Abstract
MRI has previously provided conflicting results when used to search for brain abnormalities in sufferers of chronic fatigue syndrome (CFS). Eighteen CFS patients and nine healthy volunteers each underwent MRI on two occasions, one year apart. The resulting images were examined for abnormalities in brain atrophy, deep white matter hyperintensities (WMH) and cerebral blood and cerebrospinal fluid (CSF) flow. Mean proportionate CSF volume was not significantly different between subject groups. All participants showed a slight increase in CSF between scans, but no significant difference was found between those with CFS and those without. Between-group comparisons of ventricular volume revealed no significant differences at study commencement and no significant change over the year. No significant inter-group differences were found for any of the cerebral blood and CSF flow parameters. Low levels of WMH were found in all participants. Objective scoring of WMH using Scheltens' scale revealed no change in summary components (prosencephalic deep white matter hyperintensities, basal ganglia hyperintensities and infratentorial hyperintensities) or in individual component variables between the baseline and 1 year follow-up scans. No abnormal patterns in rate and extent of brain atrophy, ventricle volume, white matter lesions, cerebral blood flow or aqueductal CSF flow were detected in the CFS population. These results throw open the debate into whether MRI scanning can reveal diagnostic signs of CFS and clinically questions the diagnoses of CFS made on the basis of previous research conclusions.
Chronic fatigue syndrome (CFS) is a symptom-based illness that lacks definitive biological markers. No precise underlying causes have been identified but extensive studies which have identified a variety of predisposing or initiating factors, which include infectious agents, genetic susceptibilities, immune system abnormalities and psychiatric disorders. Many of the typical symptoms, such as impaired short-term memory and concentration, headaches and altered sleep patterns, have led researchers to look for abnormalities within the central nervous system (CNS). Previous studies using cerebral MRI have led to conflicting reports. Abnormalities described by some researchers include a significant increase in small white matter hyperintensities (WMH) in the frontal lobes (seen on T2 weighted images) [1] and increased ventricular volume [2], but other researchers have failed to find significant differences in WMH between CFS patients and control subjects [3, 4]. Studies of cerebral blood flow using single photon computed tomography (SPECT) have also produced contradictory results, with an increase in CNS perfusion abnormalities in CFS patients reported in some studies [5, 6] but other studies failing to identify significant differences [7]. The conflicting results may be due to variations in patient selection and study design.
We report the first longitudinal study of cerebral MRI in patients selected using rigorous criteria for CFS. The study was undertaken as part of a larger study examining the effectiveness of an osteopathic treatment for CFS. The component of the study reported here was designed to detect differences in the extent or progression of cerebral atrophy, WMH, total cerebral blood flow and CSF pulsatility between CFS patients and normal controls over a period of one year.
Methods and materials
Participant recruitment
Eighteen patients with CFS and nine normal control subjects were recruited into the study. All of the CFS patients fulfilled the case definition criteria (CDC) [8] and also underwent a psychiatric assessment using the Hospital Anxiety and Depression Scale (HADS) system [9] and the more precise Schedule for Clinical Assessment in Neuropsychiatry (SCAN) [10] questionnaire to ensure the exclusion of subjects with depressive or anxiety states. The CFS patients were divided into two groups: the first group [CFS1, five men and four women: age range 20–53 years; mean age 35.3±12.6 (SD)] received only an osteopathic treatment [11] for one year, whereas patients in the other group (CFS2; five men and four women: age range 22–55 years; mean age 36.1±12.3 years) were allowed to pursue treatment regimes of their own choice, excluding osteopathic treatment. None of the CFS patients was considered to be from the extreme end of the symptomatic spectrum (i.e. bedridden or with intense sensitivity to any external stimuli). The control group (NORM, 5 men and 4 women: age range 22–53 years; mean age 36.1±12.4 years) were normal volunteers in good general health and with no history of significant neurological abnormality.
Imaging
MRI was performed using a 1.5 tesla whole-body scanner (ACS-NT PT 6000, Phillips Medical Systems, Best, The Netherlands) and a birdcage head coil receiver. MRI was performed at the beginning of the study and was repeated after 90 minutes, rest to ensure reproducibility. All imaging was repeated after 1 year to assess longitudinal changes.
The sequence for measurement of cerebral atrophy consisted of serial coronal fast spin-echo inversion-recovery images (TR 6850 ms, TE 18 ms, TI 300 ms, echo train length 9, field of view 150 mm2, matrix 256 × 256, slice thickness 3 mm, interslice gap 1 mm). A coronal fluid-attenuated inversion recovery (FLAIR) sequence was obtained for assessment of WMHs (TR 11 000 ms, TE 140 ms, TI 2600 ms, image geometry identical to that of the previous sequence).
Quantitative phase-contrast angiography was used to measure total cerebral blood flow in a single slice at a position perpendicular to the internal carotid and basilar arteries (TR 5.66–5.86 ms, TE 3.45–3.60 ms, flip angle 15°, velocity encoding profile 90 cm s−1, field of view 150 mm2, matrix 642, slice thickness 6 mm) and CSF flow through the cerebral aqueduct (TR 26.5 ms, 7.8 ms, flip angle 15°, velocity encoding profile 5 cm s−1, field of view 160 mm, matrix 48 × 64, slice thickness 6 mm). Flow velocity images were produced using flow sensitivity in each of the cardinal directions and cardiac gating to produce 15 time-points in the cardiac cycle.
Image analysis
The degree and distribution of cerebral atrophy were analysed using the method described by Thacker et al [12]. In this method, CSF is segmented from the spin-echo inversion-recovery images and the resultant CSF image is registered into a standard orientation. A co-ordinate system, bounded by the extremes of the CSF space, is then applied and the proportion of CSF in each of 12 equally sized arbitrary divisions of the co-ordinate system is calculated. The 12 segments are defined by the mid-sagittal plane, a plane midway between the superior and inferior boundaries of the co-ordinate space and 2 planes placed equidistantly between the anterior and posterior boundaries of the co-ordinate space. This produces a measure of CSF volume and distribution normalised for head size and scaling errors.
CSF voxels representing the lateral ventricles were manually selected from the binary images for volumetric measurement of the lateral ventricles. The segmented ventricle images were manually checked by three-dimensional volume rendering to ensure that voxels extraneous to the ventricles were not included. If any such voxels were present, they were manually removed. The measured ventricle volume was again normalised to the dimensions of the co-ordinate system enclosing the CSF space to correct for variations in head size.
The severity and distribution of deep white matter hyperintensities (DWMH) were assessed by an experienced neuroradiologist (AJ) using the objective scoring scale described by Scheltens et al [13]. Scoring was performed from matched FLAIR and inversion-recovery images. All examinations performed prior to follow-up, consisting of two examinations per subject, were scored in random order with the scorer blind to the diagnosis. Follow-up scans were scored by the same observer, who remained blind to the diagnosis. Following this, initial and follow-up scans were compared directly to identify changes in individual DWMH to which the scoring system might be insensitive.
Vessel localisation in flow velocity images was performed by manual selection of a small rectangular region of interest (ROI) in the centre of each vessel. The mean grey level (and hence flow velocity) was measured for each time-point in the cardiac cycle. The resultant time curve of mean velocity for the ROI was then correlated with the corresponding time curve for every pixel in the image set. This resulted in a correlation image, in which each pixel represented the correlation coefficient (r) of the waveform for that pixel location with the waveform of the selected ROI. This image was then thresholded at an empirically determined value of 0.9 to determine the boundaries of each vessel. Flow through the vessels was calculated from heart rate, mean velocity and cross-sectional area. Cerebral blood flow was calculated as the sum of the carotid and basilar flows. Inter-group comparisons were made of the total cerebral blood flow per minute, the proportion of the cycle through which CSF flow through the aqueduct was in the caudal direction (during systole), the total CSF flow in the caudal direction, the total CSF flow in the craniocaudal direction, the net CSF flow per cycle, the maximum craniocaudal CSF flow rate and the arterial to aqueduct delay, which is defined as the time between the centre of the systolic peaks of the arterial and aqueductal flow velocity curves.
Statistical analysis
Comparisons between the three groups were performed using Friedman's analysis of variance (ANOVA) for non-parametric matched sets.
Results
Measurements of proportionate CFS volume made in scans obtained 90 min apart were very similar. When the second measurement was converted to a proportion of the first, a mean value of 0.9976±0.0505 was achieved across all 27 subjects, demonstrating good reproducibility for the technique in the short term. There was no significant difference in proportionate CFS volume between the three groups (either of the CFS patient groups and the control group) at the beginning of the study (Friedman's ANOVA, p = 0.339).
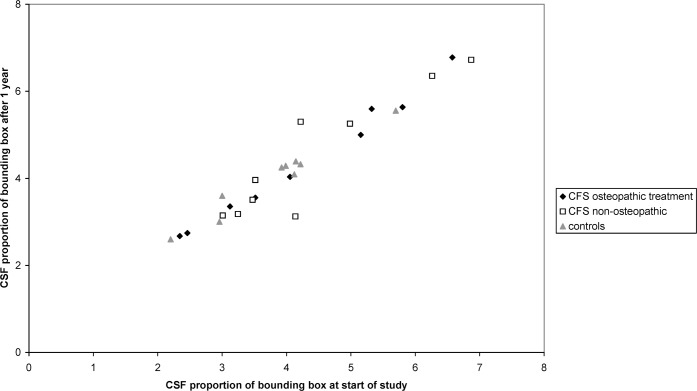
To assess and compare longitudinal changes in CSF volume, the proportion of CSF in the total bounding box after one year was converted to a proportion of that at study commencement. All three groups showed a slight increase in mean CFS proportional volume (relative values for CFS1, 1.040±0.061; CFS2, 1.025±0.133 and NORM 1.067±0.078). There were no significant differences in these increases between the control or patient groups (p = 0.73). Figure 1 shows CSF proportional volume after one year, plotted against volume at study commencement.
Figure 1.
CSF proportional volume calculated from baseline and 1 year follow-up scans.
At commencement of the study, ventricular volume showed no significant differences between the three groups (p = 0.339), and there were no significant inter-group differences in the change in ventricular volume over the course of the year (p = 0.733).
Analysis of cerebral blood flow data and CSF flow data showed no significant differences in any of the inter-group comparisons for any of the parameters described above.
All three groups showed low levels of WMH, and there was no evidence of WMH in the basal ganglia or infratentorial structures of any patient. Mean scores for periventricular hyperintensity were 0.11±0.33 for CFS1, 0.33±0.71 for CFS2 and 0.11 ± 0.33 for NORM. Mean scores for DWMH in the prosencephalon were 1.55±2.80 for CFS1, 1.67±2.78 for CFS2 and 1.11±1.45 for NORM). There was no change in any of the summary (prosencephalic DWMH, basal ganglia hyperintensities and infratentorial hyperintensities) or individual component variables that make up the score between the baseline and one year follow-up scans. Because the Scheltens' scale presents summaries of the frequency of lesions, it is possible that individual lesions may appear, resolve or change over time without a corresponding change in the resultant score. Direct comparison of baseline and one-year follow-up scans demonstrated new lesions in one subject (NORM, three lesions) and apparent resolution in two subjects (NORM, one lesion <3 mm, CFS2 one lesion <3 mm).
Discussion
The present study examined four intracranial CNS abnormalities that have previously been indicated to be of relevance in CFS and a number of additional parameters concerned with intracranial blood and CSF flow. Inter-group differences in these abnormalities between two patient groups and one control group were examined at commencement of the study. The change in these abnormalities after one year was also subjected to the same inter-group comparisons.
WMH is a brain abnormality that is often studied in patients with CFS. An increase in WMH in CFS patients was observed by Natelson et al [14] and by Buchwald et al [15], although in both of these studies some of the CFS patients showed symptoms uncharacteristic of CFS and suggestive of other disease factors. Lange et al [1] found a significant increase in WMH in CFS, but only after splitting the CFS group into those with and without DSM III-R Axis-I psychiatric disorder occurring since their CFS diagnosis, the increase being found in the group with no Axis-I psychiatric disorder. Other studies have failed to find any significant difference in the presence of WMH in CFS patients and control subjects [3, 5]. In the present study, all subjects in patient and control groups were subjected to a thorough screening to ensure the absence of psychiatric disorders, which included a detailed consultation with a psychiatrist. Scoring of DWMH with a widely used semi-quantitative scale demonstrated only small numbers of white matter abnormalities, with no difference between experimental groups and no evidence of change over time.
Distinctive patterns of brain atrophy occur in many disease processes [12] and a difference in lateral ventricle size between CFS patients and healthy control subjects has been described previously, although this difference was not quite significant [2]. The present study examined both the volume of all of the CSF within the skull vault of the prosencephalon and the volume of CSF within the lateral ventricles. In accordance with the Monro–Kellie hypothesis [16], any change in the sum of grey matter, white matter and intracranial blood volume should result in a corresponding change in the volume of CSF. The proportion of CSF volume to other structures is small, and thus the CSF volume is sensitive to small changes in brain volume. As such, measurement of CSF volume is used to detect changes in the brain tissue volume, whereas increases in ventricular volume are generally regarded as a measure of deep white matter loss [1].
No significant differences were found in total CSF or ventricular volume between the groups at commencement of the study, and no differences were found in change after one year. All three groups showed a very small increase in CSF volume over one year, consistent with a small degree of age-related atrophy. Owing to the small sample size and the non-parametric test employed, the statistical analysis may not be very sensitive to small changes in group CSF volume. However, a scatter plot of CSF proportion before and after one year gives no indication of differences between the three groups (Figure 1), although it is notable that two of the CFS patients showed a marked change in CSF volume after one year. Re-checking of the results and raw images confirms that these changes were genuine, but with scanning at only two time-points one cannot be certain whether these changes were long-term changes that occurred slowly over the course of the year or whether they represented different points in shorter-term fluctuations.
During the cardiac cycle, the transient increase in blood volume in the skull is balanced by an outflow of CSF through the cerebral aqueduct. The mechanical coupling between arterial and CSF flow pulsations has a damping effect that is dependent on the compliance of the blood vessels and surrounding tissues. Cerebrovascular disease may lead to alterations in this vascular compliance, with corresponding alterations in the pattern of CSF pulsatility in the aqueduct. This can be considered a sensitive but non-specific indicator of small vessel disease in the brain.
In this study, the temporal dynamics of blood flow into the brain and the corresponding CSF flow pulsations through the cerebral aqueduct demonstrated no significant differences for any of the flow-associated parameters tested. We were therefore unable to detect any evidence of an association between small vessel disease and CFS.
Conclusion
In summary, we were unable to detect any abnormality in brain volume, increased rate of atrophy, white matter lesions or changes in cerebral blood flow or aqueductal CSF flow in a group of patients who had undergone rigorous diagnostic and selection criteria for CFS. This does not exclude the possibility that CFS may occur as a result of some patterns of organic brain lesions that could produce distinctive imaging findings and which might represent subgroups in the CFS population.
Functional MRI or pharmacological MR will probably prove more useful than standard MRI in detecting any cerebral dysfunction. Behavioural performance and cerebral activity in CFS have been examined using rapid event-related functional MRI [17]. CFS patients were considerably slower than matched healthy control subjects in performing both motor and visual imagery tasks [17].
Clinically, there are many CFS patients who have undergone MRI scans in which no structural anomalies have been revealed. However, when no abnormality is detected, these patients are wrongly informed that they do not have CFS because the aforementioned research [1, 2, 5] has identified DWMH and CSF volume changes as a pathological entity in CFS. Our findings show that the symptom complex described as CFS can also be seen in patients with no identifiable cerebral abnormality. Thus, there are important ramifications for future diagnosis of CFS, which obviously cannot be based solely on MRI findings.
Acknowledgments
The authors would like to thank The David and Frederick Barclay Trust for providing the funds for this research, and the FORME Trust for their continuing funding and support.
Footnotes
Approved by Bury & Rochdale L.R.E.C. Study Ref. No.: BRLREC 62, Feb. 2000.
References
- 1.Lange G, DeLuca J, Maldjian JA, Lee H, Tiersky LA, Natelson BH. Brain MRI abnormalities exist in a subset of patients with chronic fatigue syndrome. J Neurol Sci 1999;171:3–7 [DOI] [PubMed] [Google Scholar]
- 2.Lange G, Holodny AI, DeLuca J, Huey-Jen L, Xiao-Hong MY, Steffener J, Natelson BH. Quantitative assessment of cerebral ventricular volumes in chronic fatigue syndrome. Appl Neuropsychol 2001;8:23–30 [DOI] [PubMed] [Google Scholar]
- 3.Cope H, Pernet A, Kendall B, David A. Cognitive functioning and magnetic resonance imaging in chronic fatigue. Br J Psychiatry 1995;167:86–94 [DOI] [PubMed] [Google Scholar]
- 4.Cope H, David AS. Neuroimaging in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry 1996;60:471–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz RB, Garada BM, Komaroff AL, Tice HM, Gleit M, Jolesz FA, Holman BL. Detection of intracranial abnormalities in patients with chronic fatigue syndrome: comparison of MR imaging and SPECT. AJR Am J Roentgenol 1994;162:935–41 [DOI] [PubMed] [Google Scholar]
- 6.Ichise M, Salit IE, Abbey SE, Chung DG, Gray B, Kirsh JC, et al. Assessment of regional cerebral perfusion by 99Tcm-HMPAO SPECT in chronic fatigue syndrome. Nucl Med Commun 1992;13:767–72 [PubMed] [Google Scholar]
- 7.Lewis DH, Mayberg HS, Fischer ME, Goldberg J, Ashton S, Graham MM, et al. Monozygotic twins discordant for chronic fatigue syndrome: regional cerebral blood flow SPECT. Radiology 2001;219:766–73 [DOI] [PubMed] [Google Scholar]
- 8.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994;121:953–9 [DOI] [PubMed] [Google Scholar]
- 9.Moorey S, Greer S, Watson M, Gorman C, Rowden L, Tunmore R, et al. The factor structure and factor stability of the hospital anxiety and depression scale in patients with cancer. Br J Psychiatry 1991;158:255–9 [DOI] [PubMed] [Google Scholar]
- 10.World HealthOrganization Schedules for clinical assessment in neuropsychiatry, Version 2. 0. Manual. Geneva: World Health Organization, 1994 [Google Scholar]
- 11.Perrin RN, Edwards J, Hartley P. An evaluation of the effectiveness of osteopathic treatment on symptoms associated with myalgic encephalitis. A preliminary report. J Med Eng Technol 1998;22:1–13 [DOI] [PubMed] [Google Scholar]
- 12.Thacker NA, Varma AR, Bathgate D, Stivaros S, Snowden JS, Neary D, et al. Dementing disorders: volumetric measurement of cerebrospinal fluid to distinguish normal from pathologic findings — feasibility study. Radiology 2002;224:278–85 [DOI] [PubMed] [Google Scholar]
- 13.Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci 1993;114:7–12 [DOI] [PubMed] [Google Scholar]
- 14.Natelson BH, Cohen JM, Brassloff I, Lee HJ. A controlled study of brain magnetic resonance imaging in patients with the chronic fatigue syndrome. J Neurol Sci 1993;120:213–7 [DOI] [PubMed] [Google Scholar]
- 15.Buchwald D, Cheney PR, Peterson DL, Henry B, Wormsley SB, Geiger A, et al. A chronic illness characterised by fatigue, neurologic and immunologic disorders, and active human herpes type 6 infection. Ann Intern Med 1992;116:103–13 [DOI] [PubMed] [Google Scholar]
- 16.Mokri B. The Monro–Kellie hypothesis: applications in CSF volume depletion. Neurology 2001;56:1746–8 [DOI] [PubMed] [Google Scholar]
- 17.De Lange FP, Kalkman JS, Bleijenberg G, Hagoort P, Van DerWerf SP, Van DerMeer JW, et al. Neural correlates of the chronic fatigue syndrome — an fMRI study. Brain 2004;127:1948–57 [DOI] [PubMed] [Google Scholar]