Abstract
The purpose of this study was to evaluate the diagnostic ability of the expanded gallbladder fossa and right posterior hepatic notch signs for hepatic fibrosis determined by double contrast-enhanced MRI. For patients with chronic viral hepatitis B (n = 96) or hepatitis C (n = 13) who underwent gadopentate dimeglumine-enhanced dynamic MRI followed by ferucarbotran-enhanced gradient-echo imaging, the degree of parenchymal fibrosis was categorised into three groups based on the extent of reticulation and nodularity: (1) pre-cirrhotic or minimal fibrosis; (2) mild to moderate fibrosis; (3) advanced cirrhosis. Each group was evaluated for the presence of a sharp notch in the posterior–medial surface of the right lobe of the liver and expanded gallbladder fossa. The expanded gallbladder fossa sign gradually increased with an increasing degree of fibrosis (Group 1, 50%; Group 2, 61%; Group 3, 78%), and there was no significant difference (p>0.5) between hepatitis B (67%) and C (73%). In the case of the right posterior hepatic notch sign, only 6% of Group 1 and Group 2 patients were positive; 27% of hepatitis B patients and 90% of hepatitis C patients in Group 3 exhibited the sign (p<0.05). Owing to its low prevalence, even in advanced cirrhosis, the right posterior hepatic notch sign is of little value in the diagnosis of cirrhosis due to chronic hepatitis B virus infection, whereas an expanded gallbladder fossa could be used as a non-specific indicator of early fibrosis before the gross appearance of advanced hepatic fibrosis.
Among imaging landmarks related to the segmental atrophy–hypertrophy complex and the secondary findings of portal hypertension [1–5], the combination of an expanded gallbladder fossa and the presence of a posteromedial notch on the right lobe of the liver has been suggested as a highly sensitive and specific sign with which to differentiate cirrhotic from normal liver [6, 7]. Particularly for chronic viral hepatitis, the early recognition of cirrhotic change is essential for patients' management of the cirrhosis itself and for monitoring the development of hepatocellular carcinoma. However, many of the previous reports mentioned only general morphological changes of the liver, irrespective of the histological grade or the cause of cirrhosis [1–7]. Recently, Aguirre et al [8] reported that double contrast-enhanced MRI (DC-MRI), including dynamic imaging using gadolinium contrast agent and subsequent delayed imaging following injection of superparamagnetic iron oxide (SPIO) particles, was similar to histological grading in scoring the hepatic fibrosis associated with cirrhotic changes. The purpose of this study was to evaluate the feasibility and usefulness of the expanded gallbladder fossa and right posterior hepatic notch signs in patients with viral-induced cirrhosis according to the degree of hepatic fibrosis as determined by DC-MRI.
Methods and materials
Approval for this retrospective study was obtained from our institutional review board, which waived the requirement for informed consent for the review of images. A retrospective search of electronic records for those patients who underwent hepatic MRI during an 18 month period (from August 2005 to March 2007) revealed 306 patients examined by DC-MRI.
After obtaining standard informed consent, MRI was performed with a 3 T unit (Signa EXCITE; GE Medical Systems, Milwaukee, WI). Fat-suppressed T2 weighted fast spin echo images (repetition time/echo time (TR/TE), 4000/98 ms; echo train length, 14) and double echo chemical shift gradient-echo images (TR/first-echo TE, second-echo TE, 160/2.4 ms (in-phase), 5.8 ms (opposed-phase); flip angle, 70°; matrix, 352 × 300; slice thickness, 8 mm; interslice gap, 1.5 mm) were obtained before DC-MRI. DC-MRI was performed using a three-dimensional gradient-echo sequence (LAVA; GE Medical Systems, Milwaukee, WI) using parallel imaging algorithms (ASSET factor 2) in the axial plane (TR/TE, 3.5–4.2/1.0–1.2 ms; flip angle, 10°; matrix, 320 × 256; slice thickness, 5 mm; slice spacing, 2.5 mm; number of slices, 64) during a 16 s breath-holding period. A dynamic series consisted of one pre-contrast series followed by three post-contrast series, including arterial, portal and 5 min delayed phase imaging, after administering a bolus injection of gadopentetate dimeglumine (0.1 mmol kg–1 Magnevist; Bayer HealthCare Berlin, Germany) at a rate of 2 ml s–1. After the dynamic imaging, SPIO (8 μmol kg–1 of Resovist; Bayer HealthCare) was administrated intravenously as the second contrast agent. After 10 min, DC-MRI comprising T2 weighted fast spin echo and T1 weighted double-echo-chemical-shift gradient-echo images were obtained using the same parameters as those for pre-contrast imaging.
A search through the medical records of 306 patients for both viral antigen test and antibody titration results yielded 128 patients positive for hepatitis B antigen and 18 patients positive for hepatitis C antibody. Digitally stored image data from these 146 patients were retrieved and preliminarily reviewed in conjunction with the medical records for initial case selection by an attending radiologist with 12 years of experience in hepatic MRI. After exclusion of those with a previous history of partial hepatectomy (n = 5) or interventional treatment involving the left lobe or posterior segment of the right lobe of the liver (n = 22) and eight additional patients with focal lesions >2 cm near the inferior portion of the left lobe or the posteromedial portion of the right lobe of the liver, a total of 111 patients (hepatitis B, n = 96; hepatitis C, n = 13; hepatitis B and C, n = 2) remained. The two patients infected with both hepatitis B and C were also excluded to further simplify analysis, resulting in 109 patients enrolled: 84 men and 25 women aged 22–78 years (mean age: 54 years).
Image analysis
The right posterior notch sign was considered to be present when there was a sharp indentation in the right medial posterior surface of the liver, and the expanded gallbladder fossa sign was considered present if the gallbladder was bound medially by the edge of the lateral segment of the left hepatic lobe below the lowermost portion of the medial segment. The portal or delayed phase images (2.5 mm slice thickness) from the dynamic imaging were used to determine the presence of the morphological signs by two independent readers — one attending radiologist with 12 years of hepatic MRI experience (Observer 1) and one junior resident (Observer 2) in the radiology department.
The degree of hepatic fibrosis was determined on DC-MRI by two attending radiologists with 14 years’ and 5 years’ experience with hepatic MRI according to the liver texture score used by Aguirre et al [8] as follows:
1. Reticulation score: from 0 (reticulations not visible on any section) to 4 (diffuse reticulations obvious on all sections)
2. Nodularity score: from 0 (nodules not visible on any section) to 4 (innumerable nodules obvious on all sections).
Using the summated value of the reticulation and nodularity scores (ranging from 0 to 8), each patient was categorised into one of three groups: Group 1 (0–2, pre-cirrhotic or minimal fibrosis); Group 2 (3–5, mild to moderate fibrosis); and Group 3 (6–8, advanced cirrhosis) (Figures 1–4). The presence of portal hypertensive stigmata (i.e. ascites, collateral veins and/or splenomegaly) was also evaluated by the same radiologists. Splenomegaly was considered present if the longest splenic dimension was greater than 11 cm in any direction [9–12].
Figure 1.
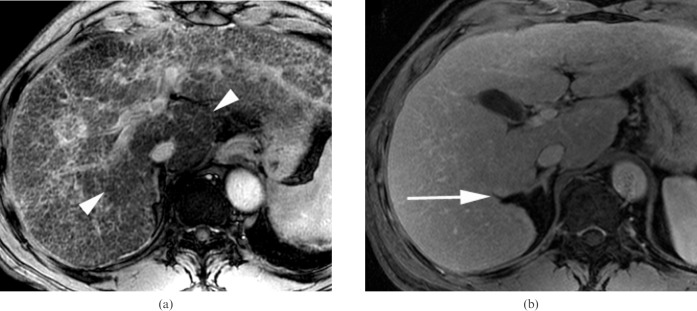
A 46-year-old man with chronic viral hepatitis B categorised into Group 1 who was negative for the two morphological signs. (a) Axial gradient-echo image (repetition time/echo time (TR/TE) = 160/5.8 ms) shows fine reticulations (arrowheads) at the peripheral portion of the liver parenchyma (reticulation score = 1) without any nodularity (nodularity score = 0). (b) Right posterior hepatic margin is smoothly curvilinear without any notch sign (arrowheads), and the left side of the gallbladder (asterisk) is partially abutting to the medial segment (arrow) on the axial contrast-enhanced three-dimensional gradient-echo image (TR/TE = 4.2/1.2 ms). There were no portal hypertensive stigmata (not shown).
Figure 4.
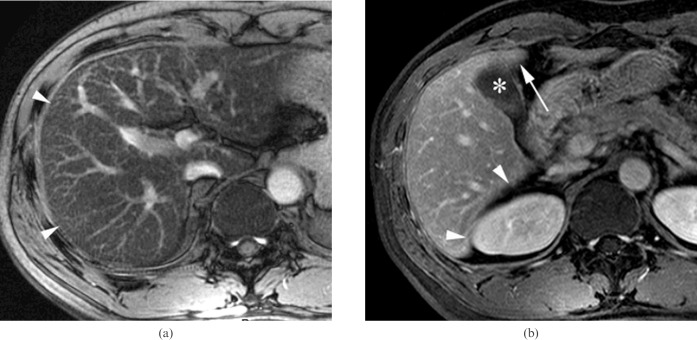
A 59-year-old man with chronic viral hepatitis C categorised into Group 3 who was positive for the right posterior hepatic notch sign. (a) The entire liver parenchyma consists of fibrotic reticulations with variable thickness and micronodules, as seen on an axial gradient-echo image (repetition time/echo time (TR/TE) = 160/5.8 ms) (reticulation score = 4, nodularity score = 4). The caudate lobe is relatively spared from the fibrotic changes and shows darker signal intensity (arrowheads) than the remaining parenchyma. (b) The right posterior hepatic notch (arrow) is well defined on the axial contrast-enhanced three-dimensional gradient-echo image (TR/TE = 4.2/1.2 ms). The expanded gallbladder fossa sign was negative, and the spleen was enlarged up to 13 cm (not shown).
Statistical analysis
The prevalence of both morphological signs in the two populations of patients was compared using the McNemar test, and weighted kappa (κ) values for interobserver variation were obtained. The likelihood ratio test was performed for the linear trend between the prevalence of each morphological sign with the degree of fibrosis. χ2 tests were used to evaluate the prevalence of each morphological sign between the patients with and without the imaging features of portal hypertensive stigmata, and between the cirrhosis caused by hepatitis B and hepatitis C.
Results
Among the 109 patients, the prevalence of right posterior hepatic notch was much lower (p<0.001) than that of expanded gallbladder fossa: 23 (21%) vs 74 (68%) for Observer 1; 21 (19%) vs 73 (67%) for Observer 2. The interobserver agreement was “excellent” for the presence of an expanded gallbladder fossa (κ = 0.812), whereas it was “good” for the presence of a right posterior hepatic notch (κ = 0.772).
The prevalence of the two morphological signs increased with an increasing degree of cirrhotic change (Table 1). The right posterior hepatic notch was demonstrated in only 6% (3/50) of patients in Groups 1 and 2 but markedly increased (34%, Observer 1; 31%, Observer 2) in Group 3 (Figures 3 and 4), whereas the expanded gallbladder fossa sign gradually increased with an increasing degree of cirrhotic change (Figures 2 and 3) (Table 1). In patients with portal hypertensive stigmata (n = 82), the prevalence of both morphological signs was significantly higher (p<0.05) than in other patients (n = 27) (Table 2).
Table 1. Prevalence of right posterior hepatic notch and expanded gallbladder fossa signs in patients with chronic viral hepatitis according to the degree of hepatic fibrosis determined by DC-MRI (n = 109).
| Right Posterior hepatic notch (+) |
Expanded gallbladder fossa (+) |
|||
| Hepatitis B (n = 96) | Hepatitis C (n = 13) | Hepatitis B (n = 96) | Hepatitis C (n = 13) | |
| Observer 1 | ||||
| Group 1 (n = 22) | 1/21 (4.8%) | 0/1 (0%) | 10/21 (48%) | 0/1 (0%) |
| Group 2 (n = 28) | 0/21 (0%) | 2/7 (29%) | 11/21 (52%) | 6/7 (86%) |
| Group 3 (n = 59) | 15/54 (28%) | 5/5 (100%) | 43/54 (80%) | 4/5 (80%) |
| p = 0.0009 | p = 0.0021 | |||
| Observer 2 | ||||
| Group 1 (n = 22) | 1/21 (4.8%) | 0/1 (0%) | 12/21 (57%) | 0/1 (0%) |
| Group 2 (n = 28) | 0/21 (0%) | 2/7 (29%) | 11/21 (52%) | 6/7 (86%) |
| Group 3 (n = 59) | 14/54 (26%) | 4/5 (80%) | 41/54 (76%) | 3/5 (60%) |
| p = 0.0021 | p = 0.0635 | |||
Group 1 (pre-cirrhotic or minimal fibrosis), Group 2 (mild to moderate fibrosis) and Group 3 (advanced cirrhosis) patients are subjectively determined by grading the reticulation and nodularity of the liver on double contrast material-enhanced MRI.
All of the p-values were calculated using the likelihood ratio test for linear trend in the overall data, regardless of the cause of cirrhosis.
+ = presence of each morphological sign.
Figure 3.
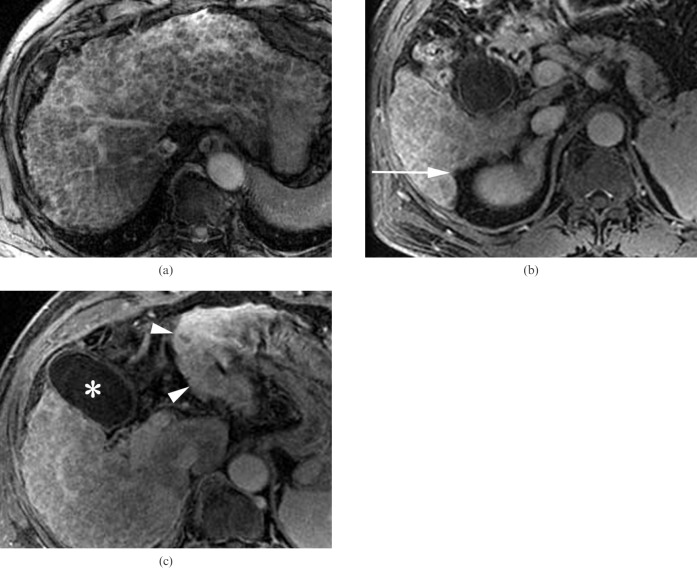
A 60-year-old man with chronic viral hepatitis B categorised into Group 3 who was positive for the two morphological signs. (a) The entire liver parenchyma consists of thick fibrotic reticulations and macronodules, as seen on an axial gradient-echo image (repetition time/echo time (TR/TE) = 160/5.8 ms) (reticulation score = 4, nodularity score = 4). (b,c) The right posterior hepatic notch (arrow) and expanded gallbladder fossa between the gallbladder (asterisk) and lateral segment (arrowheads) are well demonstrated on the axial contrast-enhanced three-dimensional gradient-echo images (TR/TE = 4.2/1.2 ms). The spleen was enlarged up to 16 cm (not shown).
Figure 2.
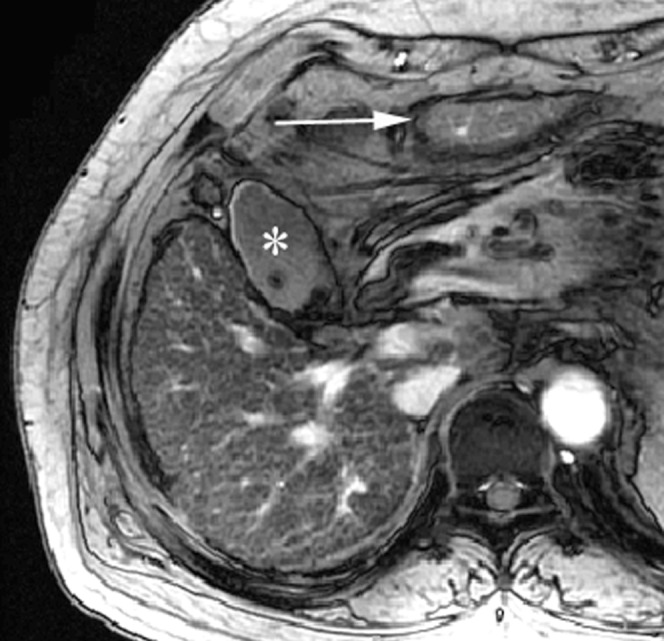
A 55-year-old woman with chronic viral hepatitis B categorised into Group 2 who was positive for the expanded gallbladder sign. Axial gradient-echo image (repetition time/echo time (TR/TE) = 160/5.8 ms) shows fine reticulations and innumerable nodules in the entire liver parenchyma (reticulation score = 2, nodularity score = 3); the inferior portion of the lateral segment (arrow) is demonstrated on the same plane as the gallbladder (asterisk) owing to the atrophy of the medial segment. The right posterior hepatic notch was not defined, and there were no portal hypertensive stigmata (not shown).
Table 2. Prevalence of right posterior hepatic notch and expanded gallbladder fossa signs in patients with chronic viral hepatitis according to the presence of portal hypertensive stigmata determined by MRI (n = 109).
| Right posterior hepatic notch (+) | Expanded gallbladder fossa (+) | |
| Observer 1 | ||
| Portal hypertensive stigmata (−) (n = 27) | 1 (3.7%) | 11 (41%) |
| Portal hypertensive stigmata (+) (n = 82) | 22 (27%) | 63 (77%) |
| p = 0.0225 | p = 0.0012 | |
| Observer 2 | ||
| Portal hypertensive stigmata (−) (n = 27) | 1 (3.7%) | 10 (37%) |
| Portal hypertensive stigmata (+) (n = 82) | 22 (27%) | 64 (78%) |
| p = 0.0373 | p = 0.0003 |
Portal hypertensive stigmata include ascites, collateral veins and splenomegaly.
All of the p-values were calculated using the χ2 test.
+ = presence of each morphologic sign or portal hypertensive stigmata.
− = absence of portal hypertensive stigmata.
The prevalence of a right posterior hepatic notch was significantly lower in patients with hepatitis B than in those with hepatitis C (hepatitis B/C: 17/54% for Observer 1 (p = 0.007); 16/46% for Observer 2 (p = 0.025)), whereas there was no significant difference between hepatitis B and C for the prevalence of an expanded gallbladder fossa (hepatitis B/C: 67/77% for Observer 1 (p = 0.670); 67/69% for Observer 2 (p = 0.897)).
Discussion
Other than chronic ethanol abuse, which is the leading cause of cirrhosis in Western countries, hepatitis B and C are primary risk factors for cirrhosis and hepatocellular carcinoma [13–16], and imaging recognition of virus-induced cirrhosis might be more important for these patients than for those with alcoholic cirrhosis [17]. The cirrhotic change is a gradual and regional process, and the correct diagnosis is not reliable without the whole specimen of the explanted liver [18]. Although it has limited value in determining the presence and severity of cirrhotic change, percutaneous or laparascopic biopsy has been used as the gold standard for evaluating the degree of hepatic fibrosis in the entire liver [19]. In addition, DC-MRI allows a feasible discrimination of the fibrotic reticulations from the regenerating nodules in the cirrhotic liver [8]. The delayed phase enhancement of fibrotic reticulations by gadolinium contrast material is well distinguished from the background parenchyma consisting of regenerative nodules with decreased signal intensity owing to SPIO uptake [8, 20, 21].
In the present study, the incidence of gallbladder fossa expansion was similar to previous reports [6, 7]; however, the appearance of a right posterior notch was limited. The low prevalence of a right posterior hepatic notch in viral-induced cirrhosis compared with alcoholic cirrhosis was highlighted by Okazaki et al [22]. In their study, however, the prevalence of a right posterior hepatic notch approximated 70%, even in patients with viral-induced cirrhosis. Although their report contained no comparative data between hepatitis B and C [22], we found that hepatic fibrosis caused by hepatitis B showed a significantly lower prevalence of the right posterior hepatic notch than the changes caused by hepatitis C, regardless of the degree of disease process.
The right posterior hepatic notch appears as if it originated from a distortion of the liver surface at the segmental border between the medially hypertrophied caudate lobe and the atrophied right posterior segment [7, 22]. Pathologically, macronodular cirrhosis is the representative disease process for hepatitis B, whereas the size of the cirrhotic nodule in hepatitis C is relatively small (micronodular cirrhosis) [22, 23]. As it is unknown, we may only speculate that there may be a different mechanism resulting in an increased tendency for caudate lobe hypertrophy and a right posterior hepatic notch related to the micronodular cirrhosis [23]. During the process of gradual and inherently non-uniform hepatic fibrosis, thick septal fibrosis in patients with micronodular cirrhosis would induce regional variation in portal perfusion within the liver [24]. The different perfusion gradients between the peripheral and central portions of the liver would result in gross morphological changes such as hypertrophy of the central portion, including the caudate lobe, owing to the relatively higher perfusion pressure. Macronodular cirrhosis, however, consists of thin fibrotic septa and larger regenerating nodules, and thus the regional variation in portal perfusion would be negligible.
Expansion of gallbladder fossa is closely related to the medial segment atrophy and other combined factors of counterclockwise rotation of the liver [6]. As observed by Rosenthal et al [25] using colour Doppler ultrasound, flow in the portal venous branches to the lateral segment and caudate lobe is uniformly hepatopetal, whereas the medial segment often receives circular flow from the right side of the umbilical segment with a lower amount of blood than other segments. Even in early fibrosis, which cannot be grossly depicted on MRI, overall portal venous perfusion is usually reduced throughout the entire liver, and the flow to the medial segment may become more reduced to cause segmental atrophy. Thus, it is not strange to observe the relatively higher prevalence of expanded gallbladder fossa, even in Group 1 and 2 patients, regardless of the cause of cirrhosis, as in the present study.
This study has several limitations. Firstly, we did not have a histological gold standard in this retrospective study [26]. Even though more studies are needed to confirm its “solid” validity, previously published MRI grading [8] was used as a matter of convenience to compare the prevalence of two morphological signs related to the different degree of fibrosis defined on DC-MRI. Secondly, despite using MRI, the size of cirrhotic nodules, the thickness of the fibrotic reticulations and the geographic distribution of the degree of the cirrhotic changes were not quantitatively categorised in the present study. A more organised access to the regional distribution of hepatic fibrosis will be necessary to obtain more sophisticated data for the relationship between the morphological signs and substantial parenchymal changes. Another limitation concerns the small size of the cirrhosis caused by hepatitis C. The high prevalence of the two morphological signs in patients consisting mostly of those with cirrhosis from hepatitis C virus infection has already been reported [7, 22]. Therefore, despite the smaller number of hepatitis C patients, our data showing the significantly low prevalence of the right posterior hepatic notch sign in hepatic fibrosis caused by hepatitis B compared with those from hepatitis C seem to be valid.
In conclusion, using the degree of hepatic fibrosis determined by DC-MRI as a reference standard, the results of this study suggest that the right posterior hepatic notch sign cannot be used as a tool to diagnose early stage viral-induced cirrhosis. Patients with a positive right posterior hepatic notch sign already possessed the stigmata of portal hypertension, suggesting an advanced disease process and bestowing very little value upon this sign as a morphological marker to predict early cirrhotic change of the liver. The right posterior hepatic notch sign may be used as a reliable tool in the diagnosis of advanced cirrhosis from hepatitis C virus infection, but it has very limited value in patients with hepatitis B virus infection owing to its low prevalence, regardless of the degree of hepatic fibrosis. The expanded gallbladder fossa sign, regardless of its increasing prevalence with increasing severity of hepatic fibrosis, could be a non-specific indicator of early fibrosis before the gross appearance of advanced fibrosis determined by DC-MRI in a number of patients.
References
- 1.Harbin WP, Robert NJ, Ferrucci JT., Jr Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology 1980;135:273–83 [DOI] [PubMed] [Google Scholar]
- 2.Fisher MR, Gore RM. Computed tomography in the evaluation of cirrhosis and portal hypertension. J Clin Gastroenterol 1985;7:173–81 [DOI] [PubMed] [Google Scholar]
- 3.Brown JJ, Naylor MJ, Yagan N. Imaging of hepatic cirrhosis. Radiology 1997;202:1–16 [DOI] [PubMed] [Google Scholar]
- 4.Lafortune M, Matricardi L, Denys A, Favret M, Dery R, Pomier-Layrargues G. Segment 4 (the quadrate lobe): a barometer of cirrhotic liver disease at US. Radiology 1998;206:157–60 [DOI] [PubMed] [Google Scholar]
- 5.Giorgio A, Amoroso P, Lettieri G, Fico P, de Stefano G, Finelli L, et al. Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology 1986;161:443–45 [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Mitchell DG, Gabata T, Hussain SM. Expanded gallbladder fossa: simple MR imaging sign of cirrhosis. Radiology 1999;211:723–6 [DOI] [PubMed] [Google Scholar]
- 7.Ito K, Mitchell DG, Kim MJ, Awaya H, Koike S, Matsunaga N. Right posterior hepatic notch sign: a simple diagnostic MR finding of cirrhosis. J Magn Reson Imaging 2003;18:561–6 [DOI] [PubMed] [Google Scholar]
- 8.Aguirre DA, Behling CA, Alpert E, Hassanein TK, Sirlin DB. Liver fibrosis: non-invasive diagnosis with double contrast material-enhanced MR imaging. Radiology 2006;239:425–37 [DOI] [PubMed] [Google Scholar]
- 9.Frank K, Linhart P, Kortsik C, Wohlenberg H. Sonographic determination of spleen size: normal dimensions in adults with a healthy spleen [German]. Ultrashall Med 1986;7:134–7 [DOI] [PubMed] [Google Scholar]
- 10.Lamb PM, Lund A, Kanagasabay RR, Martin A, Webb JA, Reznek RH. Spleen size: how well do liner ultrasound measurements correlate with three-dimensional CT volume assessment? Br J Radiol 2002;75:573–7 [DOI] [PubMed] [Google Scholar]
- 11.Dodd GD, III, Baron RL, Oliver JH, III, Federle MP. Spectrum of imaging findings of the liver in end-stage cirrhosis. 1. Gross morphology and diffuse abnormalities. AJR Am J Roentgenol 1999;173:1031–6 [DOI] [PubMed] [Google Scholar]
- 12.Spielmann AL, DeLong DM, Kliewer MA. Sonographic evaluation of spleen size in tall healthy athletes. AJR Am J Roentgenol 2005;184:45–9 [DOI] [PubMed] [Google Scholar]
- 13.Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, et al. A multivariate analysis of risk factor for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology 1993;18:47–53 [PubMed] [Google Scholar]
- 14.Chen DS. Natural history of chronic hepatitis B virus infection: new light on an old story. J Gastroenterol Hepatol 1993;8:470–5 [DOI] [PubMed] [Google Scholar]
- 15.Kato Y, Nakata K, Omagari K, Furukawa R, Kusumoto Y, Mori I, et al. Risk of hepatocellular carcinoma in patients with cirrhosis in Japan. Cancer 1994;74:2234–8 [DOI] [PubMed] [Google Scholar]
- 16.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis 2000;20:17–35 [DOI] [PubMed] [Google Scholar]
- 17.Ince N, Wands JR. The increasing incidence of hepatocellular carcinoma. N Engl J Med 1999;340:798–9 [DOI] [PubMed] [Google Scholar]
- 18.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet 1986;1:523–5 [DOI] [PubMed] [Google Scholar]
- 19.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495–500 [DOI] [PubMed] [Google Scholar]
- 20.Semelka RC, Chung JJ, Hussain SM, Marcos HB, Woosley JT. Chronic hepatitis: correlation of early patchy and late linear enhancement patterns on gadolinium-enhanced MR images with histopathology initial experience. J Magn Reson Imaging 2001;13:385–91 [DOI] [PubMed] [Google Scholar]
- 21.Lucidarme O, Baleston F, Cadi M, Bellin MF, Charlotte F, Ratziu V, et al. Non-invasive detection of liver fibrosis: is superparamagnetic iron oxide particle-enhanced MR imaging a contributive technique? Eur Radiol 2003;13:467–74 [DOI] [PubMed] [Google Scholar]
- 22.Okazaki H, Ito K, Fujita T, Koike S, Takano K, Matsunaga N. Discrimination of alcoholic from virus-induced cirrhosis on MR imaging. AJR Am J Roentgenol 2000;175:1677–81 [DOI] [PubMed] [Google Scholar]
- 23.Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer P, Sobin LH. The morphology of cirrhosis. J Clin Pathol 1978;31:395–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, et al. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology 2006;44:1511–17 [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal SJ, Hannisson LA, Baxter KG, Wetxel LH, Cox GG, Batnitzky S. Doppler US of helical flow in the portal vein. RadioGraphics 1995;15:1103–15 [DOI] [PubMed] [Google Scholar]
- 26.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C: the OBSVIRC, METAVIR, CLINIVIR and DOSVIRC groups. Lancet 1997;349:825–32 [DOI] [PubMed] [Google Scholar]