Abstract
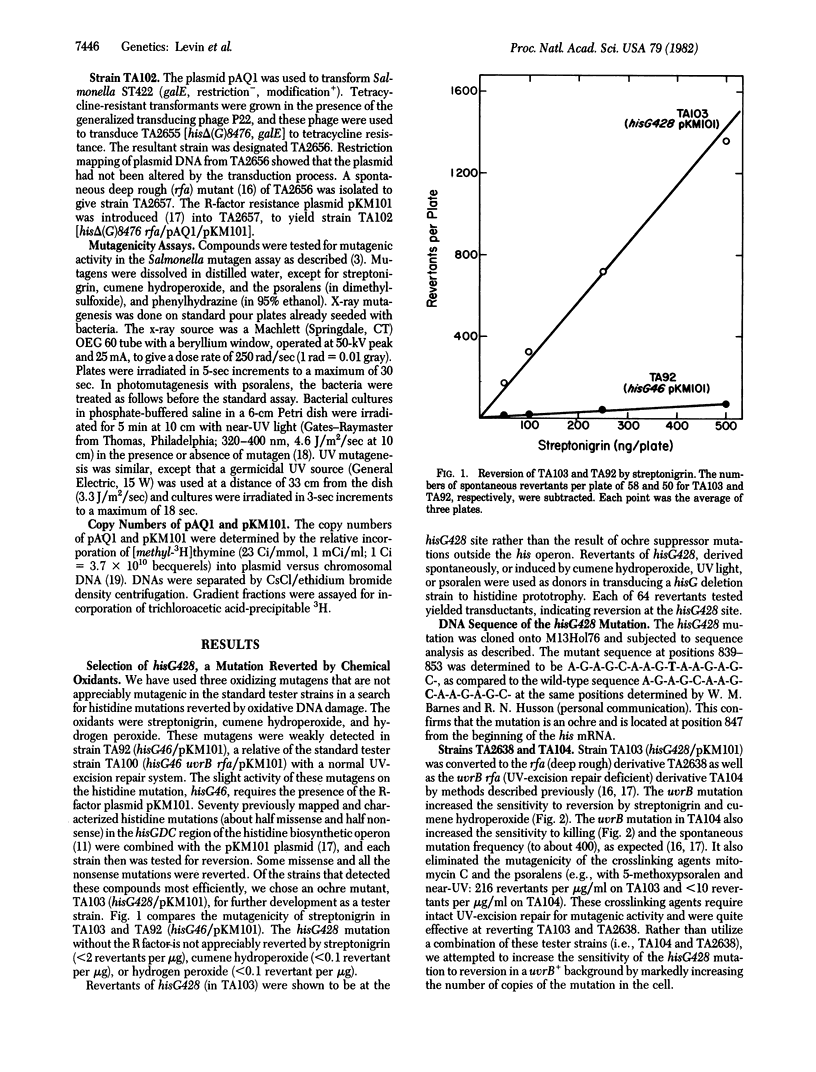
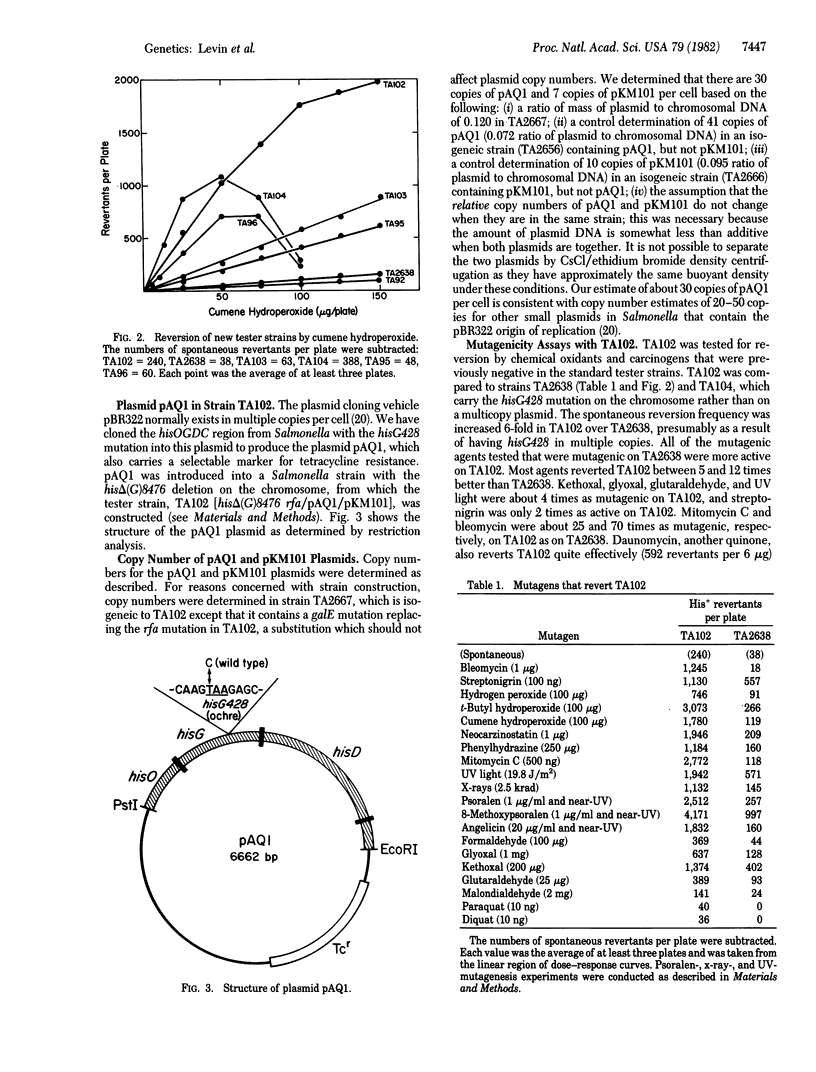
A new tester strain, TA102, is described as an addition to the set of strains for the Salmonella/microsome mutagenicity test. This strain contains A X T base pairs at the site of the mutation (determined by DNA sequence analysis) in contrast to the other Salmonella tester strains that detect mutagens damaging G X C base pairs. This strain differs from previous tester strains in that the mutation has been introduced into a multicopy plasmid, so that approximately equal to 30 copies of the mutant gene are available for back mutation. The new strain detects a variety of oxidative mutagens, including x-rays, bleomycin, hydrogen peroxide and other hydroperoxides, streptonigrin and other quinones, and phenylhydrazine; a variety of aldehydes, including formaldehyde, glyoxal, kethoxal, glutaraldehyde, and malondialdehyde; a number of psoralens (in the presence of near-UV light), mitomycin C, neocarzinostatin, and UV light. Some of these mutagens have been previously shown to damage thymine in DNA. Several auxiliary tester strains also are described, including TA96, a frameshift tester strain with a hot spot for mutation at a run of five A X T base pairs with a specificity similar to that of TA102. The importance of oxidative mutagens is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Identifying environmental chemicals causing mutations and cancer. Science. 1979 May 11;204(4393):587–593. doi: 10.1126/science.373122. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., McCann J. Validation of the Salmonella test: a reply to Rinkus and Legator. Cancer Res. 1981 Oct;41(10):4192–4203. [PubMed] [Google Scholar]
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Auerbach C., Moutschen-Dahmen M., Moutschen J. Genetic and cytogenetical effects of formaldehyde and related compounds. Mutat Res. 1977;39(3-4):317–361. doi: 10.1016/0165-1110(77)90011-2. [DOI] [PubMed] [Google Scholar]
- Bjeldanes L. F., Chew H. Mutagenicity of 1,2-dicarbonyl compounds: maltol, kojic acid, diacetyl and related substances. Mutat Res. 1979 Aug;67(4):367–371. doi: 10.1016/0165-1218(79)90034-x. [DOI] [PubMed] [Google Scholar]
- Bossi L., Roth J. R. Four-base codons ACCA, ACCU and ACCC are recognized by frameshift suppressor sufJ. Cell. 1981 Aug;25(2):489–496. doi: 10.1016/0092-8674(81)90067-2. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P., Knowles A. Mutation induction and killing of Escherichia coli by DNA adducts and crosslinks: a photobiological study with 8-methoxypsoralen. Chem Biol Interact. 1979 Oct;27(2-3):221–233. doi: 10.1016/0009-2797(79)90127-3. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Wolf M., Goldberg I. H. Mutagenesis by neocarzinostatin in Escherichia coli and Salmonella typhimurium: requirement for umuC+ or plasmid pKM101. J Bacteriol. 1980 Nov;144(2):656–660. doi: 10.1128/jb.144.2.656-660.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel K., Goldstein M. S., Duker N. J., Teebor G. W. Identification of the cis-thymine glycol moiety in oxidized deoxyribonucleic acid. Biochemistry. 1981 Feb 17;20(4):750–754. doi: 10.1021/bi00507a014. [DOI] [PubMed] [Google Scholar]
- Goldberg I. H., Friedman P. A. Antibiotics and nucleic acids. Annu Rev Biochem. 1971;40:775–810. doi: 10.1146/annurev.bi.40.070171.004015. [DOI] [PubMed] [Google Scholar]
- Grant E. L., von Borstel R. C., Ashwood-Smith M. J. Mutagenicity of cross-links and monoadducts of furocoumarins (psoralen and angelicin) induced by 360-nm radiation in excision-repair-defective and radiation-insensitive strains of Saccharomyces cerevisiae. Environ Mutagen. 1979;1(1):55–63. doi: 10.1002/em.2860010112. [DOI] [PubMed] [Google Scholar]
- Hartman P. E., Hartman Z., Stahl R. C. Classification and mapping of spontaneous and induced mutations in the histidine operon of Salmonella. Adv Genet. 1971;16:1–34. doi: 10.1016/s0065-2660(08)60352-1. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Hollstein M., McCann J., Angelosanto F. A., Nichols W. W. Short-term tests for carcinogens and mutagens. Mutat Res. 1979 Sep;65(3):133–226. doi: 10.1016/0165-1110(79)90014-9. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Isono K., Yourno J. Chemical carcinogens as frameshift mutagens: Salmonella DNA sequence sensitive to mutagenesis by polycyclic carcinogens. Proc Natl Acad Sci U S A. 1974 May;71(5):1612–1617. doi: 10.1073/pnas.71.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose J. G. Photomutagenesis by chlorinated phenothiazine tranquilizers. Proc Natl Acad Sci U S A. 1979 Jan;76(1):469–472. doi: 10.1073/pnas.76.1.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H., Liesch J. M. Identification of thymidine-5'-aldehyde at DNA strand breaks induced by neocarzinostatin chromophore. Proc Natl Acad Sci U S A. 1982 Feb;79(3):744–748. doi: 10.1073/pnas.79.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Yamasaki E., Ames B. N. A new Salmonella tester strain, TA97, for the detection of frameshift mutagens. A run of cytosines as a mutational hot-spot. Mutat Res. 1982 Jun;94(2):315–330. doi: 10.1016/0027-5107(82)90294-9. [DOI] [PubMed] [Google Scholar]
- Marnett L. J., Tuttle M. A. Comparison of the mutagenicities of malondialdehyde and the side products formed during its chemical synthesis. Cancer Res. 1980 Feb;40(2):276–282. [PubMed] [Google Scholar]
- McCann J., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals: discussion. Proc Natl Acad Sci U S A. 1976 Mar;73(3):950–954. doi: 10.1073/pnas.73.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Spingarn N. E., Kobori J., Ames B. N. Detection of carcinogens as mutagens: bacterial tester strains with R factor plasmids. Proc Natl Acad Sci U S A. 1975 Mar;72(3):979–983. doi: 10.1073/pnas.72.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk D. L., Horwitz S. B., Peisach J. Redox potential of iron-bleomycin. Biochemistry. 1981 Sep 1;20(18):5327–5331. doi: 10.1021/bi00521a036. [DOI] [PubMed] [Google Scholar]
- Moody C. S., Hassan H. M. Mutagenicity of oxygen free radicals. Proc Natl Acad Sci U S A. 1982 May;79(9):2855–2859. doi: 10.1073/pnas.79.9.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B. K. Irreversible binding of reductively activated streptonigrin to nucleic acids in the presence of metals. Chem Biol Interact. 1981 Aug;36(2):179–188. doi: 10.1016/0009-2797(81)90019-3. [DOI] [PubMed] [Google Scholar]
- Swenberg J. A., Kerns W. D., Mitchell R. I., Gralla E. J., Pavkov K. L. Induction of squamous cell carcinomas of the rat nasal cavity by inhalation exposure to formaldehyde vapor. Cancer Res. 1980 Sep;40(9):3398–3402. [PubMed] [Google Scholar]
- Takeshita M., Kappen L. S., Grollman A. P., Eisenberg M., Goldberg I. H. Strand scission of deoxyribonucleic acid by neocarzinostatin, auromomycin, and bleomycin: studies on base release and nucleotide sequence specificity. Biochemistry. 1981 Dec 22;20(26):7599–7606. doi: 10.1021/bi00529a039. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]



