Abstract
Acute pancreatitis is a common condition (thought to be increasing in incidence worldwide), which has a highly variable clinical course. The radiologist plays a key role in the management of such patients, from diagnosis and staging to identification and treatment of complications, as well as in determining the underlying aetiology. The aim of this article is (i) to familiarise the reader with the pathophysiology of acute pancreatitis, the appearances of the various stages of pancreatitis, the evidence for the use of staging classifications and the associated complications and (ii) to review current thoughts on optimising therapy.
The International Symposium on Acute Pancreatitis (AP) in Atlanta defined AP as inflammation of the pancreas with variable secondary involvement of remote organs [1]. The incidence ranges from 5 to 80 per 100 000, with the highest incidence occurring in the USA and Finland [2]. In the UK, the incidence of AP requiring hospital admission has doubled in the past three decades, from 4.9 per 100 000 (1963–1974) to 9.8 per 100 000 (1987–1998) [3]. The severity of AP is highly variable; it can range from mild and self-limiting to fulminant. The latter occurs in 20–30% of all cases of AP and is associated with a protracted clinical course, often complicated by sepsis, multiorgan failure and a mortality rate of up to 50% [1]. It is widely accepted that these two subgroups are separate entities; mild pancreatitis (also known as oedematous AP) rarely progresses to the fulminant necrotising subtype. Clearly, the prognosis and management for these two subgroups of AP are very different. In mild oedematous AP, management is primarily supportive, whereas necrotising AP usually requires care in an intensive unit setting with a combination of surgical and radiological interventions.
The commonest aetiological factors for AP are cholelithiasis and alcohol; the former is more prevalent in southern Europe, whereas alcohol-induced pancreatitis is more common in northern Europe. Alcohol is also known to be associated with a higher incidence of acute fulminant pancreatitis [4]. Other less common causes for AP include iatrogenic causes such as endoscopic retrograde cholangiopancreatography (ERCP), abdominal surgery, trauma, congenital pancreatic divisum, hyperlipidaemia, hypercalcaemia and various infections.
The initial diagnosis for AP is made clinically from signs and symptoms of an acute abdomen and an elevation of pancreatic enzymes, such as amylase and lipase, in the blood or urine. Once the diagnosis is confirmed, it is usually evident clinically within the first 48–72 h as to whether the condition will be mild or fulminant [5]. Mild pancreatitis is characterised by minimal or absent systemic organ dysfunction and tends to abate by the third day. In contrast, fulminant pancreatitis demonstrates progressive clinical symptoms and signs with associated metabolic and multiorgan dysfunction. Since the 1980s, many clinical scoring systems, such as Ranson's criteria [6] and the APACHE II (Acute Physiology and Chronic Health Evaluation) score [7], have been used to provide an objective assessment of the severity of pancreatitis.
Pathophysiology of acute pancreatitis
The theory of AP that is the most widely accepted is of an initial insult to the pancreatic acinar cells. Following the insult, there is inappropriate intracellular activation of trypsinogen and other digestive or proteolytic enzymes, with production of oxygen free radicals. These activated enzymes and free radicals injure the acinar cell, which results in the release of cytokines (interleukin (IL)-1, IL-6 and IL-8), tumour necrosis factor-α and vasoactive mediators, such as nitric oxide. The released inflammatory molecules have local effects, including disturbance of the pancreatic microcirculation [8] and stimulation of apoptosis [9], leading to oedema and further cell death.
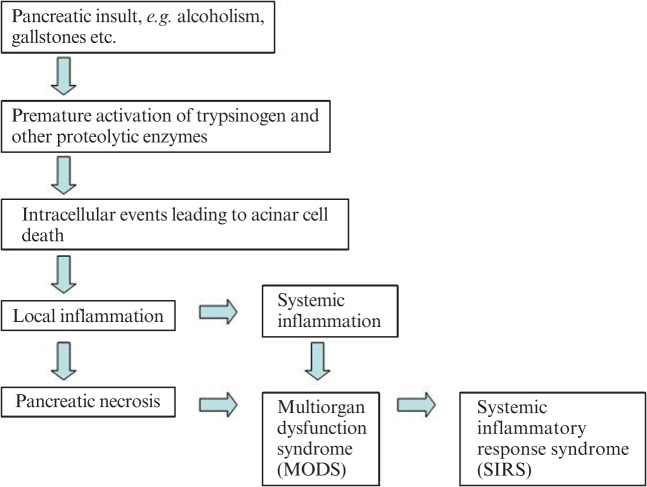
Depending on the severity of pancreatitis, local inflammation can progress to a systemic inflammatory response; if the patient deteriorates further, multiorgan dysfunction syndrome develops, which accounts for the increased morbidity and mortality [10] (Figure 1).
Figure 1.
Cascade of events in the development of severe acute pancreatitis.
Imaging features
Imaging plays a vital role in the management of pancreatitis. It enables diagnosis and differentiation of the severity of this condition. This is crucial for guiding clinical management and has prognostic value. In addition, it helps to identify and manage the associated complications with image-guided drainage and aspiration. Ultrasound is frequently the first investigation performed on admission; although it has little value in the diagnosis of pancreatitis or its complications, the early identification of gallstones and biliary dilatation may help identify those patients with a possible impacted calculus in the bile duct. Early identification and treatment of these calculi may have a significant positive impact on outcome. When imaging pancreatitis, contrast-enhanced CT is the most clinically useful investigation.
Mild acute pancreatitis
In mild pancreatitis, the CT features range from a normal-appearing pancreas with no peripancreatic abnormalities to diffuse enlargement and heterogeneous attenuation of the gland with ill-definition of the border. Peripancreatic inflammation results in hazy or reticular stranding of the surrounding fat [11] (Figure 2). In up to 18% of cases, mild pancreatitis demonstrates segmental–focal involvement, usually of the pancreatic head [12] and uncinate process. This can result in groove pancreatitis with scar tissue forming in the space between the dorsocranial part of the head of the pancreas, the duodenum and the common bile duct (Figure 3). This focal pancreatitis is common in patients with a history of chronic alcohol abuse.
Figure 2.
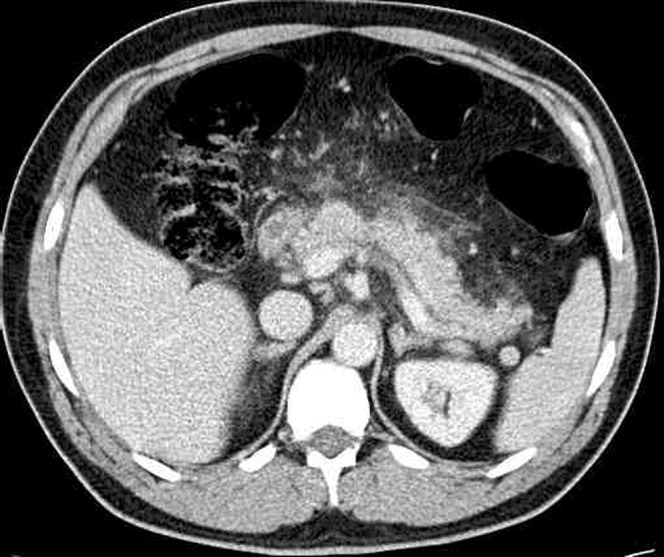
Mild pancreatitis with stranding of the surrounding fat and normal pancreatic enhancement.
Figure 3.
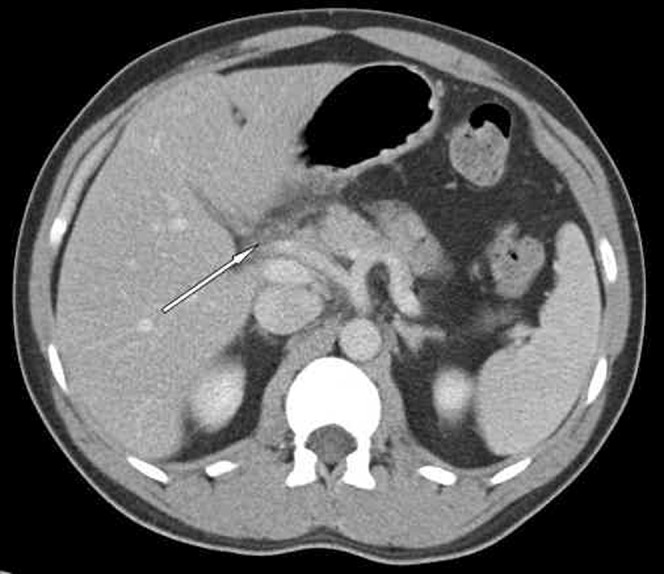
Groove pancreatitis with pancreatic inflammation localised to the area adjacent to the duodenum (arrow).
Severe acute pancreatitis
In severe pancreatitis, there is, in addition to the features stated above, a lack of normal enhancement of part of the pancreas or the entire pancreas, in keeping with necrosis (Figure 4). This usually becomes evident a few days after the onset of symptoms, and can therefore be missed if the patient is imaged too early [5]. Areas of non-enhancement, especially when >3 cm or >30% of the pancreatic volume, are considered a reliable CT sign for necrosis. However, in minor necrosis (<30% of the gland), CT has a false-negative rate of 21% [12]. Pancreatic necrosis is an important prognostic feature on CT and has been shown to correlate well with the clinical severity of AP, with an increased incidence of complications, morbidity and mortality [13].
Figure 4.
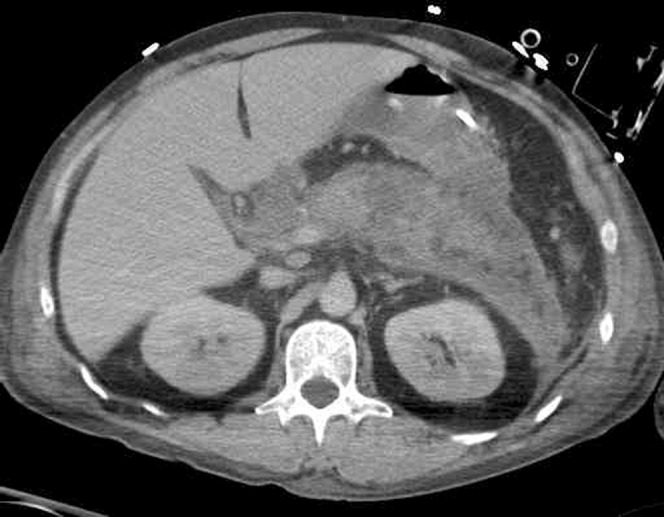
Areas of non-enhancement of the pancreas, in keeping with necrosis. Also note the stranding and thickening of the flank soft tissues — Grey Turner's sign.
Other characteristics of severe pancreatitis include more extensive peripancreatic inflammatory change than is seen in mild acute pancreatitis. Usually, this is associated with focal fluid collections, which consist of sterile pancreatic fluid rich in pancreatic enzymes (Figure 5). These fluid collections occur in approximately 40% of cases; around half of these will resolve spontaneously. Those that do not resolve may be complicated by infection or haemorrhage. Others may evolve to become pseudocysts [12].
Figure 5.
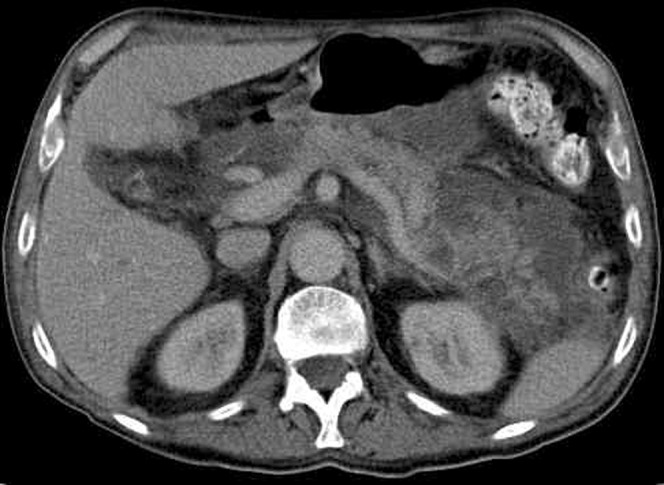
Multiple peripancreatic fluid collections with patchy non-enhancement of the pancreas.
Staging
In addition to the diagnostic value of imaging in AP, CT enables one to determine the severity of this condition. This adds an early prognostic value to the study and helps clinicians decide on whether intensive monitoring and specific therapies are required. Balthazar et al [12] developed a grading system (Table 1) for pancreatic inflammation and later refined it with the CT severity index (CTSI) score (Table 2). In addition to the degree of pancreatic inflammation, the index also takes into account the amount of necrosis. These two features are the most important prognostic factors in pancreatitis imaging and are associated with a protracted clinical course, a higher complication rate and increased mortality. In our institution, the timing of the initial study depends on the clinical scenario. Early studies may be indicated if there is uncertainty about the diagnosis or a high degree of clinical suspicion that there may be a significant complication. However, pancreatic necrosis becomes more obvious after 72 h [12, 14]. A statistically significant correlation between the rate of morbidity and mortality and the CTSI score has been established. Patients scoring 0–1 point exhibit no morbidity or mortality, whereas those with an index of 2 have a morbidity rate of 4% and no mortality. In contrast, an index of 7–10 yields a 17% mortality and 92% complication rate [12]. Although the CTSI is a widely accepted staging system, it does have its limitations. It is recognised to have only moderate interobserver correlation of up to 75% [15]. There is also variable correlation with established clinical scoring systems such as Ranson and APACHE II [5].
Table 1. CT grading system for acute pancreatitis.
| Grade | CT finding |
| A | Normal pancreas |
| B | Pancreatic enlargement |
| C | Pancreatic inflammation and/or peripancreatic fat |
| D | Single peripancreatic fluid collection |
| E | Two or more fluid collections and/or retroperitoneal air |
Table 2. CT grading system for acute pancreatitis.
| Necrosis |
||||
| CT grade | Points | Percentage necrosis | Additional points | Severity Indexa |
| A | 0 | 0 | 0 | 0 |
| B | 1 | 0 | 0 | 1 |
| C | 2 | <30% | 2 | 4 |
| D | 3 | 30–50% | 4 | 7 |
| E | 4 | >50% | 6 | 10 |
aCT grade points are added to points assigned for percentage of necrosis.
Serial imaging should not be performed routinely in mild pancreatitis, with repeat CT studies indicated primarily where there is a change in clinical status suggesting that there is an evolving complication. In severe cases, follow-up studies should be performed routinely in order that complications may be identified and managed at an early stage. It should be borne in mind that radiological resolution will lag behind clinical improvement.
MRI potentially offers some advantages over CT in AP, over and above radiation considerations. Several studies have shown that MRI is similar to contrast-enhanced CT for staging the severity of AP and providing diagnostic and prognostic value [16, 17]. There is superior tissue characterisation of the pancreatic parenchyma, and the use of heavily T2 weighted sequences enables evaluation of ductal integrity, an important prognostic factor [18]. MRI may help in the differentiation of fluid collections from partly liquefied necrotic tissue; the former can be drained percutaneously, whereas the latter may require necrosectomy. It does, however, have significant practical limitations in acutely ill patients who are usually connected to multiple lines and monitoring devices. Furthermore, the duration of an MRI study is far longer than CT, and many patients have difficulty co-operating with breath-hold sequences. Hence, the major role of MRI in AP is in determining the aetiology, particularly calculi and pancreas divisum (Figure 6).
Figure 6.
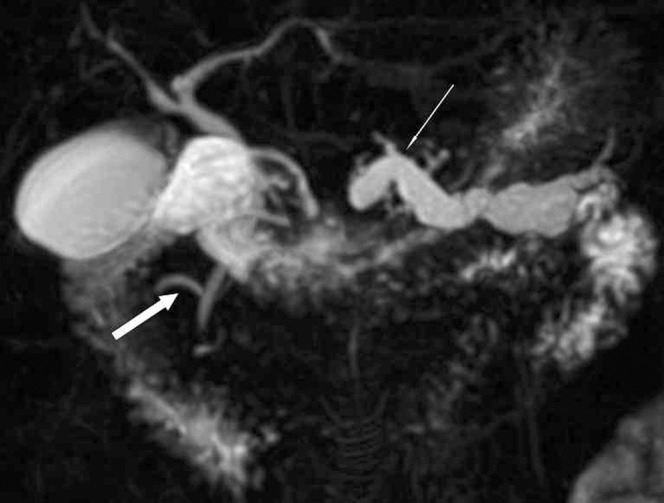
Three-dimensional maximum intensity projection image from a magnetic resonance cholangiopancreatography study demonstrating pancreas divisum (thick arrow) and a dilated obstructed pancreatic duct (thin arrow).
Endoscopic ultrasound (EUS) has evolved into an important technique for assessing pancreatico-biliary disease, but is not commonly used in the setting of AP. However, it has a sensitivity of 91% for detecting common bile duct calculi compared with 50% for transabdominal ultrasound [19]. In patients with AP of unknown aetiology, it is useful for the detection of gallstones, occult tumours and pancreatic duct abnormalities such as pancreatic divisum.
Complications
Infected necrosis
Necrosed pancreatic and/or peripancreatic tissue may become infected. The patient is usually critically ill and septic; complicating sepsis accounts for more than 80% of deaths related to acute pancreatitis. The risk of developing infected necrotic tissue increases with prolonged illness and reaches a peak at 3 weeks, by which time 60% of patients with acute necrotising pancreatitis would have been affected [11]. The only imaging feature to help the radiologist recognise this serious complication is the presence of gas locules within areas of parenchymal necrosis (Figure 7). When this is absent, infection cannot be diagnosed unless percutaneous aspiration is performed [12]. Historically, infected necrosis has been treated with surgical necrosectomy but, increasingly, aggressive percutaneous drainage procedures are being favoured owing to the high peri-operative mortality in this condition [20].
Figure 7.
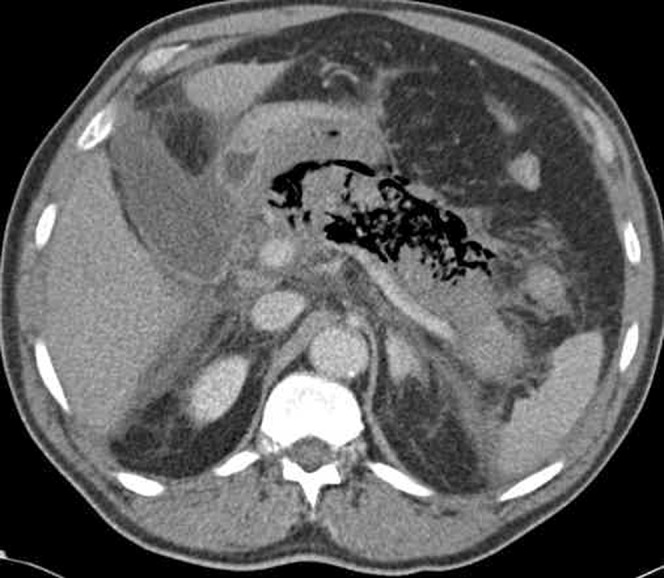
Infected pancreatic necrosis with gas locules.
Pancreatic abscess
Pancreatic abscess is far less common than infected necrosis, occurring in 3% of patients with severe acute pancreatitis. It occurs following bacterial infection of peripancreatic fluid collection. This is typically a late complication, presenting 4–6 weeks after the onset of symptoms [12]. It appears as a well-circumscribed fluid attenuation collection with no pancreatic necrosis detected within. It usually has a thick enhancing wall and contains locules of gas (Figure 8). Although frequently located in close proximity to the pancreas, these abscesses can be found anywhere in the abdomen and pelvis. Pancreatic abscesses are typically managed with percutaneous drainage.
Figure 8.
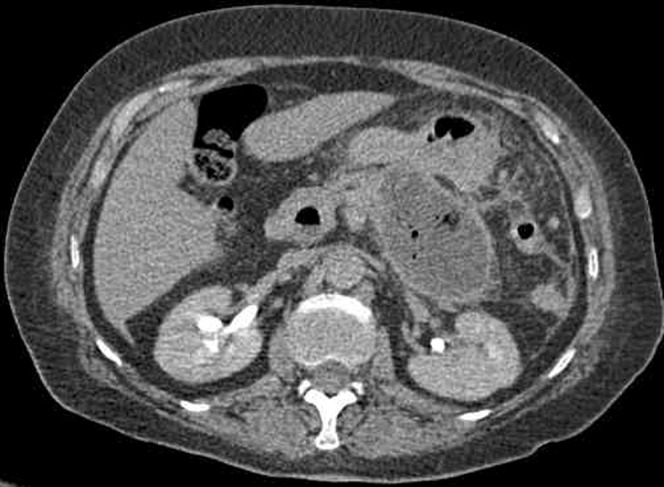
Pancreatic abscess with a typical enhancing rim and gas locules.
Pseudocysts
Pseudocysts evolve from acute peripancreatic fluid collections and occur in 2–18% of patients following AP [21]. They are most commonly found in the pararenal space or the lesser sac. Occasionally, these collections can track distally into other locations, such as the mediastinum (Figure 9). Pseudocysts consist of sterile pancreatic fluid, initially constrained by anatomical fascial planes, which become encapsulated by an inflammatory wall. This process takes at least 4 weeks to occur, and thus the diagnosis should not be made earlier than this. The typical CT features are of a fluid collection (<15 Hounsfield units) with a smooth thin wall forming a round or ovoid configuration [22]. Most pseudocysts, especially if <6 cm, are asymptomatic and either resolve spontaneously (40%) or remain stable in size [23]. In a small but significant number of patients, the pseudocyst continues to enlarge and may become symptomatic with abdominal pain. They can become complicated with infection (abscess), haemorrhage or gastric outlet obstruction (Figure 10). EUS is increasingly used for performing endoscopic cystgastrostomy in patients with a persistent pseudocyst (Figure 11).
Figure 9.
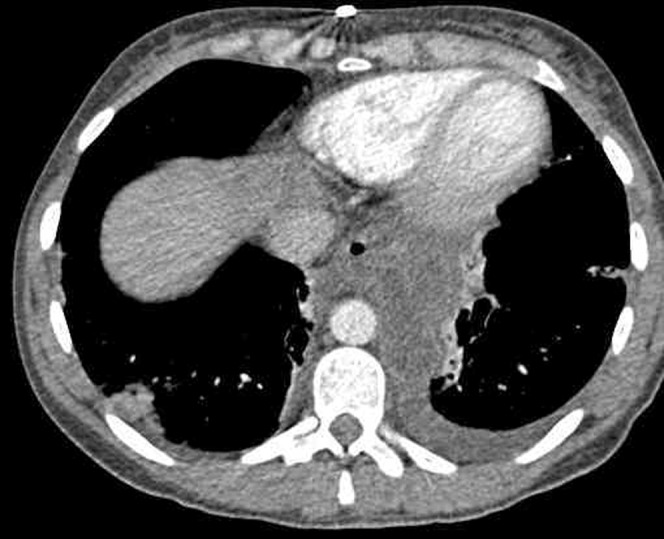
Mediastinal pseudocyst in a patient who had been treated for acute severe pancreatitis.
Figure 10.
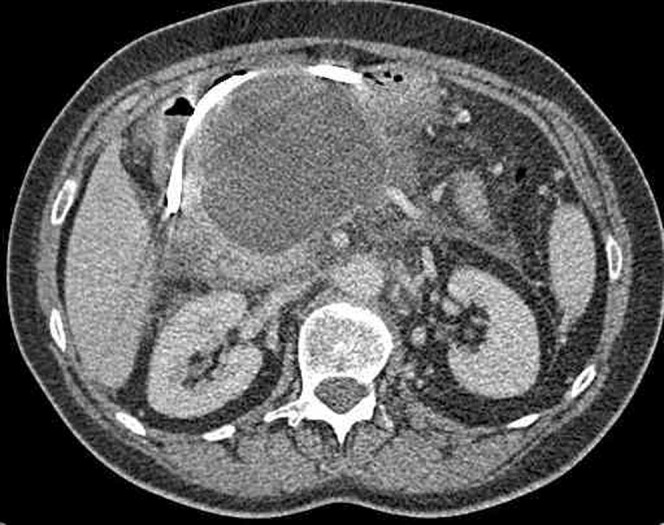
Large pseudocyst that is displacing and compressing the stomach anteriorly. There is a nasogastric tube within the gastric lumen.
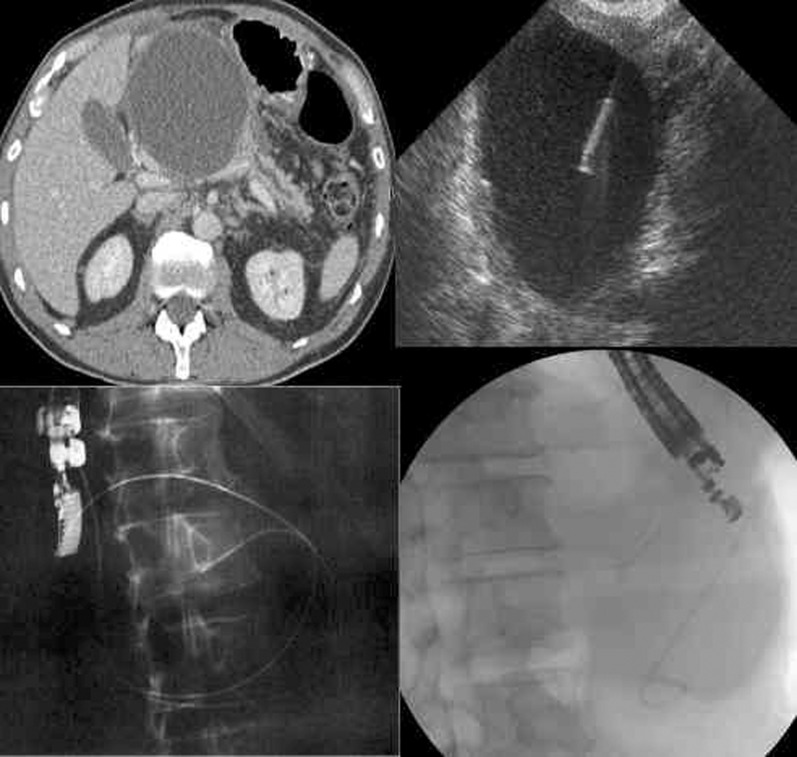
Figure 11.
CT image demonstrating a large pseudocyst (top left), EUS-guided puncture (top right) and the final position of the cyst–gastrostomy stent immediately (bottom right) and at follow-up (bottom left). Images courtesy of Dr N R Carroll.
Vascular
Venous thrombosis is relatively common owing to secondary inflammation or extrinsic compression by the oedematous gland or pseudocyst. As the splenic vein lies immediately adjacent to the pancreas, it is the most commonly affected vein. With time, collateral vessels shunt blood returning from the spleen via other routes, and gastric varices may develop [24]. Extension of this thrombosis to include the portal vein and superior mesenteric vein is also occasionally seen (Figure 12).
Figure 12.
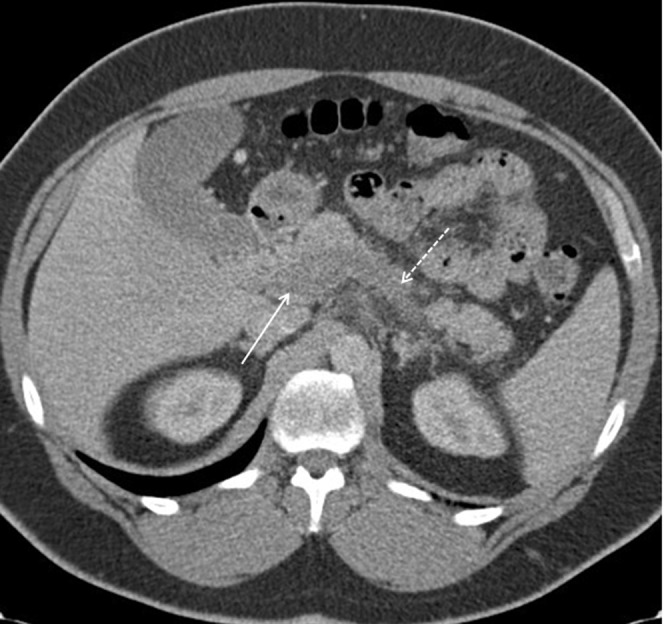
Portal phase CT study demonstrating a hypodense splenic vein (dashed arrow), indicating thrombosis of the vein up to the confluence of the portal vein (solid arrow).
Haemorrhage in AP is caused by enzymatic damage to surrounding vessels, such as the splenic artery and gastroduodenal artery. A pseudoaneurysm may form; subsequent rupture may result in a potentially catastrophic bleed into the pancreatic bed or retroperitoneum [24]. Unenhanced CT demonstrates heterogeneous high-attenuation material corresponding to haematoma and, occasionally, the extravasation of contrast medium on arterial phase images. Conventional angiography may be required for more detailed assessment and embolisation of the bleeding vessel.
Bile duct obstruction and strictures
Pancreatitis-related biliary obstruction can occur as a result of common bile duct strictures or external compression secondary to mass effect and surrounding fibrosis. Image-guided percutaneous drainage and biliary stenting is a safe and effective treatment option.
Radiological interventions in AP
The role of interventional radiology in managing AP has expanded, largely owing to the advances and technical refinements seen in interventional radiology in recent years.
Endoscopic or percutaneous drainage or aspiration of fluid collections, abscesses and pseudocysts is regularly performed to aid the diagnosis of infection or for therapeutic management [11].
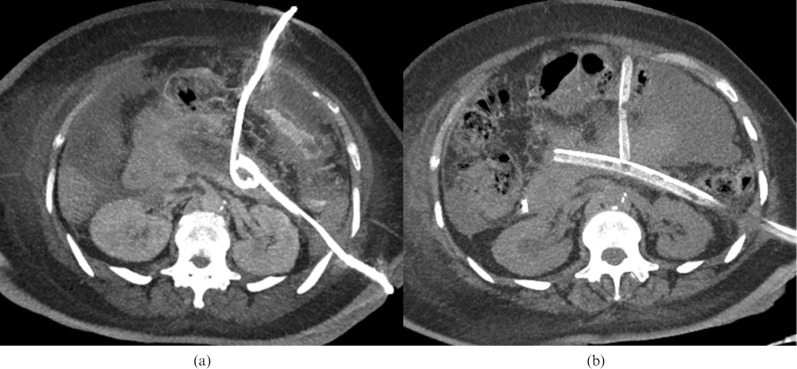
Aggressive percutaneous drainage of acute necrotising pancreatitis is increasingly used either as the primary treatment or as an adjunct to surgery. This often entails placing multiple large bore (8–30 Fr) drains into the collections. Careful pre-procedure planning of the access routes is crucial for ensuring successful placement and minimising complications [25]. Common routes include traversing the gastrocolic ligament to access the pancreatic head and via the left anterior pararenal space to the pancreatic tail (Figure 13) [20]. Image guidance is provided by CT or ultrasound and, although the latter has the advantage of being portable, deeper collections may be more difficult to visualise, especially if there is overlying bowel gas.
Figure 13.
Maximum intensity projection CT image following CT-guided drainage of pancreatic fluid collection via both the left anterior pararenal space (a) and an anterior approach across the gastrocolic ligament (b). Once the tracks are mature, these are replaced with large bore drains for improved irrigation and, ultimately, percutaneous necrosectomy.
Following catheter insertion, meticulous catheter care with regular flushing and aspiration is crucial to ensure patency and successful drainage. This usually requires close co-operation with the clinicians and nursing staff.
The use of minimally invasive necrosectomy procedures is also increasingly common. Using either endoscopic or percutaneous approaches, a flexible endoscope is inserted in to the necrotic tissue cavity (usually requires a sheath of up to 30 Fr). Necrotic tissue may then be removed under direct visualisation using irrigation, snares and dormia baskets [26]. The published literature consists largely of relatively small retrospective case series, but clinical results are consistently being improved and the morbidity of the procedure is relatively low.
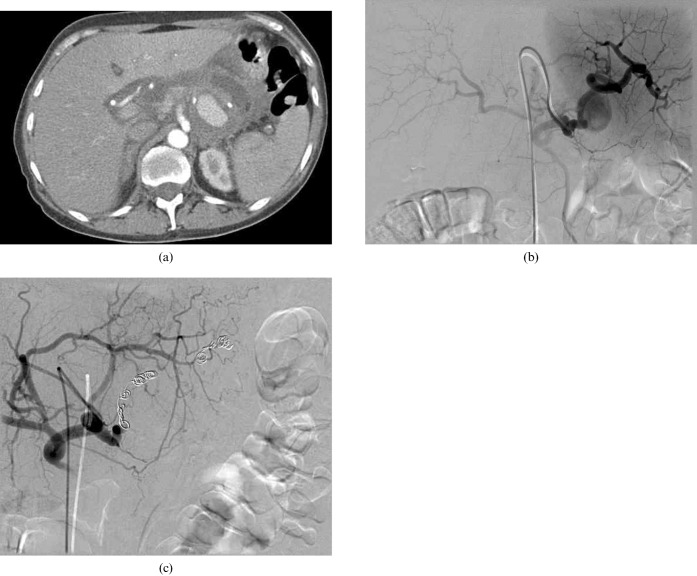
Interventional procedures in the management of AP also extend to treating remote complications and supportive procedures, such as chest drains for pleural effusions, insertion of peripherally inserted central catheter (PICC) lines and placement of gastrojejunostomy tubes in patients who need nutritional support. Vascular complications such as haemorrhage or pseudoaneurysm require urgent angiography and embolisation (Figure 14).
Large splenic artery pseudoaneurysm treated with coil embolisation. (a) CT of the upper abdomen demonstrates a large pseudoaneurysm on the left. (b) The digital subtraction angiogram confirms this arises from the splenic artery. (c) Following coil embolisation of the splenic artery, no filling of the pseudoaneurysm is seen.
Conclusions
AP can be divided into two subgroups that follow a markedly different clinical course. Imaging with contrast-enhanced CT plays a vital role in diagnosing, staging and identifying the associated complications of AP. Other imaging modalities such as EUS and MRI have a subsidiary role in the early stages of AP but are better at evaluating the aetiology. Interventional radiological procedures in managing AP are expanding; it is important to be familiar with their indications and limitations. To ensure the best possible outcome for the patient, close co-operation with the clinical team is crucial.
Acknowledgments
The authors would like to acknowledge the NIHR Cambridge Biomedical Research Centre.
References
- 1.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993;128:586–90 [DOI] [PubMed] [Google Scholar]
- 2.Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointest Endosc 2002;56:S226–30 [DOI] [PubMed] [Google Scholar]
- 3.Goldacre MJ, Roberts SE. Hospital admission for acute pancreatitis in an English population, 1963–98: database study of incidence and mortality. BMJ 2004;328:1466–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lankisch PG, Assmus C, Pflichthofer D, Struckmann K, Lehnick D. Which etiology causes the most severe acute pancreatitis? Int J Pancreatol 1999;26:55–7 [DOI] [PubMed] [Google Scholar]
- 5.Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002;223:603–13 [DOI] [PubMed] [Google Scholar]
- 6.Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Localio SA. Objective early identification of severe acute pancreatitis. Am J Gastroenterol 1974;61:443–51 [PubMed] [Google Scholar]
- 7.Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet 1989;2:201–5 [DOI] [PubMed] [Google Scholar]
- 8.Klar E, Werner J. [New pathophysiologic knowledge about acute pancreatitis]. Chirurg 2000;71:253–64 [DOI] [PubMed] [Google Scholar]
- 9.Karne S, Gorelick FS. Etiopathogenesis of acute pancreatitis. Surg Clin North Am 1999;79:699–710 [DOI] [PubMed] [Google Scholar]
- 10.Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, et al. Pathophysiology of acute pancreatitis. Pancreatology 2005;5:132–44 [DOI] [PubMed] [Google Scholar]
- 11.Maher MM, Lucey BC, Gervais DA, Mueller PR. Acute pancreatitis: the role of imaging and interventional radiology. Cardiovasc Intervent Radiol 2004;27:208–25 [DOI] [PubMed] [Google Scholar]
- 12.Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology 1994;193:297–306 [DOI] [PubMed] [Google Scholar]
- 13.Kivisaari L, Somer K, Standertskjold-Nordenstam CG, Schroder T, Kivilaakso E, Lempinen M. Early detection of acute fulminant pancreatitis by contrast-enhanced computed tomography. Scand J Gastroenterol 1983;18:39–41 [DOI] [PubMed] [Google Scholar]
- 14.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331–6 [DOI] [PubMed] [Google Scholar]
- 15.Lecesne R, Taourel P, Bret PM, Atri M, Reinhold C. Acute pancreatitis: interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology 1999;211:727–35 [DOI] [PubMed] [Google Scholar]
- 16.Arvanitakis M, Koustiani G, Gantzarou A, Grollios G, Tsitouridis I, Haritandi-Kouridou A, et al. Staging of severity and prognosis of acute pancreatitis by computed tomography and magnetic resonance imaging-a comparative study. Dig Liver Dis 2007;39:473–82 [DOI] [PubMed] [Google Scholar]
- 17.Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology 2004;126:715–23 [DOI] [PubMed] [Google Scholar]
- 18.Lau ST, Simchuk EJ, Kozarek RA, Traverso LW. A pancreatic ductal leak should be sought to direct treatment in patients with acute pancreatitis. Am J Surg 2001;181:411–15 [DOI] [PubMed] [Google Scholar]
- 19.Chak A, Hawes RH, Cooper GS, Hoffman B, Catalano MF, Wong RC, et al. Prospective assessment of the utility of EUS in the evaluation of gallstone pancreatitis. Gastrointest Endosc 1999;49:599–604 [DOI] [PubMed] [Google Scholar]
- 20.Segal D, Mortele KJ, Banks PA, Silverman SG. Acute necrotizing pancreatitis: role of CT-guided percutaneous catheter drainage. Abdom Imaging 2007;32:351–61 [DOI] [PubMed] [Google Scholar]
- 21.Lee MJ, Wittich GR, Mueller PR. Percutaneous intervention in acute pancreatitis. Radiographics 1998;18:711–24; discussion 728 [DOI] [PubMed] [Google Scholar]
- 22.Balthazar EJ. Complications of acute pancreatitis: clinical and CT evaluation. Radiol Clin North Am 2002;40:1211–27 [DOI] [PubMed] [Google Scholar]
- 23.Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg 1997;21:130–5 [DOI] [PubMed] [Google Scholar]
- 24.Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008;31:957–70 [DOI] [PubMed] [Google Scholar]
- 25.Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol 1998;170:969–75 [DOI] [PubMed] [Google Scholar]
- 26.Bruennler T, Langgartner J, Lang S, Zorger N, Herold T, Salzberger B, et al. Percutaneous necrosectomy in patients with acute, necrotizing pancreatitis. Eur Radiol 2008;18:1604–10 [DOI] [PubMed] [Google Scholar]