on our mammographic reporting performance, in particular the effects on our recall rate, biopsy rate and cancer detection rate. The case notes and imaging of all patients discussed at the weekly indeterminate imaging multidisciplinary team meeting were reviewed retrospectively before, immediately after and at a delayed interval following the installation of Premium View post-processing software. Factors recorded included the mammographic abnormality, further investigations and final histology. The indeterminate mammogram rate increased significantly from a baseline of 5.7% (before Premium View) to 8.7% in the time period immediately after the installation of Premium View (p _ 0.002). The stereotactic biopsy rate also increased from 0.8% to 2.4% (p _ 0.001), with a significant increase in the overall cancer detection rate from 3.4% to 4.4% (p _ 0.02). In the follow-up period several months after the installation of Premium View, the indeterminate mammogram rate returned to a level similar to that before Premium View (6%; p _ 0.7). The stereotactic biopsy rate remained significantly higher at 1.6% (p _ 0.07), as did the overall cancer detection rate of 5.0% (p _ 0.003). In conclusion, the use of Premium View may lead to higher cancer detection rates, at the expense of an initial increase in recall rate. Although prospective studies are suggested, this result is of interest in light of the proposed installation of digital mammography across the NHS Breast Screening Programme.
Image quality is of paramount importance in mammography, and it has long been recognised that full-field digital mammography (FFDM) has many potential advantages over conventional screen–film mammography (SFM) [1–5]. A number of large studies have fully evaluated the diagnostic performance of this technology, notably the Digital Mammographic Imaging Screening Trial (DMIST), which showed the overall diagnostic accuracy of digital and film mammography as a means of screening for breast cancer to be similar, but digital mammography as more accurate in women under the age of 50 years, women with radiographically dense breasts and pre- or peri-menopausal women [5]. Several other studies have also looked at the use of digital mammography in screening, supporting the fact that FFDM is at least equal to SFM; the effect on recall rate, however, has varied. The Oslo I study comprised 3683 women aged 50–60 years and found no significant difference in cancer detection rates [3]. Direct side-by-side cancer conspicuity was equal; however, the recall rate for FFDM was slightly higher (4.6% vs 3.5%). Another paired screening study by Lewin et al [6] involving 6736 paired screen–film and digital mammography examinations performed in 4489 women again found no significant difference in cancer detection rates between the two modalities. In this study, however, the recall rate for FFDM was significantly lower than for SFM (11.8% vs 14.9%; p<0.001). More recently, the Oslo II study [4], a randomised trial involving more than 25 000 women attending for screening, found that the cancer detection rate for FFDM was superior to SFM in women aged 50–60 years (0.83% vs 0.54%). This almost reached statistical significance (p _ 0.053). For younger women aged 45–49 years, cancer detection rate was almost equal (0.27% vs 0.22%); however, the recall rate was significantly higher in both age groups for FFDM (p<0.05). Initial Food and Drug Administration (FDA) trials comparing FFDM and SFM in symptomatic patients found no significant difference in sensitivity or specificity [7, 8]. However, faster image acquisition associated with FFDM leads to increased productivity of a department and is of particular benefit for patients undergoing needle localisation procedures. By decoupling the tasks of image capture and display, both dynamic range and contrast resolution can be optimised, without the typical trade-off encountered in SFM [9, 10]. It avoids the problem of film artefact encountered in SFM and allows multiple ways of enhancing image quality after processing: the ability to change window width and lengths, roam and zoom images and apply post-processing algorithms to equalise tissue thickness or highlight specific features. As well as having the potential to improve image quality, this may impact favourably on overall dose to the patient. FFDM allows more efficient storage and transmission of data, enabling comparison of images across sites, and facilitates the use of computer-aided detection [11–13].
One area in which FFDM is surpassed by SFM is that of spatial resolution: SFM limiting resolution is typically 12–16 lpmm–1; that for FFDM is 5–10 lp mm–1. However, because lesions such as microcalcification become fainter as they become smaller, it is contrast resolution rather than spatial resolution that limits detectability [14, 15].
At our institution, we perform daily symptomatic breast clinics and have used GE FFDM equipment for more than seven years, thereby making us one of the most experienced units in using this technology in the UK. A year ago, we implemented “Premium View” software as part of a system and workstation upgrade. Premium View is a state-of-the-art post-processing breast algorithm developed by GE Medical Systems (GEMS, Waukesha, WI) to increase radiologists' diagnostic confidence by optimising mammographic image contrast resolution. It is offered as standard software on the Senographe DS unit and is additional to the thickness equalisation algorithm originally introduced with the Senographe 2000D unit. The aim of this study was to identify the effect of installation of Premium View on our mammographic reporting performance, in particular the effects on our recall rate for indeterminate mammography, as well as our biopsy rate and subsequent cancer detection rate.
Methods and materials
Audit committee approval was obtained for this study. Two GE Senographe DS mammogram machines (GE Medical Systems) were installed in our department in January 2007. Premium View software was implemented on a new mammographic workstation, with staff training occurring during January and February 2007. Premium View was applied as a post-processing algorithm and, although available to the radiologist and visible as standard on all mammograms viewed on the workstation, it could be toggled on or off manually at the touch of a button.
We reviewed retrospectively the case notes and imaging of all patients discussed at the weekly indeterminate imaging multidisciplinary team meeting. Mammographic reporting conventionally includes the use of a grading system from M1 to M5. A mammogram is recorded as M1 if it is normal, M2 if there is an abnormality that is deemed to be benign, M3 for an indeterminate abnormality, M4 for an abnormality that is felt likely to be malignant and M5 for an unequivocal malignancy. Patients with a mammographic grade of M3 and M4 were therefore discussed in the indeterminate imaging meeting.
Patients comprised those referred from symptomatic clinics and high- and moderate-risk family history clinics and those undergoing post-treatment follow-up mammograms. To assess the practice pre-software upgrade, data were collected from a three-month period immediately before the installation of Premium View (September to November 2006). To allow time for training and familiarity, data were not collected for January or February 2007. Instead, data were collected from March to May 2007 and also four months later (September to November 2007) in order to show whether practice varied with further experience. The total number of patients referred to the meeting for these time periods were 139 for September to November 2006, 138 for March to May 2007 and 136 for September to November 2007. Patients referred to that meeting owing to indeterminate clinical findings or indeterminate ultrasound findings or for any other reason other than a new indeterminate mammographic report (M3 or M4) were excluded from analysis. Patients discussed at the meeting on the basis of a mammographic abnormality performed at an outside hospital were also excluded from our study. Patients with a mammogram graded as M5 or highly suspicious for cancer were not discussed at this meeting, although overall mammographic cancer detection rates were available for analysis, and so effects on this rate were evaluated. The proportion of family history, symptomatic and wide local and mastectomy follow-up in each time period were relatively constant, as was the patient age range (30–88 years). The final number of patients assessed for each time period was 88 for September to November 2006, 114 for March to May 2007 and 88 for September to November 2007.
In the remaining study group of patients, factors recorded included the reported mammographic abnormality (soft tissue density/calcifications/distortion/asymmetry), further investigations (ultrasound/further mammographic views), biopsy (clinical/ultrasound-guided/stereotactic) and final histology. In particular, the effect of this software on our recall rate, biopsy rate and cancer detection rate was evaluated. Follow-up data were also available for most patients.
All film readers of the symptomatic mammograms were consultant radiologists with at least two years’ experience of FFDM reporting. All film readers performed regular breast radiology sessions and satisfied Royal College of Radiologists guidelines for symptomatic breast radiology practice [16]. All films were single read, with the exception of patients in family history screening, for whom the second read was undertaken by a breast clinician with more than 10 years’ mammographic experience and who satisfied Royal College of Radiologist guidelines for screening breast radiology practice [16]. In addition, all film readers regularly participated in breast audit and undertook the Personal Performance in Mammographic Screening (PERFORMS) test to evaluate individual performance [17].
Data were analysed using Fisher’s exact test, with our null hypothesis being that Premium View allows the radiologist to differentiate lesions from superimposed parenchyma with greater ease, and thus allows improved cancer detection rates.
Results
Before Premium View
In the initial time period before Premium View (September to November 2006), a total of 1556 mammograms were performed. The total discussed at the indeterminate mammogram meeting was 88, constituting a discussion rate of 5.66%.
The reported abnormalities necessitating discussion are shown in Table 1. 16 patients were subsequently downgraded at the indeterminate imaging multidisciplinary team meeting, without recall. Of the recalled patients, 33 in total underwent vacuum biopsy/core biopsy/fine-needle aspiration (FNA), of which 19 were ultrasound-guided biopsies, 13 were stereotactic biopsies and 1 was open surgical biopsy. This resulted in a total biopsy rate of 2.1%, with a stereotactic biopsy rate of 0.84%. In total, 28 non-malignant pathologies and 5 malignancies (1 ductal carcinoma in situ (DCIS), 1 invasive ductal carcinoma (IDC) and 3 DCIS + IDC) were detected. Our cancer detection rate for these indeterminate mammogram-detected cases during this time period was 0.32%. The overall mammographic cancer detection rate in the unit during this time period was 3.4%.
Table 1. Mammographic abnormality requiring discussion before Premium View.
| Abnormality | Number | % |
| Soft tissue density | 52 | 59.1 |
| Microcalcifications | 21 | 23.9 |
| Distortion | 5 | 5.7 |
| Asymmetry | 5 | 5.7 |
| Multiple abnormalities: | ||
| Microcalcification + distortion | 1 | 1.1 |
| ST density + distortion | 3 | 3.4 |
| Bilateral – ST density + microcalcification | 1 | 1.1 |
| Total | 88 | 100 |
ST, soft tissue.
After Premium View — early
In the time period immediately after the installation of Premium View (March to May 2007), a total of 1307 mammograms were performed. The number discussed at the indeterminate mammogram meeting was 114, constituting a discussion rate of 8.72%. The reported abnormalities necessitating discussion are shown in Table 2. 16 patients were subsequently downgraded at the indeterminate imaging multidisciplinary team meeting, without recall. Of the recalled patients, 54 underwent vacuum biopsy/core biopsy/FNA, of which 17 were ultrasound-guided biopsies (1 of which was bilateral), 3 clinical biopsies, 32 stereotactic biopsies and 2 ultrasound-guided biopsy followed by a stereotactic biopsy. The total biopsy rate for this period was 4.1%, with a stereotactic biopsy rate of 2.4%. There were, in total, 36 non-malignant pathologies and 14 malignancies (6 DCIS, 7 IDC and 1 IDC + DCIS) detected. The cancer detection rate for these indeterminate mammogram-detected cases during this time period was 1.1%. The overall mammographic cancer detection rate in the unit during this time period was 4.4%.
Table 2. Mammographic abnormality requiring discussion immediately after Premium View .
| Abnormality | Number | % |
| Soft tissue density | 54 | 47.4 |
| Microcalcifications | 44 | 38.5 |
| Distortion | 5 | 4.4 |
| Asymmetry | 2 | 1.8 |
| Multiple abnormalities | ||
| ST density + microcalcification | 3 | 2.6 |
| Asymmetry + distortion | 1 | 0.9 |
| ST density + distortion | 2 | 1.8 |
| Bilateral – ST density + microcalcification | 3 | 2.6 |
| Total | 114 | 100 |
ST, soft tissue.
After Premium View — delayed
In the final time period (September to November 2007), several months after the installation of Premium View, a total of 1460 mammograms were performed. The number discussed at the indeterminate mammogram meeting was 88, constituting a discussion rate of 6.03%. The reported abnormalities necessitating discussion are shown in Table 3. 21 patients were subsequently downgraded at the indeterminate imaging multidisciplinary team meeting, without recall. Of the recalled patients, 45 underwent vacuum biopsy/core biopsy/FNA, of which 22 were ultrasound-guided biopsies and 23 stereotactic biopsies. One further stereotactic biopsy was advised but was declined by the patient. The total biopsy rate for this group was 3.1%, with a stereotactic biopsy rate of 1.6%. There were, in total, 26 non-malignant pathologies and 19 malignancies (4 DCIS, 7 IDC, 5 DCIS + IDC and 3 invasive lobular carcinoma (ILC)) detected. The cancer detection rate for these indeterminate mammogram-detected cases during this time period was 1.3%. The overall mammographic cancer detection rate in the unit during this time period was 5.0%.
Table 3. Mammographic abnormality requiring discussion after Premium View (delayed) .
| Abnormality | Number | % |
| Soft tissue density | 38 | 43.2 |
| Microcalcifications | 30 | 34.1 |
| Distortion | 6 | 6.8 |
| Asymmetry | 3 | 3.4 |
| Multiple abnormalities | ||
| Microcalcification + distortion | 1 | 1.1 |
| ST density + distortion | 1 | 1.1 |
| ST density + microcalcification | 3 | 3.4 |
| ST density + asymmetry | 3 | 3.4 |
| Bilateral (ST density + microcalcification, bilateral microcalcifications, microcalcification + distortion) | 3 | 3.4 |
| Total | 88 | 99.9 |
ST, soft tissue.
When comparing the different time blocks (Table 4), it can be seen that the indeterminate mammography discussion rate significantly increased initially (p _ 0.002), but reverted to a level similar to that before Premium View after the delay (p _ 0.7). Overall and ultrasound-guided biopsy rates behaved similarly, showing an initial increase (p _ 0.002) followed by reversion after the delay to a level similar to that before Premium View (p _ 0.1). The stereotactic biopsy rate increased significantly initially (p _ 0.001) and, although decreasing after the delay, it remained higher than levels before Premium View (p _ 0.07). Interestingly, cancer detection rates in these indeterminate mammograms showed a steady increase both initially (p _ 0.02) and then again after the delay when compared with original levels (p _ 0.003).
Table 4. Indeterminate mammogram discussion rates, biopsy rates and cancer detection rates before and after Premium View.
| Discussion rate | Biopsy rate | Stereotactic biopsy rate | Cancer detection rate | |
| Before Premium View | 5.7% | 2.1% | 0.8% | 0.3% |
| After Premium View (early) | 8.7% | 4.1% | 2.4% | 1.1% |
| After Premium View (delayed) | 6% | 3.1% | 1.6% | 1.3% |
| p-value (before vs after early) | 0.002 | 0.002 | 0.001 | 0.02 |
| p-value (before vs after delayed) | 0.7 | 0.1 | 0.07 | 0.003 |
The breakdown of mammographic abnormalities that were subsequently found to be cancerous is shown in Table 5. This demonstrates an increase in the number of cancers found in both the microcalcification and soft tissue density groups.
Table 5. Mammographic abnormalities in the cancer cases.
| Mammographic abnormality | Before Premium View | After Premium View (early) | After Premium View (late) |
| Soft tissue density | 1 | 3 | 9 |
| Microcalcifications | 2 | 8 | 4 |
| Distortion | 1 | 2 | 1 |
| Asymmetry | 1 | ||
| Soft tissue density + microcalcifications | 1 | 2 | |
| Distortion + asymmetry | 1 | ||
| Soft tissue density + distortion | 1 | ||
| Microcalcifications + distortion | 1 | ||
| Total | 5 | 14 | 19 |
Discussion
There is little literature assessing the effect of Premium View on mammographic reporting and its subsequent radiological and clinical effects. A study by Muller et al [18] showed that the addition of Premium View led to increased conspicuity of lesions, as well as improved visualisation comfort, in a small reader preference study. A study performed by Kolb et al [19] and presented at the American Roentgen Ray Society (ARRS) in Miami in 2004 found that radiologists were able to review images more quickly, because of less time spent adjusting the window width and level, and with a greater level of diagnostic confidence when using Premium View. In addition, up to 46.6% more microcalcifications were detected compared with the standard tissue equalisation algorithm alone. A higher number of cancers were detected, although this was not statistically significant. However, previous work assessing the effect of post-processing algorithms on mammographic reader performance showed an unfavourable effect compared with SFM [20]. Premium View works as follows (GE, personal communication). The large-scale density variations in the image (e.g. density variations between glandular and fatty tissue) are isolated using a low spatial frequency filter; the resulting image is set as a mask. Small-scale contrast variations in the image (e.g. structure within glandular tissue) are isolated by subtracting the mask from the original image; a frequency-enhanced image is obtained. The mask is further processed and the frequency-enhanced image is further processed and weighted. The resulting images are then added together. The final image exhibits reduced contrast between different tissue types, but enhanced contrast of small-scale anatomical architecture.
Studies looking at the use of digital mammography in screening populations have so far failed to demonstrate any definite clinical benefits for women aged 50–70 years screened in the NHS Breast Screening Programme (NHSBSP) [3–5]. However, in women with dense breasts, and particularly those in the under-50 age group, this technology is evidenced to improve cancer detection rate [5]. It is now, however, government policy in the form of the new cancer reform strategy to roll out FFDM countrywide in the NHSBSP over the next few years [21]. Undoubtedly, the cost of installing FFDM (and therefore possibly Premium View) for the screening population would be significant, with current breast diagnostic units using FFDM in the UK being rather sparse. Although this small study reviewed only patients with a higher risk than those asymptomatic women screened through NHSBSP, the results are still applicable to a future NHSBSP population, as we were essentially recording a change in our practice in our stable population.
Clearly, the interim results and high recall rate represent a technical learning curve that is not surprising, as many new technologies in medicine can have this effect until operators have embedded them into practice. However, if these results were to be repeated in an NHSBSP unit, a similar increase in recall and biopsies may, in the short term, have devastating service implications, particularly in light of the new 62 day pathway, as outlined in the Cancer Reform Strategy [20]. Our results suggest that the increase in recall rate was primarily caused by an increased sensitivity for detecting microcalcifications: before Premium View, microcalcification was the reported abnormality necessitating recall in 23.9% of mammograms; this increased to 38.5% after the installation of Premium View and remained high at 34.1% (Figure 1). These results are in line with the only available data on Premium View (presented at the ARRS in 2004) [19], which also demonstrated a higher number of microcalcifications. In contrast to our data. however, that study did not find an increase in the cancer detection rate.
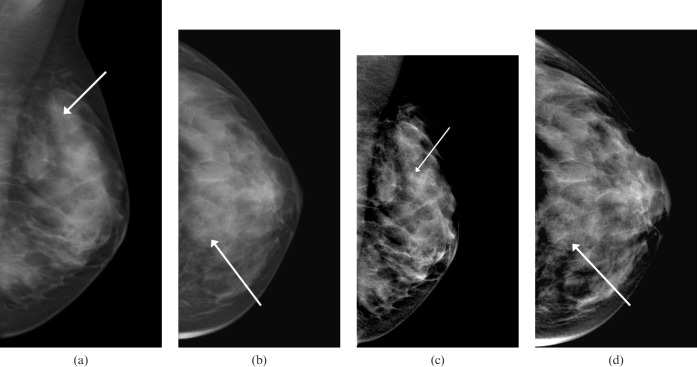
Figure 1.
A left-sided mammogram of a 45-year-old woman shows faint microcalcifications in the upper breast (arrows). (a,b) Images without Premium View show calcifications, but these are very faint and difficult to quantify. (c,d) The same mammographic images with Premium View applied show the calcifications to be much more contrasted, and easier to quantify in terms of appearance and extent. An increased soft tissue contrast in overlying breast tissue may allow the visibility of these to be further enhanced. These were subjected to a stereotactic biopsy, and high-grade ductal carcinoma in situ was obtained pathologically.
In addition to showing microcalcifications more conspicuously, Premium View also makes soft tissue more contrasted and, in theory, would make soft tissue densities (particularly in dense breast tissue) more evident. This, however, did not translate into a higher recall rate for possible soft tissue lesions; indeed, the converse was true. The rate dropped slightly from before Premium View to “early after” and then again to “delayed after” Premium View (Figure 2). It is difficult to explain this result with certainty, although perhaps a few more soft tissue lesions were recalled with Premium View owing to the increased contrast, counterbalanced by others that would have been called in patients with dense breasts that were dismissed owing to better visualisation of the breast tissue as a whole. This could, therefore, leave the recall rates fairly stable. Nevertheless, the cancer detection rate increased in the soft tissue density recall subgroup, and such a trend supports our hypothesis that Premium View is helping the radiologist differentiate between lesions and awkwardly superimposed normal parenchyma.
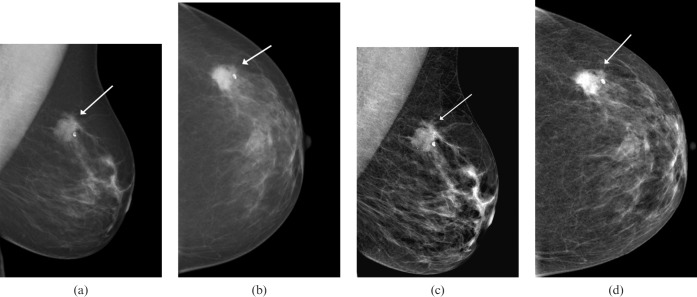
Figure 2.
A left-sided mammogram in a 65-year-old woman presenting with a left breast lump. (a,b) Images without Premium View show a highly suspicious lesion in the outer breast that is consistent with a carcinoma (arrows). The background breast pattern is largely fatty density. (c,d) The same mammographic images with Premium View applied show the lesion appearing slightly less suspicious on the mediolateral oblique (MLO) view than on the non-Premium View MLO image (arrows). In fatty breast parenchyma, this software probably adds little diagnostic value.
As well as the introduction of Premium View, our mammography units were changed from the GE Senographe 2000D system to the Senographe DS system. This in itself may have had a bearing on our diagnostic performance. The only way to have assessed this intervention alone would have been not to use Premium View at all, and reviewed only the standard processed images. This was not done in this study, but of course could be performed in future research. However, the exposure protocols performed on the DS system were matched as closely as possible to the exposure protocols performed on the 2000D system in terms of patient doses. In practical terms, this meant using “standard mode” up to 5 cm breast thickness and contrast thereafter; on the 2000D, we had used standard throughout.
Premium View was applied to the workstation as a toggle feature, such that the reader would automatically scroll through images with Premium View applied. At reader discretion, a button could be depressed to remove Premium View, showing only a standard processed image. All mammography was reported from the workstation and not the hospital's picture archive communication system (PACS), as Premium View is available only as part of the GE workstation. This means that only the reporting radiologists can see the Premium View images, with other clinicians (e.g. the surgeons) able to see only the standard processed images. Archived comparison mammography would not have had Premium View applied, and images often appeared quite different, even when there had clearly been no change in the woman's breast parenchyma. It was at this stage that Premium View would usually be toggled off briefly, in order to allow a closer comparison (if needed) of asymmetrical breast parenchyma. We did not assess reporting speeds during this process, as our small unit fortunately allowed us sufficient time to report these examinations in a timely fashion. However, it is suggested that, with such functionality available to the radiologist at reporting, in addition to the standard digital functions such as invert, magnification etc., this may be an issue in busier units with less experience of FFDM.
It is very likely in the impending digital mammography revolution that, unless a single manufacturer wins a nationwide contract, there will be a variety of post-processing software packages on the different manufacturers' mammogram workstations. Premium View needs both a linear raw image and a grey level-equalised image to work, and would most probably not work, as it is, on any other manufacturer's images. A key aspect of our study, however, is that the image processing package may affect diagnostic performance and it may be as important an aspect of the purchasing decision as the actual mammography unit itself.
References
- 1.James JJ. The current status of digital mammography. Clin Radiol 2004;59:1–10 [DOI] [PubMed] [Google Scholar]
- 2.Del Turco MR, Mantellini P, Ciatto S, Bonardi R, Martinelli F, Lazzari B, et al. Full-field digital versus screen-film mammography: comparative accuracy in concurrent screening cohorts. AJR Am J Roentgenol 2007;189:860–6 [DOI] [PubMed] [Google Scholar]
- 3.Skaane P, Young K, Skjennald A. Population based mammography screening: comparison of screen-film and full-field digital mammography with soft-copy reading: Oslo I study. Radiology 2003;229:877–84 [DOI] [PubMed] [Google Scholar]
- 4.Skaane P, Skjennald A. Screen film mammography versus full field digital mammography with soft copy reading: randomized trial in a population-based screening program: the Oslo II study. Radiology 2004;132:197–204 [DOI] [PubMed] [Google Scholar]
- 5.Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005;353:1773–83 [DOI] [PubMed] [Google Scholar]
- 6.Lewin JM, D'Orsi CJ, Hendrick RE, Moss LJ, Isaacs PK, Karellas A, et al. Clinical comparison of full-field digital mammography and screen-film mammography for detection of breast cancer. AJR Am J Roentgenol 2002;179:671–7 [DOI] [PubMed] [Google Scholar]
- 7.Hendrick RE, Lewin JM, D'Orsi CJ. Non-inferiority study of FFDM in an enriched diagnostic cohort: comparison with screen-film mammography in 625 women. In: Yaffe MJ, editor. IWDM 2000: 5th International workshop on Digital Mammography. Madison, WI: Medical Physics, 2001;475–81 [Google Scholar]
- 8.Cole E, Pisano ED, Brown M, Kuzmiak C, Braeuning MP, Kim HH, et al. Diagnostic accuracy of Fischer Senoscan digital mammography versus screen-film mammography in a diagnostic mammography population. Acad Radiol 2004;11:879–86 [DOI] [PubMed] [Google Scholar]
- 9.Pisano ED, Yaffe MJ. Digital mammography. Radiology 2005;234:353–62 [DOI] [PubMed] [Google Scholar]
- 10.Obenauer S, Luftner-Nagel S, von Heyden D, Munzel U, Baum F, Grabbe E. Screen film vs. full-field digital mammography: image quality, detectability and characterisation of lesions. Eur Radiol 2002;12:1697–702 [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Moon WK, Cho N, Cha JH, Kim SM, Im JG. Computer-aided detection in full-field digital mammography: sensitivity and reproducibility in serial examinations. Radiology 2008;246:71–80 [DOI] [PubMed] [Google Scholar]
- 12.Khoo LA, Taylor P, Given-Wilson RM. Computer-aided detection in the United Kingdom National Breast Screening Programme: prospective study. Radiology 2005;237:444–9 [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa RM. Computer-aided diagnosis complements full-field digital mammography. Diagn Imaging (San Franc) 1999;21:47–51 [PubMed] [Google Scholar]
- 14.Fischer U, Hermann KP, Baum F. Digital mammography: current state and future aspects. Eur Radiol 2006;16:38–44 [DOI] [PubMed] [Google Scholar]
- 15.Hermann KP, Obenauer S, Funke M, Grabbe EH. Magnification mammography: a comparison of full-field digital mammography and screen-film mammography for the detection of simulated small masses and microcalcifications. Eur Radiol 2002;12:2188–91 [DOI] [PubMed] [Google Scholar]
- 16.Royal CollegeofRadiologistsBreastGroup Guidelines for reporting breast imaging. Available from: http://www.rcr.ac.uk/docs/radiology/pdf/BreastSSC.pdf [Accessed 2 June 2009] [Google Scholar]
- 17.Scott HJ, Gale AG. doi: 10.1259/bjr/25049149. Breast screening: PERFORMS identifies key mammographic training needs. Br J Radiol. 2006 Dec; 79 Spec No2:SI27–33. [DOI] [PubMed] [Google Scholar]
- 18.Muller S, Lordache R, Levy L. Glandular tissue enhancement: improved display of digital mammography images. Eur Radiol 2003; 13(Suppl 1):149. ECR, Vienna, 7–11 March 2003. [Google Scholar]
- 19.Kolb TM, Lichy J, Mercado C, Koenigsberg T, Brown M. A novel post-processing algorithm for digital mammography: effect on conspicuity, sensitivity, speed and confidence. Am J Roentgenol 2004;182(4)(Suppl):11 [Google Scholar]
- 20.Cole EB, Pisano ED, Zeng D, Muller K, Aylward SR, Park S, et al. The effects of gray scale image processing on digital mammography interpretation performance. Acad Radiol 2005;12:585–95 [DOI] [PubMed] [Google Scholar]
- 21.Department ofHealth The Cancer Reform Strategy. December 2007. Available from: http://www.dh.gov.uk/publications [Accessed 2 June 2009] [Google Scholar]