Abstract
The purpose of this study was to evaluate the enhancement pattern of the normal facial nerve at 3.0 T temporal MRI. We reviewed the medical records of 20 patients and evaluated 40 clinically normal facial nerves demonstrated by 3.0 T temporal MRI. The grade of enhancement of the facial nerve was visually scaled from 0 to 3. The patients comprised 11 men and 9 women, and the mean age was 39.7 years. The reasons for the MRI were sudden hearing loss (11 patients), Méniàre's disease (6) and tinnitus (7). Temporal MR scans were obtained by fluid-attenuated inversion-recovery (FLAIR) and diffusion-weighted imaging of the brain; three-dimensional (3D) fast imaging employing steady-state acquisition (FIESTA) images of the temporal bone with a 0.77 mm thickness, and pre-contrast and contrast-enhanced 3D spoiled gradient record acquisition in the steady state (SPGR) of the temporal bone with a 1 mm thickness, were obtained with 3.0 T MR scanning. 40 nerves (100%) were visibly enhanced along at least one segment of the facial nerve. The enhanced segments included the geniculate ganglion (77.5%), tympanic segment (37.5%) and mastoid segment (100%). Even the facial nerve in the internal auditory canal (15%) and labyrinthine segments (5%) showed mild enhancement. The use of high-resolution, high signal-to-noise ratio (with 3 T MRI), thin-section contrast-enhanced 3D SPGR sequences showed enhancement of the normal facial nerve along the whole course of the nerve; however, only mild enhancement was observed in areas associated with acute neuritis, namely the canalicular and labyrinthine segment.
Imaging of the facial nerve is useful for the evaluation of pathological conditions. MRI of the facial nerve is usually performed selectively in cases of peripheral facial nerve palsy in patients with an atypical presentation or delayed recovery to exclude space-occupying lesions. The gadolinium-diethylene triamine pentaacetic acid (Gd-DTPA) contrast pulse sequence is the most informative MRI procedure for evaluation of facial nerve pathology [1]. Although many studies involving MRI of normal and paralysed facial nerves have been performed [2–10], there are no reports on the enhancement pattern of normal and abnormal facial nerves with 3.0 T MR scanning. The 3.0 T MR scan provides a higher signal-to-noise ratio (SNR), which allows a higher imaging matrix, thinner slices and a shorter time for scanning [11].
The purpose of this retrospective study was to evaluate the enhancement pattern of normal facial nerves, bilaterally, with 3.0 T temporal MRI.
Methods and materials
Contrast-enhanced 3.0 T temporal MRI was performed on 55 consecutive patients from February 2006 to March 2008. Among them, 35 patients were excluded from the study with one or more of the following conditions: facial nerve dysfunction, central facial paralysis, mass lesion of the eighth nerve or cerebellopontine angle, trauma, central nervous system or temporal infection or inflammation, neurofibromatosis and/or previous cranial surgery of any type. 20 patients were enrolled with clinically normal facial nerves bilaterally, and we retrospectively reviewed the enhancement pattern of 40 facial nerves. There were 11 men and 9 women, with an age range from 11 years to 60 years 3 months (mean age, 39.7 years). The reasons for undergoing MRI included sudden hearing loss (n _ 11), Méniàre's disease (n _ 6) and tinnitus (n _ 7).
Temporal MR scans were obtained with a 3.0 T MR scanner with the use of a head coil (SIGNA HDx®; GE, Milwaukee, WI). For the evaluation of the brain, T2 fluid-attenuated inversion-recovery (FLAIR) axial (repetition time/echo time/inversion time (TR/TE/IT) _ 9500/120/2250 ms), diffusion-weighted axial (TR/TE _ 8000/63.6 ms), and T1 FLAIR axial (TR/TE/IT _ 2600/8.9/920 ms) images with a 3 mm slice thickness and 0.1 mm slice gaps were obtained. For evaluation of the temporal bone, axial three-dimensional (3D) FIESTA (TR/TE _ 6.35/2.34 ms; flip angle _ 60°) images with a 0.77 mm slice thickness without gaps, with reformatted coronal and oblique sagittal images, were obtained. Pre-contrast 3D spoiled gradient record acquisition in the steady state (SPGR) images (TR/TE _ 6.06/2.6 ms; flip angle _ 120°) with a 22 cm field of view and a 1 mm slice thickness without gaps were obtained, and post-contrast 3D SPGR images were acquired immediately after an intravenous bolus injection of 0.1 mmol kg–1 Gd-DTPA (Gadovist® 1.0, Bayer Schering Pharma, Germany). The reformatted axial, coronal and bilateral oblique sagittal images were obtained (field of view _ 18 cm).
The facial nerve was divided into five anatomical segments for the evaluation of the enhancement patterns: (i) cisternal–internal auditory canalicular, (ii) labyrinthine, (iii) geniculate ganglion, (iv) tympanic and (v) mastoid or vertical. The signal intensities of the enhanced facial nerves on the 3D SPGR images were graded from 0 to 3: 0 _ no enhancement; 1 _ mild but definite enhancement; 2 _ moderate, but less than vascular enhancement; and 3 _ intense, similar to the signal intensity of vasculature.
The enhancement patterns of facial nerves were analysed by two radiologists at different times; the differences were then resolved in consensus.
Results
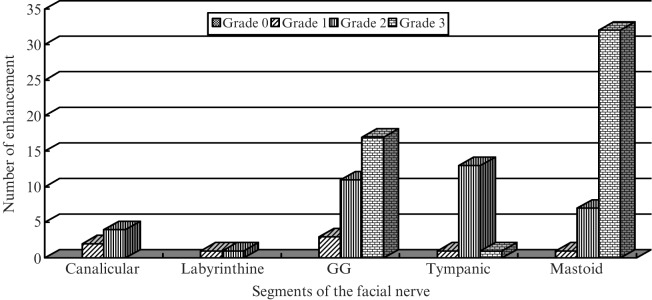
40 nerves (100%) were visibly enhanced along at least one segment of the facial nerve (Figures 1 and 2): the geniculate ganglion (77.5%; Grade 1, 3/40; Grade 2, 11/40; Grade 3, 17/40), tympanic segment (37.5%; Grade 1, 1/40; Grade 2, 13/40; Grade 3, 1/40), and mastoid segment (100%; Grade 1, 1/40; Grade 2, 7/40; Grade 3, 32/40). The results showed that even the facial nerve in the internal auditory canal (15%; Grade 1, 2/40; Grade 2, 4/40) and labyrinthine segments (5%; Grade 1, 1/40; Grade 2, 1/40) showed mild enhancement.
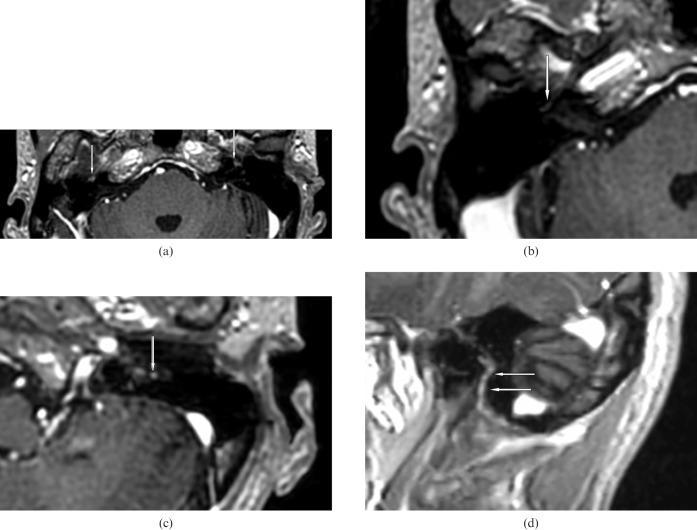
Figure 1.
A 46-year-old man with Méniàre's disease and normal facial nerves. (a) Post-contrast axial three-dimensional spoiled gradient record acquisition in the steady state MR scan demonstrates intense enhancement of the geniculate ganglion bilaterally (arrows) (Grade 3). (b) Right proximal tympanic segment (arrow) (Grade 1). (c) Axial scan of the left mastoid segment (arrow) shows a high-signal spot as intense as a visualised vessel (Grade 3). (d) Left parasagittal scan — the mastoid segment (arrows) is intensely enhanced and better visualised than in the axial scan.
Figure 2.
Graph illustrating the enhancement pattern of the normal facial nerve from the canalicular to mastoid segment. GG, geniculate ganglion.
Discussion
There have been many studies on the enhancement patterns of the normal facial nerve [1, 2, 9]. The normal facial nerve has been reported to enhance along at least one segment in 76% of cases, excluding the distal intrameatal and the labyrinthine segments [2]. The geniculate ganglion, greater superficial petrosal nerve and the proximal tympanic segment generally demonstrate the most easily detected enhancement [2–4]. The cisternal, intracanalicular and labyrinthine segments normally do not enhance [2, 9]. Contrary to our results, the distal tympanic, mastoid and parotid segments also usually do not enhance. This pattern of contrast enhancement can be explained by the flux of contrast material in the lush circumneural arteriovenous plexus supplying the facial nerve in the facial canal, which abruptly terminates at the labyrinthine segment and the stylomastoid foramen. The markedly reduced flow of contrast material into the compressed endoneural capillaries in the labyrinthine segment may prevent flow of contrast material into this segment, resulting in minimal enhancement [2].
The intense enhancement of the meatal segment is considered pathological and is observed in Bell's palsy [3–10]. In this condition, enhancement of the facial nerve is caused by a breakdown of the blood–peripheral nerve barrier and subsequent diffusion of contrast material into the endoneural space. In addition, venous congestion may contribute to the enhancement. Balkany et al [12] in their study on human temporal bones and fresh cadaver nerves demonstrated that the labyrinthine segment of the facial nerve, similar to the cat [13], contains fewer and smaller intrinsic blood vessels than do the mastoid and tympanic segments. They suggested that the labyrinthine segment of the facial nerve is the most vulnerable portion. In addition, the fallopian canal is the narrowest in the labyrinthine segment; the nerve fills the entire canal in this region, which contributes to the vulnerability of this particular segment [13–15]. In our study, mild enhancement of the labyrinthine segment and distal meatal segment was observed in normal facial nerves. A higher grade of intense enhancement is seen only in pathological conditions, such as Bell's palsy (Figure 3). This may be explained by the poor vascularity of this segment, as demonstrated by Balkany et al [12, 13]. In previous studies, the descending portion of the nerve appeared to enhance much less frequently. However, in our study, the mastoid segment was an intensely enhanced segment (32/40; 80.0%) compared with the geniculate ganglion (17/40; 42.5%). In previous studies, only axial scans were obtained; because the orientation of the mastoid segment is perpendicular to the plane of MRI, the axial scans resulted in a low detection rate. We obtained thinner slices without gaps, in addition to parasagittal planes along the facial nerve; these technical factors contributed to the improved detection rate. Balkany et al [12, 13] showed a statistically different capillary density when comparing tympanic and mastoid segments; the mastoid segment showed a higher capillary density than did the tympanic segment. In addition to the advances in MR technique, these findings show that the entire mastoid segment is enhanced and higher grade enhancement is frequent. It is important to take into consideration the variation in the scanners used in order to explain some of the differences reported in studies on the facial nerve.
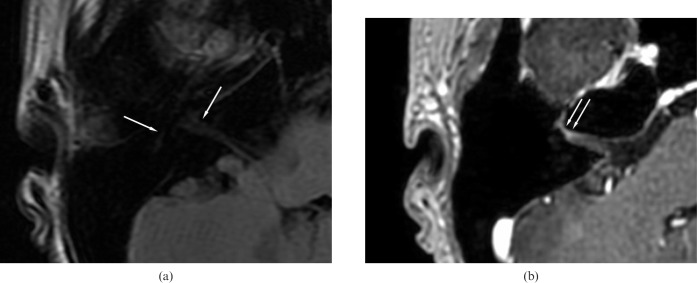
Figure 3.
A 32-year-old man with right facial palsy: MR scan 7 days after onset of symptoms. (a) Pre-contrast axial T1 fluid-attenuated inversion-recovery axial image of the facial nerve (arrows) at the cerebellopontine angle (CP) angle. (b) Contrast-enhanced three-dimensional spoiled gradient record acquisition in the steady state scan shows intense enhancement of the distal meatal and labyrinthine segments of the right facial nerve (arrows) (Grade 3).
In addition to normal enhancement of the facial nerve, the facial nerve may also enhance in association with many pathological conditions, including acute idiopathic peripheral facial paralysis (Bell's palsy), infections, inflammation (e.g. post-radiation injury), cholesteatoma, trauma, previous surgery, neoplasms and neurofibromatosis [3–10, 14]. In our experience with Bell's palsy, the distal meatal and labyrinthine segments are characteristically intensely enhanced; therefore, intense enhancement of these segments is considered pathological. Ipsilateral facial nerve enhancement, predominantly at the meatal and labyrinthine segments, has been reported previously in Bell's palsy [16]. In the labyrinthine segment, the nerve occupies nearly the entire fallopian canal, and at the tympanic and mastoid segment it occupies approximately 30–50% of its bony canal [14, 15]. In patients with Bell's palsy, oedema and haemorrhagic infarction occur regularly in the labyrinthine segment [10, 14]. This may indicate that the labyrinthine segment might be more vulnerable to ischaemic damage, consistent with the previously demonstrated narrowness of the labyrinthine and fallopian canal [13–15]. These results explain the intense enhancement of the distal meatal and labyrithine segments in Bell's palsy.
Conclusions
In conclusion, the results of this study show that MR images from 3.0 T scanners demonstrate enhancement of the normal facial nerve along the whole course of the nerve. However, only mild enhancement was observed in the areas associated with acute neuritis, namely the canalicular and labyrinthine segments.
References
- 1.Kumar A, Mafee M, Mason T. Value of imaging in disorders of the facial nerve. Topics Magn Reson Imag 2000;11:38–51 [DOI] [PubMed] [Google Scholar]
- 2.Gebarski SS, Telian SA, Niparko JK. Enhancement along the normal facial nerve in the facial canal: MR imaging and anatomic correlation. Radiology 1992;183:391–4 [DOI] [PubMed] [Google Scholar]
- 3.Tien R, Dillon WE, Jackler RK. Contrast-enhanced MR imaging of the facial nerve in 11 patients with Bell's palsy. AJNR Am J Neuroradiol 1990;155:573–9 [PMC free article] [PubMed] [Google Scholar]
- 4.Sartoretti-Schefer S, Wichmann W, Valavanis A. Idiopathic, herpetic and HIV-associated facial nerve palsies: abnormal MR enhancement patterns. AJNR Am J Neuroradiol 1994;15:479–85 [PMC free article] [PubMed] [Google Scholar]
- 5.Kohysu H, Aoyagi M, Tojima H, Tada Y, Inamura H, Ikarashi T, et al. Facial nerve enhancement in Gd-MRI in patients with Bell's palsy. Acta Otolaryngol Supp1 1994;511:165–9 [DOI] [PubMed] [Google Scholar]
- 6.Saatci I, Sahinturk F, Sennaroglu L, Boyvat F, Gursel B, Besim A. MRI of the facial nerve in diopathic facial palsy. Eur Radiol 1996;6:631–6 [DOI] [PubMed] [Google Scholar]
- 7.Sartoretti-Schefer S, Brandle P, Wichmann W, Valavanis A. Intensity of MR contrast enhancement does not correspond to clinical and electroneurographic findings in acute inflammatory facial nerve palsy. AJNR Am J Neuroradiol 1996;17:1229–36 [PMC free article] [PubMed] [Google Scholar]
- 8.Jager L, Reiser M. CT and MR imaging of the normal and pathologic conditions of the facial nerve. Eur J Radiol 2001;40:133–46 [DOI] [PubMed] [Google Scholar]
- 9.Kress BPJ, Griesbeck F, Efinger FK, Solbach T, Gottschalk A, Kornhuber AW, et al. doi: 10.1007/s00234-001-0738-y. Bell's palsy: what is the prognostic value of measurements of signal intensity increases with contrast enhancement on MRI? Neuroradiology 2002:44:428–33. [DOI] [PubMed] [Google Scholar]
- 10.Kress B, Griesbeck F, Stippich C, Bahren W, Sartor K. Bell palsy: quantitative analysis of MR imaging data as amethod of predicting outcome. Radiology 2004;230:504–9 [DOI] [PubMed] [Google Scholar]
- 11.Runge VM, Case RS, Sonnier HL. Advances in clinical 3-Tesla neuroimaging. Invest Radiol 2006;41:63–7 [DOI] [PubMed] [Google Scholar]
- 12.Balkany T, Fradis M, Jafek BW, Rucker NC. Intrinsic vasculature of the labyrinthine segment of the facial nerve — implications for site of lesion in Bell's palsy. Otolaryngol Head Neck Surg 1991;104:20–3 [DOI] [PubMed] [Google Scholar]
- 13.Balkany T. The intrinsic vasculature of the cat facial nerve. Laryngoscope 1986;96:70–7 [DOI] [PubMed] [Google Scholar]
- 14.Fisch U, Esslen E. Total intratemporal exposure of the facial nerve. Arch Otolaryngol 1972;95:335–41 [DOI] [PubMed] [Google Scholar]
- 15.Ogawa A, Sando I. Spatial occupancy of vessels and facial nerve in the facial canal. Ann Otol rhinol Laryngol 1982;91:14–19 [DOI] [PubMed] [Google Scholar]
- 16.Engstrom M, Abdsaleh S, Ahlstrom H, Johansson L, Stalberg E, Jonsson L. Serial GD-enhanced MRI and assessment of facial nerve involvement in Bell's palsy. Otolaryngol Head Neck Surg 1997;117:559–66 [DOI] [PubMed] [Google Scholar]