Abstract
Desmoid tumours are rare, poorly circumscribed tumours that have a firm consistency and, although benign, have a remarkable tendency to infiltrate into surrounding structures. Extra-abdominal desmoid tumours involve mainly the extremities or the chest wall and are usually managed by wide radical resection. Moreover, desmoid tumours involving the chest wall are locally aggressive tumours with a high recurrence rate. We report a case of a pathologically proven desmoid tumour of the chest wall in a patient with a history of bilateral breast cancer and oesophageal cancer. We discuss the imaging appearances of this tumour on positron emission tomography combined with computed tomography (PET/CT) and magnetic resonance imaging.
Desmoid tumours are poorly circumscribed tumours that are firm, rubbery and have a remarkable tendency to infiltrate into surrounding structures with a strong propensity to recur locally after resection [1]. Desmoid tumours of the chest wall are uncommon tumours that have been described extensively in the pathological and surgical literature.
18F-fluoro-2-deoxy-d-glucose (FDG) positron emission tomography combined with computed tomography (FDG-PET/CT) has been shown to be very useful in the staging of patients with breast cancer and oesophageal cancer, as well as in the evaluation of treatment response [2]. It has a specific ability to discriminate responders from non-responders more accurately and earlier than conventional imaging methods [2]. PET/CT also plays an important role in the assessment of malignant soft-tissue tumours of the chest wall, such as sarcoma, by improving the accuracy of staging and helping to determine the appropriate therapy [3]. The PET/CT imaging characteristics of chest wall desmoid fibromatosis, a benign condition that may clinically mimic metastatic disease or sarcomatous degeneration, has not yet been reported to our knowledge.
We describe the PET/CT and MRI appearances of a biopsy-proven desmoid tumour of the chest wall in a patient with a history of bilateral breast cancer and oesophageal cancer.
Case report
We present the case of a 72-year-old female who had a left-sided breast cancer treated with lumpectomy in 1976, and a right-sided breast cancer treated with lumpectomy and radiotherapy in 1984. The patient was doing well until May 2004, when she presented with odynophagia. Oesophagogastroduodenoscopy and biopsy performed at that time were consistent with oesophageal squamous cell carcinoma and she was further treated with a three-hole oesophagectomy in 2004. Subsequently, two years later, the patient had a recurrent high-grade infiltrating ductal and lobular tumour in her left breast treated with total mastectomy and chemoradiation. Approximately one year ago, the patient noticed a lump underneath her right arm which, according to her, slowly progressed over time. She also began having some discomfort, particularly in the right upper arm and lateral chest area. On physical exam, there was a 7 × 6 cm firm subcutaneous mass in the right lateral chest wall beneath the scars from her thoracotomy and mastectomy. The mass was slightly mobile and tender on manipulation. Neurological symptoms such as numbness, tingling or weakness were not present. The differential diagnosis at this point included recurrent breast cancer, oesophageal cancer or radiation-induced sarcoma, considering the known increased risk of secondary neoplasm following radiotherapy [4].
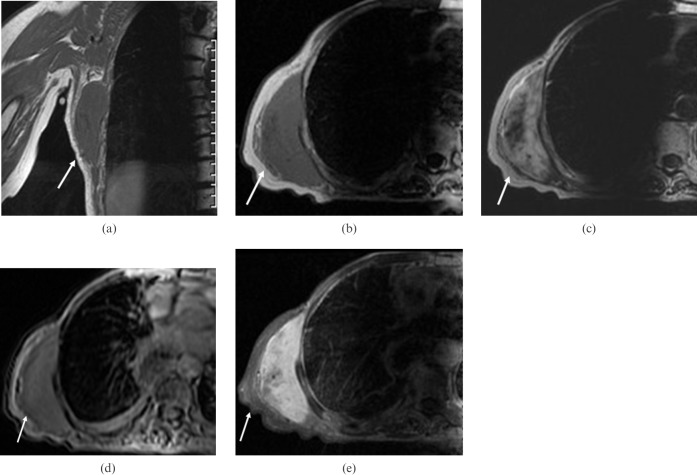
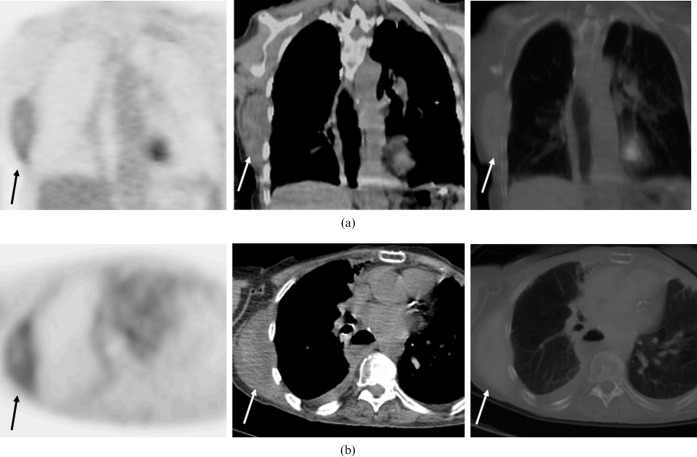
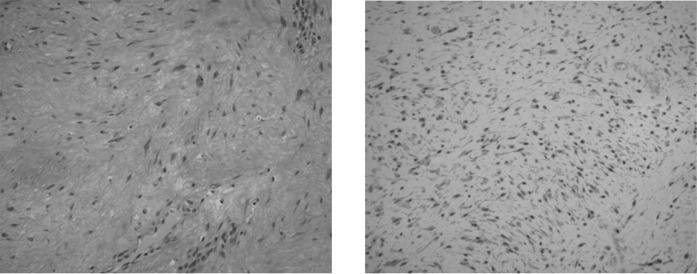
Magnetic resonance imaging (MRI) of the chest wall with and without contrast was performed and revealed an elliptical soft-tissue mass measuring 9 cm (long)×8 cm (anterior to posterior)×3 cm (transaxial) that was isointense to the muscle on T1-weighted images and hyperintense on T2-weighted images (Figure 1a). After gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ) administration, the mass showed homogeneous and early enhancement, without evidence of extension to the pleural or intercostal spaces (Figure 1b). The patient subsequently underwent FDG-PET/CT examination, which showed a large homogeneous soft-tissue mass measuring approximately 9.0 × 8.0 × 3.2 cm located between the chest wall and the latissimus dorsi muscle, beneath the inferior border of the scapula. This mass demonstrated only mild FDG tracer uptake, with a maximum standardised uptake value (SUVmax) of 1.7 (Figure 2). The degree of FDG uptake within the mass was less than that expected for a malignant process, such as sarcoma or metastasis, and was more suggestive of an indolent process. The patient subsequently underwent an ultrasound-guided core biopsy of the chest wall mass that revealed fibrous tissue with haemosiderin deposition, suggestive of a desmoid fibrous tumour. There was no evidence of malignancy. As the patient suffered discomfort in the posterior chest wall region, and because her concern for malignancy was still high, the decision was made to excise the mass. The resected mass was firm, well-circumscribed and embedded within skeletal muscle (Figure 3). Histological and immunohistochemical examination revealed that the tumour was composed of collagen fibres intermixed with scattered, bland-looking spindle cells that stained positive for β-catenin, consistent with desmoid fibromatosis (Figure 4).
Figure 1.
A 72-year-old woman with a history of breast and oesophageal cancer underwent MRI of the chest wall. (a) Coronal T1 and (b) axial T1 fast spin echo (FSE) weighted sequences. (c) Axial T2 single-shot fast spin echo (SSFSE) weighted sequences. (d) Heavily weighted T2 SSFSE and (e) T1 liver acquisition with volume acceleration (LAVA) with fat saturation post-contrast. On T1-weighted MRI images, the tumour shows a signal intensity that is less than or equal to that of muscle, whereas T2-weighted images have increased signal intensity with central areas of low signal that are thought to result from high collagen content. After contrast administration, the mass demonstrates intense and homogeneous enhancement.
Figure 2.
A 72-year-old woman with a history of breast and oesophageal cancer underwent FDG-PET/CT for further evaluation of a right-sided posterior chest wall mass. (a) Coronal images: FDG-PET alone, CT alone and fused FDG-PET/CT (from left to right). (b) Axial images: FDG-PET alone, CT alone and fused FDG-PET/CT (from left to right). There is a large soft-tissue homogeneous mass within the right posterior chest wall that measures approximately 8×3.2 cm located between the chest wall and the latissimus dorsi muscle right beneath the inferior border of the scapula. This mass shows only mild FDG tracer uptake, with an SUVmax of 1.7.
Figure 3.
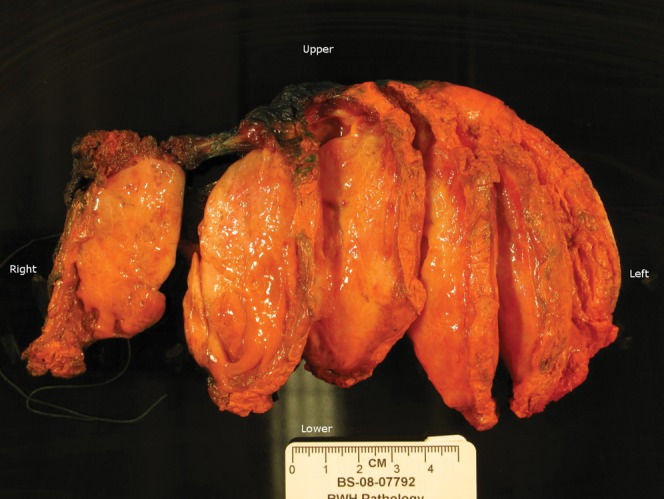
“Right chest wall mass” consists of a soft-tissue resection specimen (11.5 × 9.5 × 4.5 cm).
Figure 4.
Histological specimen with (a) haematoxylin and eosin and (b) β-catenin staining. The tumour is composed of collagen fibres and bland-looking spindle cells that stain positive for β-catenin, consistent with a desmoid fibrous tumour.
Discussion
Tumours of the chest wall are uncommon. They can be benign or malignant [4–6] and can be divided based on skeletal versus soft-tissue origin. Common soft-tissue neoplasms and non-neoplastic chest wall masses include peripheral nerve tumours, lipomas, liposarcomas, haemangiomas, elastofibromas, lymphoma, metastases from distant tumours, infectious lesions, desmoid tumours and malignant fibrous histiocytoma [7].
Desmoid tumours, also known as aggressive fibromatosis, are rare connective tumours that account for only 0.03% of all the neoplasms [8]. In an imaging and histological review of 44 soft-tissue tumours and mass-like lesions of the chest wall, O'Sullivan et al [7] found that only two cases (4.5%) represented desmoid tumours. These tumours are also locally aggressive and produce large exuberant masses characterised by local invasion and frequent recurrences, but they rarely metastasise [8, 9]. Desmoid tumours are usually non-inflammatory asymptomatic masses that become symptomatic when they compress surrounding structures, especially nerves. These tumours arise from connective tissue of fascia, aponeurosis or muscle striae; they do not show any significant mitotic activity or cytological features of malignancy [10].
Prior studies describing the CT findings of chest wall desmoid tumours have shown that these lesions have variable appearance and depend on the tumour composition, including the collagen content and amount of solid or necrotic tissue present [5]. Lesions with higher solid tissue components have greater attenuation and enhancement, and most lesions are confined by the surrounding fascia. CT usually reveals the size and location of the tumour precisely, but MRI is probably more sensitive in detecting soft-tissue infiltration and evidence of local recurrence [11, 12]. On MRI, these lesions have a similar signal to muscle on T1-weighted images, with very high signal on T2-weighted images [7]. Central areas of low signal are also seen on T2-weighted images, which is thought to be a result of the high collagen content [5]. In our patient, the MRI study demonstrated such an imaging appearance on T1- and T2-weighted images, with mild heterogeneity seen on the post-contrast images.
To the authors' knowledge, this is the first description of the FDG-PET/CT appearance a pathologically proven desmoid fibromatosis of the chest wall. In our study, mild FDG tracer localisation was seen in the mass located between the chest wall and latissimus dorsi muscle, beneath the inferior border of the scapula. The suggestion of a more indolent process was made based on the low level of tracer uptake (mean SUV of 1.4; SUVmax of 1.7), as well as on the well-defined appearance of the mass. We considered other possible causes of low-level FDG uptake in that area. Elastofibroma dorsi was a consideration; however, the typical layered appearance of interposed strands of lower density (attributed to fat) that have been previously described on CT [13] were not seen in this case. Lymphoma could have involved the skeletal muscle, but the extremely low tracer uptake was not that expected for an aggressive lymphomatous aetiology. Similarly, metastatic disease and soft-tissue sarcoma were considered less likely among our differential diagnosis owing to the low-level FDG uptake.
The widespread availability of FDG-PET and FDG-PET/CT hybrid systems can help in assessing masses of unclear aetiology and predicting benign versus malignant aetiology, and can aid in subsequent appropriate treatment planning, although pathological confirmation remains the gold standard. In this case, FDG-PET/CT helped to confirm the benign nature of a desmoid tumour suggested on MRI. It may also have a valuable role in monitoring the effect of systemic pharmacotherapy in patients with recurrent progressive disease after unsuccessful local–regional treatment [14]. However, as this is a rare entity with imaging features that may mimic other benign or malignant processes, biopsy or short-term interval follow-up should always be considered.
References
- 1.Dominguez-Malagon HR, Alfeiran-Ruiz A, Chavarria-Xicotencatl P, Duran-Hernandez MS. Clinical and cellular effects of colchicine in fibromatosis. Cancer 1992;69:2478–83 [DOI] [PubMed] [Google Scholar]
- 2.Eubank WB, Mankoff DA. Evolving role of positron emission tomography in breast cancer imaging. Semin Nucl Med 2005;35:84–99 [DOI] [PubMed] [Google Scholar]
- 3.Tateishi U, Yamaguchi U, Seki K, Terauchi T, Arai Y, Kim E. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 2007;245:839–47 [DOI] [PubMed] [Google Scholar]
- 4.Chapelier AR, Bacha EA, de Montpreville VT, Dulmet EM, Rietjens M, Margulis A, et al. Radical resection of radiation-induced sarcoma of the chest wall: report of 15 cases. Ann Thorac Surg 1997;63:214–19 [DOI] [PubMed] [Google Scholar]
- 5.Tateishi U, Gladish G, Kusumoto M, Hasegawa T, Tsuchiya R, Moriyama N, et al. Chest wall tumors: radiologic findings and pathologic correlation: part 1. Benign tumors. Radiographics 2003;23:1477–90 [DOI] [PubMed] [Google Scholar]
- 6.Tateishi U, Gladish GW, Kusumoto M, Hasegawa T, Tsuchiya R, Moriyama N, et al. Chest wall tumors: radiologic findings and pathologic correlation: part 2. Malignant tumors. Radiographics 2003;23:1491–508 [DOI] [PubMed] [Google Scholar]
- 7.O'Sullivan P, O'Dwyer H, Flint J, Munk P, Muller N. Soft tissue tumours and mass-like lesions of the chest wall: a pictorial review of CT and MR findings. Br J Radiol 2007;80:574–80 [DOI] [PubMed] [Google Scholar]
- 8.Kinzbrunner B, Ritter S, Domingo J, Rosenthal CJ. Remission of rapidly growing desmoid tumors after tamoxifen therapy. Cancer 1983;54:2011–14 [DOI] [PubMed] [Google Scholar]
- 9.Tsuchishima S, Nishizawa H, Matsumoto Y, Kitagawa S, Shimizu T, Yuasa K. A desmoid tumor of the chest wall. Kyobu Geka 1998;51:1055–9 [PubMed] [Google Scholar]
- 10.Kabiri EH, Al Aziz S, El Maslout A, Benosman A. Desmoid tumors of the chest wall. Eur J Cardiothoracic Surg 2001;19:580–3 [DOI] [PubMed] [Google Scholar]
- 11.Klein DL, Gamsu G, Gant TD. Intrathoracic desmoid tumor of the chest wall. AJR Am J Roentgenol 1977;129:524–5 [DOI] [PubMed] [Google Scholar]
- 12.Munden RF, Kemp BL. Desmoid tumor of the chest wall. Am J Roentgenol 1999;172:1149. [DOI] [PubMed] [Google Scholar]
- 13.Pierce JC, Henderson R. Hypermetabolism of elastofibroma dorsi on PET–CT. Am J Roentgenol 2004;183:35–7 [DOI] [PubMed] [Google Scholar]
- 14.Basu S, Nair N, Banavali S. Uptake characteristics of fluorodeoxyglucose (FDG) in deep fibromatosis and abdominal desmoids: potential clinical role of FDG-PET in the management. Br J Radiol 2007;957:750–6 [DOI] [PubMed] [Google Scholar]