Abstract
The aim of this study was to evaluate the imaging features of hepatic angiomyolipoma (AML) on contrast-enhanced ultrasound (CEUS). The imaging features of 12 pathologically proven hepatic AML lesions in 10 patients who had undergone baseline ultrasound (BUS) and CEUS examinations were evaluated retrospectively. The enhancement extent, pattern and dynamic change, along with the enhancement process, on CEUS were analysed. The diagnostic results of BUS and CEUS before pathological examination were also recorded. The results showed that 75% (9/12) of the AML lesions exhibited mixed echogenicity on BUS and most showed remarkable hyperechogenicity in combination with a hypoechoic or anechoic portion. Arterial flow signals were detected in 75% (9/12) of the lesions on colour Doppler imaging. On CEUS, 66.7% (n = 8) of the 12 lesions exhibited hyperenhancement in the arterial phase, slight hyperenhancement (n = 2) or isoenhancement (n = 6) in the portal phase, and slight hyperenhancement (n = 1) or isoenhancement (n = 7) in the late phase. Three (25%) lesions exhibited hyperenhancement in the arterial phase and hypoenhancement in both portal and late phases. One (8.3%) lesion exhibited hypoenhancement throughout the CEUS process. Before pathological examination with BUS, only 3 (25%) lesions were correctly diagnosed as hepatic AML. Conversely, on CEUS, correct diagnoses were made for 66.8% (8/12) of hepatic AMLs. Therefore, arterial hyperenhancement and subsequent sustained enhancement on CEUS were found in the majority of hepatic AMLs. The combination of BUS and CEUS leads to the correct diagnosis in the majority of hepatic AMLs, and is higher than the success rate achieved by BUS alone.
Hepatic angiomyolipoma (AML) is generally considered a rare benign mesenchymal tumour of the liver [1]. With the increasing clinical application of imaging, more and more hepatic AMLs are being detected [2, 3]. Baseline ultrasound (BUS) is the first-line imaging modality for liver use owing to its relatively low cost, non-invasiveness, easy manipulation and ready availability; however, its ability to characterise focal liver lesions (FLLs) cannot meet the requirement in the clinical setting [4]. The advent of the second-generation ultrasound contrast agents and contrast-specific ultrasound techniques allows depiction of the micro- and macro-circulation of FLLs, which has facilitated a great improvement in the characterisation of FLLs [5, 6]. However, until now, few reports in the literature have described the use of contrast-enhanced ultrasound (CEUS) in hepatic AML [7–11]. Herein we analyse retrospectively the imaging features of 12 hepatic AMLs on CEUS; the diagnostic results of BUS and CEUS before pathological examination are also recorded.
Methods and materials
Patients
From June 2004 to January 2009, 12 pathologically proven hepatic AML lesions from 10 patients who had undergone CEUS in the institute were chosen retrospectively for inclusion into the study. The patients comprised one man and nine women, aged 25–50 years (mean age, 35±8 years). Hepatitis B surface antigen (HBsAg) was seropositive in one patient. α-Fetoprotein (AFP) and carcinoembryonic antigen (CEA) were seronegative in all of the 10 patients. The nature of the 12 lesions was proved by pathological examination using specimens obtained from surgery (n = 7) or percutaneous ultrasound-guided biopsy (n = 5). Biopsy was performed with an 18 gauge automated biopsy device with a 2.2 cm cutting needle (Magnum; Bard Peripheral Technologies, Covington, GA); two or three cores were obtained from each lesion. Pathological examination was performed by an experienced pathologist who has specialised in liver pathology for more than 30 years. The basic characteristics of the 12 lesions are presented in Table 1. Written informed consent was obtained from all patients and the study was approved by the ethic committee of the institution.
Table 1. Basic characteristics of 12 hepatic AMLs and the original diagnoses of BUS, CEUS and CECT before pathological examination.
| Case no. | Gender (M/F) | Age (years) | Lesion no. | Lesion |
Specimen-obtaining method | Original diagnosis before pathological examination |
|||
| Locationa | Diameter (cm) | BUS | CEUS | CECT | |||||
| 1 | F | 42 | 1 | S7 | 3.4 | Biopsy | Uncertain | Focal nodular hyperplasia | NA |
| 2 | F | 32 | 2 | S7 | 3.1 | Biopsy | Uncertain | Hepatic AML | Uncertain |
| 3 | M | 25 | 3 | S7 | 3.5 | Surgery | Uncertain | Hepatic AML | NA |
| 4 | F | 38 | 4 | S5.8 | 11.2 | Biopsy | Hepatic AML | Hepatic AML | Hepatic AML |
| 5 | S4 | 4.2 | Biopsy | Hepatic AML | Hepatic AML | Hepatic AML | |||
| 5 | F | 36 | 6 | S8 | 4.8 | Biopsy | Uncertain | Hepatic AML | Hepatic AML |
| 6 | F | 34 | 7 | S4 | 2.5 | Surgery | HCC | HCC | HCC |
| 7 | F | 35 | 8 | S7 | 5.3 | Surgery | Uncertain | Cystoadenoma | NA |
| 8 | F | 31 | 9 | S7.8 | 8.3 | Surgery | HCC | HCC | HCC |
| 9 | F | 50 | 10 | S5 | 2.9 | Surgery | Hepatic AML | Hepatic AML | NA |
| 10 | F | 25 | 11 | S6 | 2.5 | Surgery | Uncertain | Hepatic AML | NA |
| 12 | S3.4 | 8.8 | Surgery | Uncertain | Hepatic AML | NA | |||
AML, angiomyolipoma; CECT, contrast-enhanced CT; CEUS, contrast-enhanced ultrasound; BUS, baseline ultrasound; HCC, hepatocellular carcinoma; NA, not available; M, male; F, female.
aLocation is expressed as the segment of the liver (e.g. S7 means segment 7).
Ultrasound contrast agent and equipment
The contrast agent used in this study was SonoVue (Bracco, Milan, Italy) — a sulphur hexafluoride-filled microbubble contrast agent. 2.4 ml of contrast agent was injected through a 20 gauge intravenous cannula into the antecubital vein in a bolus fashion, followed by a flush of 5 ml of 0.9% sodium chloride solution.
Two ultrasound machines were used in this study, depending on their availability. One was an Acuson Sequoia 512 US machine (Siemens Medical Solutions, Mountain View, CA) equipped with a 4V1 vector transducer with a frequency range of 1.0–4.0 MHz, in which a contrast-specific imaging mode of contrast pulse sequencing (CPS) was installed. The other was an Aplio XV machine (Toshiba Medical Systems, Tokyo, Japan) equipped with a 375BT convex transducer with a frequency range of 1.9–6.0 MHz; the contrast-specific imaging mode was contrast harmonic imaging (CHI).
BUS and CEUS examinations
All BUS and CEUS examinations were performed by one of three experienced radiologists who had more than five years of experience in liver CEUS. The entire liver was scanned thoroughly using BUS, and the target lesions were identified. The location, size and echogenicity of the lesion were recorded. Afterwards, the transducer was kept in a stable position and the imaging mode was shifted to low acoustic power contrast-specific imaging mode. In the contrast-enhanced study, low mechanical index (MI) values were used (ranging from 0.15 to 0.21 for CPS in Acuson Sequoia 512 and from 0.05 to 0.08 for CHI in Aplio XV). Imaging settings, such as the gain, depth and focal zone, were optimised to ensure sufficient tissue cancellation with the maintenance of adequate depth penetration.
Subsequently, the SonoVue was injected into the antecubital vein in a bolus fashion. The timer was activated promptly from the beginning of ultrasound contrast agent administration and the lesion was observed continuously until the clearance of the contrast agent from the hepatic parenchyma. The imaging process was observed continuously for 6 min after the initiation of contrast injection, and the entire process was recorded and stored on the hard disk within the scanner.
Data analysis
The baseline and contrast-enhanced images were analysed retrospectively by two independent investigators who were not involved in the ultrasound examination and were unaware of the relevant clinical and laboratorial information, the histopathological results and the results of other imaging modalities. Disagreements over the enhancement pattern and extent were solved by consensus of the two reviewers. The CEUS phase was classified into arterial (8–30 s from contrast agent injection), portal (31–120 s) and late (121–360 s) phases according to the previous literature [10, 12, 13]. The enhancement extent of the hepatic AMLs was referenced to the adjacent liver parenchyma and was divided into hyper-, iso-, hypo- and non-enhancement. The enhancement patterns were divided into homogeneous and heterogeneous enhancement [14].
To evaluate the initial diagnostic ability of CEUS for hepatic AML, the original diagnostic results given by the radiologists from BUS and CEUS before pathological examination were also recorded. The diagnostic criteria for hepatic AML were: normal liver background, mixed echogenicity with a remarkable hyperechoic portion, hypervascularity with arterial flow on BUS and remarkable hyperenhancement during the arterial phase and sustained enhancement during the portal or late phase on CEUS [8–12].
Results
BUS
The liver background of all of the patients was normal, and no liver cirrhosis was found. 75% (9/12) of the AML lesions exhibited mixed echogenicity on BUS and most showed remarkable hyperechogenicity in combination with a hypoechoic or anechoic portion. On colour Doppler imaging, arterial flow signal was detected in 75% (9/12) of the lesions, with 2 being punctiform and 7 filiform in their vascular distribution pattern. The maximal blood flow velocity was 55.7±21.3 cm s–1 (range, 38.3–140 cm s–1) and the resistive index was 0.53±0.1 (range, 0.40–0.62) (Table 2).
Table 2. BUS and CEUS features of the 12 hepatic AML lesions.
| Case no. | Lesion no. | BUS |
CEUS |
|||
| Echogenicity | Vascularity on colour Doppler imaging | Arterial phase | Portal phase | Late phase | ||
| 1 | 1 | Mixed | Filiform | Hyper, homogeneous | Iso | Iso |
| 2 | 2 | Mixed | Filiform | Hyper, homogeneous | Hypo | Hypo |
| 3 | 3 | Mixed | Punctiform | Hyper, homogeneous | Iso | Iso |
| 4 | 4 | Hyper | None | Hypo, homogeneous | Hypo | Hypo |
| 5 | Hyper | None | Hyper, homogeneous | Iso | Iso | |
| 5 | 6 | Mixed | Filiform | Hyper, homogeneous | Hyper | Iso |
| 6 | 7 | Mixed | Filiform | Hyper, homogeneous | Iso | Iso |
| 7 | 8 | Mixed | Filiform | Hyper, heterogeneous | Iso | Iso |
| 8 | 9 | Mixed | Filiform | Hyper, heterogeneous | Hypo | Hypo |
| 9 | 10 | Hyper | Punctiform | Hyper, homogeneous | Hypo | Hypo |
| 10 | 11 | Mixed | None | Hyper, homogeneous | Hyper | Hyper |
| 12 | Mixed | Filiform | Hyper, heterogeneous | Iso | Iso | |
CEUS, contrast-enhanced ultrasound; BUS, baseline ultrasound; hypo, hypoenhancement; iso, isoenhancement; hyper, hyperenhancement.
CEUS
On CEUS, 9 (75%) of the 12 hepatic AMLs showed homogeneous enhancement and the remaining 3 (25%) showed heterogeneous enhancement during the arterial phase (Figures 1 and 2). Of the 12 lesions, 66.7% (n = 8) exhibited remarkable hyperenhancement during the arterial phase, slight hyperenhancement (n = 2) or isoenhancement (n = 6) in the portal phase, and slight hyperenhancement (n = 1) or isoenhancement (n = 7) in the late phase (Figure 3). Three (25%) lesions exhibited hyperenhancement during the arterial phase and hypoenhancement in both the portal and late phases (Figure 5). The above-mentioned 11 (91.7%) lesions that showed hyperenhancement during the arterial phase all enhanced earlier than the surrounding liver tissue. The remaining one (8.3%) lesion exhibited hypoenhancement throughout the CEUS process (Table 2).
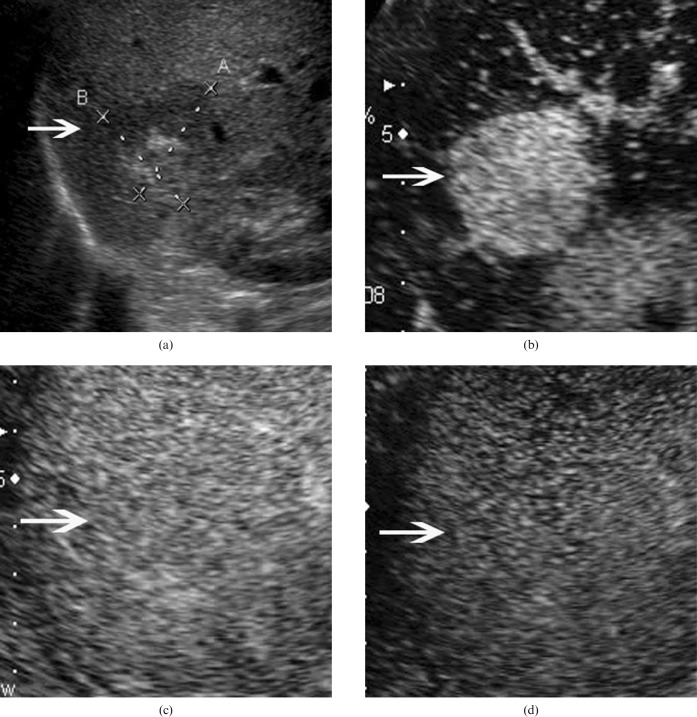
Figure 1.
Hepatic angiomyolipoma in a 42-year-old woman. (a) Baseline ultrasound scan shows a mixed echogenic lesion (arrow) 3.4 cm in diameter in segment 7 of the liver. Hyper- and hypoechoic portions are found in the lesion. (b) In the arterial phase of contrast-enhanced ultrasound, the lesion (arrow) shows homogeneous hyperenhancement 13 s after contrast agent injection. The lesion (arrow) shows isoenhancement in the (c) portal phase (100 s after contrast agent injection) and (d) late phase (130 s after contrast agent injection).
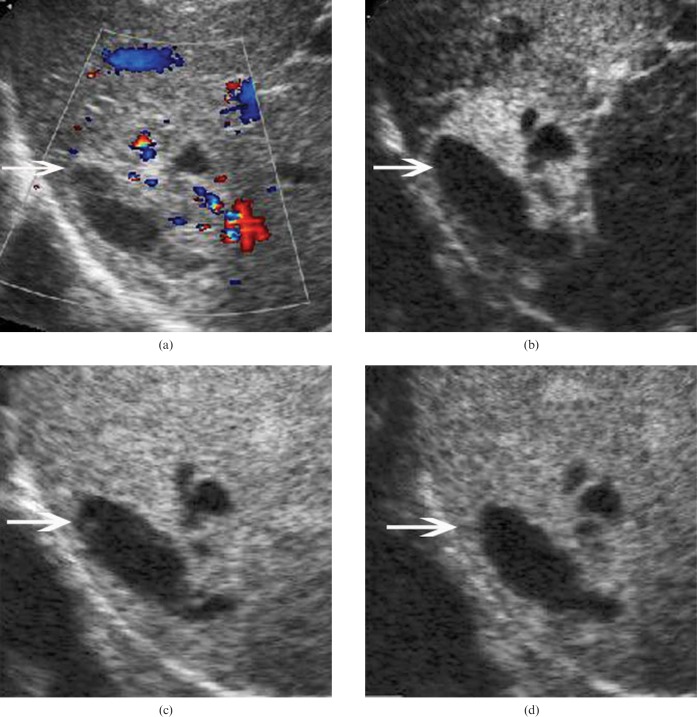
Figure 2.
Hepatic angiomyolipoma in a 35-year-old woman. (a) Baseline ultrasound scan shows a mixed echogenic lesion (arrow) 5.3 cm in diameter in segment 7 of the liver. An anechoic portion is found in the lesion. (b) In the arterial phase of contrast-enhanced ultrasound, the lesion (arrow) shows heterogeneous hyperenhancement 11 s after contrast agent injection. The lesion (arrow) shows isoenhancement in the (c) portal phase (44 s after contrast agent injection) and (d) late phase (136 s after contrast agent injection).
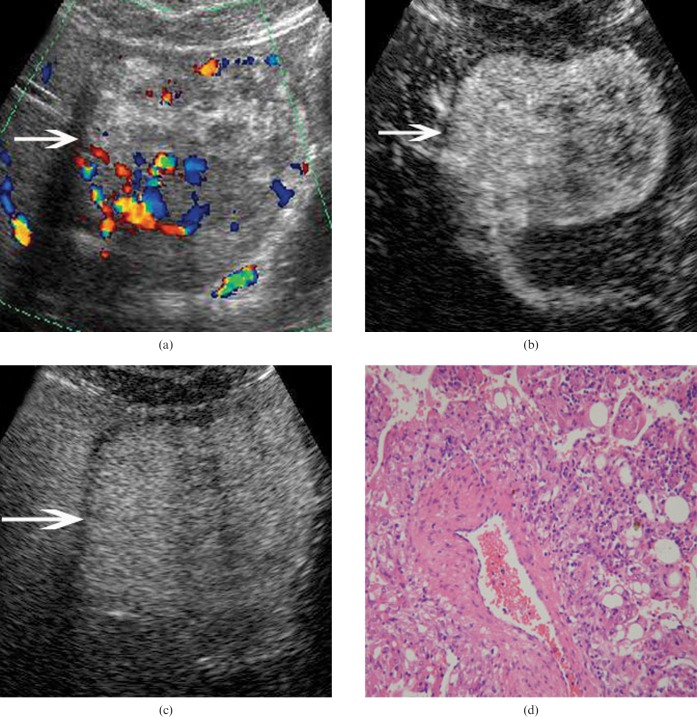
Figure 3.
Hepatic angiomyolipoma (AML) in a 25-year-old woman. (a) Baseline ultrasound scan shows a mixed echogenic lesion (arrow) 8.8 cm in diameter in segments 3 and 4 of the liver. (b) In the arterial phase of CEUS, the lesion (arrow) shows heterogeneous hyperenhancement 8 s after contrast agent injection. (c) The lesion (arrows) shows isoenhancement in the late phase (180 s after contrast agent injection). (d) Pathological examination confirms the diagnosis of hepatic AML hematoxylin-eosin stain; original magnification: ×100.
Original diagnostic results before pathological examination
Before pathological examination, 7 (58.3%) lesions on BUS were determined to be uncertain in nature, 2 (16.7%) lesions were misdiagnosed as hepatocellular carcinoma (HCC) and 3 (25%) lesions were correctly diagnosed as hepatic AML. Conversely, on CEUS, correct diagnoses were made in 66.8% (8/12) of hepatic AMLs. 1 (8.3%) lesion was misdiagnosed as cystadenoma, 1 (8.3%) as focal nodular hyperplasia, and 2 (16.6%) lesions that were presumed to be HCC on BUS were also misdiagnosed as HCC.
In this series, only five patients (six lesions) underwent contrast-enhanced CT examination and none received an MRI examination. For the 6 lesions that had CT results, 3 (50%) lesions were diagnosed correctly as hepatic AML, all of which showed hypoattenuation on unenhanced CT, compared with hypoenhancement during all of the phases for 1 lesion, heterogeneous hyperenhancement during the arterial phase and isoenhancement in both the portal and late phases for 1 lesion, and homogeneous hyperenhancement during the arterial and portal phases and isoenhancement during late phase for 1 lesion. 1 (16.7%) was determined to be uncertain in nature and showed hypoattenuation on an unenhanced CT scan, hyperenhancement during the arterial phase and hypoenhancement during the portal and late phases on enhanced CT. The remaining 2 (33.3%) lesions that were misdiagnosed as HCC on BUS and CEUS were also misdiagnosed as HCC on CECT, showing hypoattenuation on unenhanced CT, compared with homogeneous hyperenhancement during the arterial phase and isoenhancement during the portal and late phases for one lesion, and heterogeneous hyperenhancement during the arterial phase and hypoenhancement during the portal and late phases for the other (Table 1).
Discussion
AML is generally considered to be a benign tumour of mesenchymal origin, which often occurs in the kidney but is rarely found in the liver. Hepatic AML is more likely to occur in women. Most of the hepatic AMLs are solitary, and may be complicated by renal AMLs. About 5–10% of patients with tuberous sclerosis also have hepatic AML [1], which is usually asymptomatic and discovered incidentally. In a small number of cases, patients may complain of abdominal pain, abdominal distension, fever, discomfort and weight loss [15]. Laboratory results are almost normal in hepatic AML. The tumour markers AFP and CEA were serum negative in our study, and there was no history of viral hepatitis infection in the majority of cases. Although most hepatic AMLs behave as a benign tumour with almost no recurrence after surgical resection, the benign nature of hepatic AML has been challenged in recent years; some authors suggest that hepatic AML should be considered to be a mesenchymal tumour with a tendency for malignancy [16–18]. In this series, only one case was accompanied by renal AML, and nine of the 10 patients were women. No evidence for tuberous sclerosis was found. One patient was HBsAg seropositive; the others were seronegative.
Pathologically, AML is composed of varying proportions of three elements: proliferating thick-walled blood vessels, smooth muscle and mature adipose cells. According to the inner fat content, AML is classified histologically into mixed, lipomatous, myomatous and angiomatous types. The diagnosis of hepatic AML is still challenging and largely depends on identification of intratumoral fat on imaging. On CT and MRI, hepatic AML usually appears as a combination of fat and soft tissue. On unenhanced CT, most cases of hepatic AML appear as low-density lesions, whereas, on contrast-enhanced CT or MRI, AML always shows remarkable hyperenhancement during the arterial phase and sustained enhancement during the portal phase.
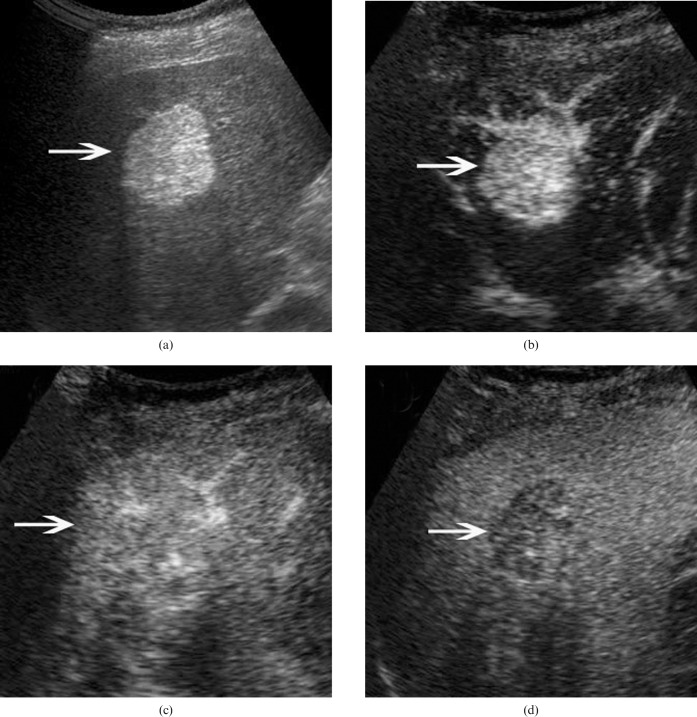
Figure 4.
Hepatic angiomyolipoma in a 50-year-old woman. (a) Baseline ultrasound scan shows a homogeneously hyperechoic lesion (arrow) 2.9 cm in diameter in segment 5 of the liver. (b) In the arterial phase of contrast-enhanced ultrasound, the lesion (arrow) shows homogeneous hyperenhancement 11 s after contrast agent injection. The lesion (arrows) shows (c) isoenhancement in the portal phase (31 s after contrast agent injection) and (d) hypoenhancement in the late phase (180 s after contrast agent injection).
On BUS, hepatic AML may show obvious hyperechogenicity because of its fat content. The fat content of hepatic AML varies from <5% to >90% of the tumour volume; thus, the lesion can show homogeneous or heterogeneous hyperechogenicity. On colour Doppler imaging, arterial signals can be detected; the maximal blood flow velocity ranged from 38.3 to 140 cm s–1 in this series. The resistive index in hepatic AML (0.53±0.1) was lower than that reported in HCC.
In this series, 11 (91.7%) of 12 lesions showed heterogeneous (n = 3) or homogeneous (n = 8) hyperenhancement during the arterial phase of CEUS. The enhancement of the lesions was seen earlier than the enhancement of adjacent liver parenchyma, consistent with evidence reported in the literature [7, 11, 19–21]. The hypervascularity in hepatic AML is associated with the proliferating thick-walled blood vessels in the lesion. The remaining lesion showed hypoenhancement throughout the CEUS phases. During the late phase, sustained enhancement (i.e. slight hyperenhancement and isoenhancement) was found in ∼67% of all AMLs, which is a critical clue for determining the benign nature of these lesions. Conversely, hypoenhancement in late phase was found in the remaining 33% of hepatic AMLs. Therefore, the characterisation algorithm of CEUS for FLLs (i.e. sustained enhancement in late phase indicates benign lesions and washout in the late phase indicates malignancies) is applicable for only 67% of AMLs. Accordingly, before pathological examination, CEUS made the correct diagnosis in 67% of AMLs. Compared with BUS, the correct diagnosis increased significantly from 25% to 67% of hepatic AMLs.
Clinically, the ultrasound appearances of hepatic AML are quite variable and may overlap with those of other benign and malignant fat-containing hepatic lesions, including haemangioma, hepatic adenoma, focal nodular hyperplasia (FNH), lipoma, focal fatty change and even HCC with fatty metamorphosis. With the aid of CEUS, haemangioma is easy to exclude because peripheral nodular hyperenhancement during the arterial phase and gradual centripetal enhancement is seen. Hepatic adenoma has similar features on CEUS to hepatic AML, whereas it is usually homogeneously hypoechoic or isoechoic on BUS, in contrast to the remarkable hyperechogenicity seen in AML. FNH also has similar characteristics to hepatic AML on CEUS; however, the isoechoic or hypoechoic feature on greyscale BUS and the spoke-wheel-shaped artery signals on colour Doppler imaging provide clues to discriminate them. Liver lipoma is an extremely uncommon benign tumour and the imaging appearance of lipoma is characteristic. On BUS, these lipomas usually appear as well-circumscribed uniformly remarkable hyperechoic lesions, in contrast to the mixed echogenicity of AMLs. Focal fatty change always shows isoenhancement in all three phases on CEUS, and thus it is easy to distinguish from hepatic AML. HCC with fatty metamorphosis is not rare clinically, especially for small early-stage HCC. The liver background of liver cirrhosis and serum biomarkers may help to make the distinction between the two lesion types [21–24]. However, in this series, one patient with AML was electropositive for HBsAg and was misdiagnosed as having HCC. Thus, it should be kept in mind that the possibility of AML should be ruled out when making a diagnosis of HCC from CEUS.
Compared with BUS, CEUS improved the rate of correctly diagnosing hepatic AML. However, the CEUS pattern of AML is not specific, as hyperenhancement during the arterial phase and sustained enhancement in the portal or late phase can be seen in FNH, liver adenoma and even HCC. Thus, combining the particular signs on BUS with typical CEUS findings could lead to an improved diagnosis rate for hepatic AML. Conversely, although it was supposed that CT would lead to the correct diagnosis in the majority of cases, thus avoiding surgery or biopsy, CT made the correct diagnosis in only 50% of hepatic AMLs in this series. Therefore, the imaging diagnosis of hepatic AML is not yet satisfactory, and further improvement in diagnostic ability is necessary.
Conclusions
In addition to the characteristics of mixed echogenicity with a remarkable hyperechoic portion in the lesion and hypervascularity on BUS, hepatic AML also has some characteristic manifestations on CEUS. Arterial hyperenhancement and subsequent sustained enhancement was found in the majority of hepatic AMLs. The combination of BUS and CEUS leads to the correct diagnosis in the majority of hepatic AMLs, which is a higher rate than that achieved by BUS alone.
Acknowledgments
This work was supported in part by Grant NCET-06-0723 from Chinese Ministry of Education and Grant 2008-2-10 of Public Welfare Research Special Project from Chinese Ministry of Health.
References
- 1.Nonomura A, Mizukami Y, Kadoya M. Angiomyolipoma of the liver: a collective review. J Gastroenterol 1994;29:95–105 [DOI] [PubMed] [Google Scholar]
- 2.Tsui WM, Colombari R, Portmann BC, Bonetti F, Thung SN, Ferrell LD, et al. Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants. Am J Surg Pathol 1999;23:34–48 [DOI] [PubMed] [Google Scholar]
- 3.Horton KM, Bluemke DA, Hruban RH, Soyer P, Fishman EK. CT and MR imaging of benign hepatic and biliary tumors. Radiographics 1999;19:431–51 [DOI] [PubMed] [Google Scholar]
- 4.Rettenbacher T. Focal liver lesions: role of contrast-enhanced ultrasound. Eur J Radiol 2007;64:173–82 [DOI] [PubMed] [Google Scholar]
- 5.Albrecht T, Blomley M, Bolondi L, Claudon M, Correas JM, Cosgrove D, et al. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med 2004;25:249–56 [DOI] [PubMed] [Google Scholar]
- 6.Bauer A, Solbiati L, Weissman N. Ultrasound imaging with SonoVue: low mechanical index real-time imaging. Acad Radiol 2002;9:S282–4 [DOI] [PubMed] [Google Scholar]
- 7.Bartolotta TV, Runza G, Minervini M, Midiri M. Hepatic angiomyolipoma: contrast-enhanced pulse inversion US in a case. Radiol Med (Torino) 2003;105:514–18 [PubMed] [Google Scholar]
- 8.Zheng RQ, Kudo M. Hepatic angiomyolipoma: identification of an efferent vessel to be hepatic vein by contrast-enhanced harmonic ultrasound. Br J Radiol 2005;78:956–60 [DOI] [PubMed] [Google Scholar]
- 9.Yen YH, Wang JH, Lu SN, Changchien CS. Contrast-enhanced ultrasonography in hepatic angiomyolipoma. J Ultrasound Med 2005;24:855–9 [DOI] [PubMed] [Google Scholar]
- 10.Xu HX, Xie XY, Lu MD, Liu GJ, Xu ZF, Liang JY, et al. Unusual benign focal liver lesions: findings on real-time contrast-enhanced sonography. J Ultrasound Med 2008;27:243–54 [DOI] [PubMed] [Google Scholar]
- 11.Ren N, Qin LX, Tang ZY, Wu ZQ, Fan J. Diagnosis and treatment of hepatic angiomyolipoma in 26 cases. World J Gastroenterol 2003;9:1856–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, et al. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound 2006;34:261–72 [DOI] [PubMed] [Google Scholar]
- 13.Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, et al. Characterization of small focal liver lesions using real-time contrast-enhanced sonography: diagnostic performance analysis in 200 patients. J Ultrasound Med 2006;25:349–61 [DOI] [PubMed] [Google Scholar]
- 14.Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) — update 2008. Ultraschall Med 2008;29:28–44 [DOI] [PubMed] [Google Scholar]
- 15.Yang CY, Ho MC, Jeng YM, Hu RH, Wu YM, Lee PH. Management of hepatic angiomyolipoma. J Gastrointest Surg 2007;11:452–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen TT, Gorman B, Shields D, Goodman Z. Malignant hepatic angiomyolipoma: report of a case and review of literature. Am J Surg Pathol 2008;32:793–8 [DOI] [PubMed] [Google Scholar]
- 17.Dalle I, Sciot R, de Vos R, Aerts R, van Damme B, Desmet V, et al. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology 2000;36:443–50 [DOI] [PubMed] [Google Scholar]
- 18.Yang CY, Ho MC, Jeng YM, Hu RH, Wu YM, Lee PH. Management of hepatic angiomyolipoma. J Gastrointest Surg 2007;11:452–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogemann D, Flemming P, Kreipe H, Galanski M. Correlation of MRI and CT findings with histopathology in hepatic angiomyolipoma. Eur Radiol 2001;11:1389–95 [DOI] [PubMed] [Google Scholar]
- 20.Guidi G, Catalano O, Rotondo A. Spontaneous rupture of a hepatic angiomyolipoma: CT findings and literature review. Eur Radiol 1997;7:335–7 [DOI] [PubMed] [Google Scholar]
- 21.Ascenti G, Gaeta M, Zimbaro G, Villari D, Blandino A, Scribano E. US power Doppler of hepatic angiomyolipoma with low fat content. Eur Radiol 2000;10:935–7 [DOI] [PubMed] [Google Scholar]
- 22.Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol 2000;33:282–9 [DOI] [PubMed] [Google Scholar]
- 23.Prasad SR, Wang H, Rosas H, Menias CO, Narra VR, Middleton WD, et al. Fat-containing lesions of the liver: radiologic-pathologic correlation. Radiographics 2005;25:321–31 [DOI] [PubMed] [Google Scholar]
- 24.Sonsuz A, Ozdemir S, Akdogan M, Senturk H, Ozbay G, Akin P, et al. Lipoma of the liver. Z Gastroenterol 1994;32:348–50 [PubMed] [Google Scholar]