Abstract
The aim of this study was to investigate whether the combination of cisplatin-eluting gelatin microspheres (GMSs) and flavopiridol enhances anti-tumour effects in a rabbit VX2 liver tumour model. Tumour-bearing rabbits (n = 21) were divided into five groups and infused from the proper hepatic artery. Group 1 (n = 5) received cisplatin-eluting GMSs (1 mg kg−1) and flavopiridol (3 mg kg−1), group 2 (n = 5) cisplatin-eluting GMSs alone (1 mg kg−1), Group 3 (n = 5) flavopiridol (3 mg kg−1), Group 4 (n = 3) GMSs alone (1 mg kg−1), and Group 5 (n = 3) was the control group receiving physiological saline (1 ml kg−1). On days 0 and 7 after procedures the liver tumour volume was measured using a horizontal open MRI system and the relative tumour volume growth rates for 7 days after treatment were calculated. On T1 weighted images, the tumours were visualised as circular, low-intensity areas just below the liver surface. After treatment, the signals remained similar. The relative tumour volume growth rate for 7 days after treatment was 54.2±22.4% in Group 1, 134.1±40.1% in Group 2,166.7±48.1% in Group 3, 341.8±8.6% in Group 4 and 583.1±46.9% in Group 5; the growth rate was significantly lower in Group 1 than the other groups (p<0.05). We concluded that in our rabbit model of liver tumours the combination of cisplatin-eluting GMSs and flavopiridol was effective.
Meliaceae are flowering plants in the Mahogany family comprising primarily trees and shrubs in the order Sapindales. A flavonoid extracted from the bark of Dysoxylum binectarferum, a species in the Meliaceae family of trees that grows in tropical and subtropical regions, has been termed flavopiridol [1, 2]. Flavopiridol has been reported to be a cyclin-dependent kinase inhibitor that induces apoptosis [3, 4], and a combination of flavopiridol and cisplatin has been shown to effectively treat platinum drug-resistant advanced malignancies [5]. Phase II trials of this combination are ongoing in patients with ovarian cancer and primary peritoneal carcinoma.
We have developed cisplatin-eluting gelatin microspheres (GMSs) and carried out experiments to test their applicability for the treatment of rabbit liver tumours; their anti-tumour properties were confirmed by demonstrating their embolisation effects and the sustained release of cisplatin [6, 7]. In patients with treatment-resistant metastatic liver tumours, cisplatin-eluting GMSs exerted some anti-tumour effects [8]. In this study, we investigated whether a combination of cisplatin-eluting GMSs and flavopiridol exhibited enhanced antitumour effects in a rabbit model of VX2 liver tumours.
Methods and materials
Materials
Acidic gelatin (molecular weight 99 kDa) with an isoelectric point of 5.0 was purchased from Nitta Gelatin Co., Ltd (Osaka, Japan), cisplatin from Nippon Kayaku Co., Ltd (Tokyo, Japan) and flavopiridol from Sanofi-Aventis KK (Tokyo, Japan). All other chemicals were of the highest commercially available purity. Sheaths, catheters, microcatheters and guidewires were from Clinical Supply Co., Ltd (Gifu, Japan). Iopamiron was from Bayer AG (Leverkusen, Germany).
All experiments were approved in advance by the Animal Experiment Ethical Committee of Shiga University of Medical Science.
Preparation of cisplatin-releasing GMSs
GMSs were prepared according to the modified method of Tabata and Ikada [9] by glutaraldehyde cross-linking of an aqueous gelatin solution dispersed in an oil phase in the absence of a surfactant. Briefly, 10 ml of acidic aqueous gelatin solution (10%) that had been pre-heated to 37°C was added dropwise to 375 ml of olive oil (Wako Pure Chemical Industries Ltd, Osaka, Japan). The mixture was stirred (400 rpm, 37°C, 10 min) to yield a water-in-oil (w/o) emulsion. Stirring was continued for 30 min at 4°C and the microspheres formed were washed three times with acetone (Wako) by centrifugation (5000 rpm, 5 min, 4°C). After air-drying, the microspheres were sized by passage through sieves with apertures of 100 μm; in this way microspheres with a diameter of 100–200 μm were obtained. Non-cross-linked dry GMSs were dispersed in 5 ml of aqueous glutaraldehyde solution (7.5 mg ml−1, 25%, Nacalai Tesque Inc., Kyoto, Japan) at 4°C for 15 h to facilitate cross-linking. Microspheres were further agitated in 5 ml of 10 mM aqueous glycine solution (Nacalai Tesque) at 37°C for 1 h to block the residual aldehyde groups of unreacted glutaraldehyde. The resultant microspheres were finally washed three times by centrifugation with double-distilled water (DDW) and freeze-dried. The GMSs used in this study were designed to degrade completely within 5 days in extravascular tissues. The degradation period was confirmed by introducing GMSs into the subcutaneous dorsal neck tissues of rabbits [10].
To prepare the cisplatin-releasing GMSs, cross-linked GMSs (1 mg) were immersed in 50 μl of cisplatin solution (0.3 mg ml−1) at 38°C for 1 h to allow conjugation to cisplatin. The samples were then repeatedly washed in DDW and centrifuged (×7) to remove uncombined cisplatin from the GMSs before freeze drying. Immersion in cisplatin solution, washing and drying was repeated four times. Finally, cisplatin-loaded GMSs contained 20 mg platinum per 1 g gelatin [6, 7].
In vivo therapeutic study
We divided 21 rabbits bearing liver tumours into 5 groups: Group 1 (n = 5) was infused with flavopiridol (3 mg kg−1) and then with cisplatin-eluting GMSs (1 mg kg−1); Group 2 (n = 5) was infused with cisplatin-eluting GMSs alone (1 mg kg−1); Group 3 (n = 5) with flavopiridol alone (3 mg kg−1); Group 4 (n = 3) with GMSs alone (1 mg kg−1); and Group 5 (n = 3), which served as the control, was infused with physiological saline (1 ml kg−1). All animals were infused from the proper hepatic artery.
The liver tumour volume was measured on days 0 and 7 after procedures using a horizontal open MRI system (0.3 T coil; Hitachi Co. Ltd, Tokyo, Japan). To obtain these images we used a spin-echo pulse sequence T1 weighted image (time to repeat 300 ms, time to echo 14 ms, average 20, field of view 150 mm, matrix size 224 × 192, slice thickness 3 mm, interslice gap 0.5 mm). Images were interpolated to a 256 × 256 matrix on a data server, saved as BMP files, and transferred to a personal computer. Liver tumour volumes were estimated using Photoshop 7.0.1 (Adobe Systems Inc., San Jose, CA). The tumours were manually outlined on each slice by the consensus of two radiologists (NN and AS) and the total number of pixels was multiplied by 1.37 mm3. In implementing the measurement of tumour sizes on MR images, the two radiologists were not informed as to which group the measured tumours belonged or whether the tumours to be measured were imaged before or after therapy. In other words, the radiologists performed their measurements blinded to the background of the tumour images.
After MRI acquisition, the drugs were infused via the proper hepatic artery using a 2.0 Fr microcatheter (Revo Sniper selective type; Clinical Supply Co. Ltd) introduced with the aid of coliac arteriography (2 ml iopamiron, 185 mg ml−1). Relative tumour volume growth rates for 7 days after treatment (total number of pixels on day 7/total number of pixels on day 0 × 100) (%) were calculated. Values of more and less than 100% were considered to reflect an increase and a decrease in the tumour size, respectively.
Statistical analysis
All results are expressed as the mean±the standard deviation of the mean (SD). They were statistically analysed using Dr SPSS II for Windows standard version (SPSS Japan Inc., Tokyo, Japan). Relative tumour volume growth rates for 7 days after treatment in the five groups were first compared by ANOVA. In cases with significant differences, Tukey's HSD test was applied for comparisons between groups.
Results
On T1 weighted MRI, tumours were visualised as circular, low-intensity areas just below the liver surface. After treatment, the signals remained similar (Figure 1). Pathological analysis of the liver tumour samples confirmed the presence of both necrotic portions and tumour remnants. The relative tumour volume growth rates for 7 days after treatment were 54.2%±22.4% in Group 1, 134.1%±40.1% in Group 2, 166.7%±48.1% in Group 3, 341.8%±8.6% in Group 4 and 583.1%±46.9% in Group 5 (Figure 2). The relative tumour volume growth rates 7 days after treatment were significantly lower in Group 1 than for the other Groups (p<0.05). The growth rate was also significantly lower in group 3 than in Groups 4 and 5 (p<0.05); there was no significant difference between Groups 2 and 3 (p = 0.65).
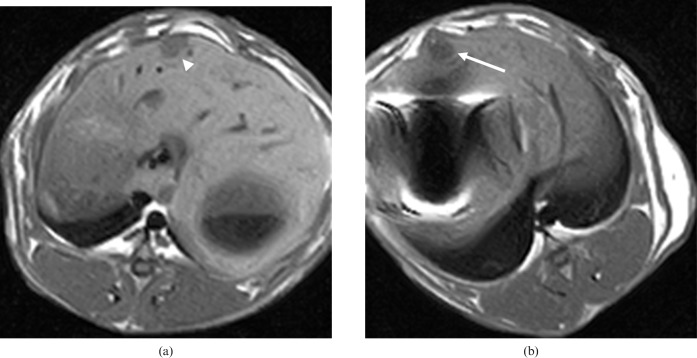
Figure 1.
Results for Group 1. (a) Pre- and (b) post-treatment T1 weighted magnetic resonance images. The tumours were visualised as circular, low-intensity areas (arrow) just below the liver surface. After treatment, the signals remained similar (arrowhead).
Figure 2.
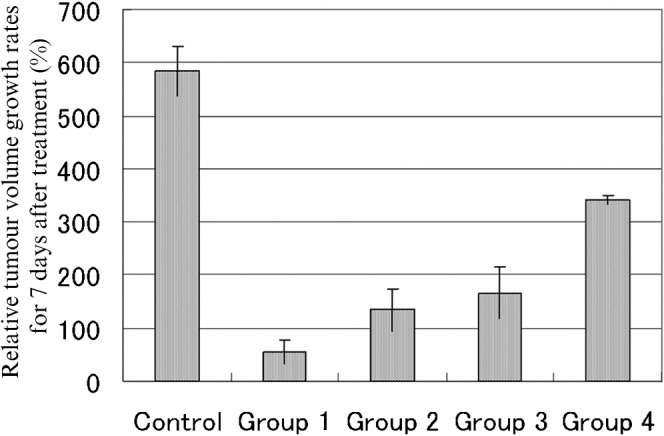
Evaluation of relative tumour volume growth rates for 7 days after treatment: Group 1 (flavopiridol with cisplatin-eluting GMSs), relative tumour volume growth rate (TGR) 54.2±22.4%; Group 2 (cisplatin-eluting GMSs) TGR 134.1±40.1%; Group 3 (flavopiridol) TGR 166.7±48.1%; Group 4 (GMSs) TGR 341.8±8.6%; Group 5 (control: physiological saline) TGR 583.1±46.9%. Relative tumour volume growth rates for 7 days after treatment were significantly lower in Group 1 than for the other groups (p<0.05). There was no significant difference in the relative tumour volume growth rates for 7 days after treatment between groups 2 and 3 (p = 0.65). There was a significant difference in the relative tumour volume growth rates for 7 days after treatment between Group 3 and Group 4 (p<0.05).
Discussion
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. Although surgery is the first-line therapy for early and small HCC, low-invasive local therapies are selected for multiple or recurrent HCC, such as transarterial chemoembolisation (TACE), percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), microwave coagulation therapy (MCT) and laser-induced thermotherapy (LITT). The tumour model used in this study has been established following many years' research by our group in this area. Because the vascularisation of our tumour model is similar to that of human liver tumour, it has been used in clinical research on liver tumour imaging, chemotherapy and tumour etiology [10, 11].
We found that in liver tumour-bearing rabbits treated with cisplatin-eluting GMSs plus flavopiridol, the tumour volume reduction was greater than in the other groups.
Flavopiridol as monotherapy and in combination with other agents has been studied in a number of haematological and solid tumour malignancies using a variety of dose schedules. Flavopiridol as a monotherapy demonstrated acceptable toxicity and signs of efficacy in several Phase I solid tumour studies [12–14]. As a result, Phase II monotherapy studies have been completed or are ongoing in patients with advanced and chemotherapy-resistant diseases (e.g. soft-tissue sarcoma, multiple myeloma, endometrial, renal, gastric, colorectal, non-small-cell lung and prostate cancers). The early signs of activity in the Phase I studies, however, have not been realised in the Phase II solid tumour studies completed to date [1, 15–20]. A recent study on relapsed chronic lymphocytic leukaemia reported the use of a novel pharmacologically derived schedule. The schedule comprised a 30 min intravenous loading dose infusion followed by 4 h continuous intravenous infusion, to compensate for the higher than expected protein binding in human serum, and led to increased drug exposure and improved efficacy [4]. Therefore, the anticancer effect of flavopiridol in solid tumours using the earlier dose infusion schedules might need to be re-examined with novel schedules to increase drug exposure. The safety and efficacy of flavopiridol in combination with other anticancer drugs, such as cisplatin, carboplatin, docetaxel, irinotecan and paclitaxel, has also been demonstrated in several Phase I studies supporting the follow-up evaluation in Phase II studies [21–25].
In our experimental studies we documented the embolisation effect of cisplatin-eluting GMSs and their sustained release of cisplatin [6, 7]. In clinical studies, we verified the embolisation effects of GMSs alone and the effects of cisplatin-eluting GMSs on metastatic liver tumours [8, 26]. Moreover, we found that cisplatin-eluting GMSs elicited milder adverse effects than systemic chemotherapy and the arterial infusion of other anticancer drugs. Although the response rate to cisplatin-eluting GMSs was only 33%, considering that the tumours of 8 of 9 patients were resistant to previous treatment and that the adverse effects were mild, we concluded that cisplatin-eluting GMSs demonstrated satisfactory anticancer effects [8].
Using a rabbit liver VX2 model, we have examined here whether the effect of the combined administration of cisplatin-eluting GMSs and flavopiridol was superior to that of cisplatin-eluting GMSs alone and whether the combination was more effective than flavopiridol alone. Ours is the first study in which flavopiridol and a cisplatin-impregnated compound have been delivered by transhepatic artery infusion to study the embolisation effect.
We found in rabbits that the administration of flavopiridol alone resulted in a tumour proliferation rate of 166.7%. This rate was not significantly different from the 134.1% observed in rabbits treated with cisplatin-eluting GMSs alone. However, in rabbits treated with cisplatin-eluting GMSs and flavopiridol, the relative tumour volume growth rates for 7 days after treatment were significantly lower (54.2%, p<0.05). Regarding tumour volume growth rates, our findings were in agreement with our expectations. The combination of cisplatin-eluting GMSs and flavopiridol inhibited tumour growth to a greater degree than cisplatin-eluting GMSs alone, suggesting that increased drug doses may improve tumour growth inhibition.
In this study, we used flavopiridol at a dose of 3 mg kg−1, which was moderately small compared with the doses that are usually used in clinical trials. It was found, however, that flavopiridol alone produced a stronger antitumour effect than GMS alone and exhibited an almost identical antitumour effect to that of cisplatin-eluting GMSs alone. An increase in the dose and/or number of doses of flavopiridol may further potentiate its antitumour effect.
Combining flavopiridol with the spheric drug-eluting embolisation materials recently used for TACE (e.g. DC beads and hepaspheres) might enhance its anti-tumour effects even further. In our study, we administered flavopiridol only once, but it has been found in some reports that its administration for several consecutive days is effective. Also, there is the possibility that the intravenous infusion of drugs over several sessions may enhance their anticancer effects on cells already damaged by embolisation. Studies are under way to examine the anticancer effects of different doses of flavopiridol and the effectiveness of different administration routes. We conducted this study to investigate human HCC from a viewpoint of clinical medicine. To achieve this aim, we used rabbit models in which VX2 tumours were implanted in the liver. These tumours were reported to easily necrose despite their hypervascularity. In this study, therapy was started at 2 weeks of implantation, although it was necessary to find the most appropriate time for initiating therapy. Furthermore, tumour implantation was associated with some degree of injury to normal liver, such that the parenchymal haemodynamics surrounding implanted tumours might be different from those associated with natural tumours; this point merits further study.
Conclusion
In conclusion, we showed that the combined administration of flavopiridol and cisplatin-eluting GMSs was effective in treating VX2 liver tumours in rabbits.
References
- 1.Shapiro GI, Supko JG, Patterson A, Lynch C, Lucca J, Zacarola PF, et al. A phase 2 trial of the cyclin-dependent kinase inhibitor flavopiridol in patients with previously untreated stage 4 non-small cell lung cancer. Clin Cancer Res 2001;7:1590–8 [PubMed] [Google Scholar]
- 2.Schwartz GK, Ilson D, Saltz L, O'Reilly E, Tong W, Maslak P, et al. Phase 2 study of the cyclin-dependent kinase inhibitor flavopiridol administered to patients with advanced gastric carcinoma. J Clin Oncol 2001;19:1985–92 [DOI] [PubMed] [Google Scholar]
- 3.Flinn IW, Byrd JC, Bartlett N, Kipps T, Gribben J, Thomas D, et al. Flavopiridol administered as a 24-hour continuous infusion in chronic lymphocytic leukemia lacks clinical activity. Leuk Res 2005;29:1253–7 [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood 2007;109:399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bible KC, Lensing JL, Nelson SA, Lee YV, Reid JM, Ames MM, et al. Phase 1 trial flavopiridol combined with cisplatin or carboplatin in patients with advanced malignancies with the assessment of pharmacokinetic and pharmacodynamic end points. Clin Cancer Res 2005;11:5935–41 [DOI] [PubMed] [Google Scholar]
- 6.Ohta S, Nitta N, Sonoda A, Seko A, Tanaka T, Takahashi M, et al. doi: 10.1016/j.ejrad.2008.07.030. Prolonged local persistence of cisplatin-loaded gelatin microspheres and their chemoembolic anti-cancer effect in rabbits. Eur J Radiol 2008: in press. [DOI] [PubMed] [Google Scholar]
- 7.Ohta S, Nitta N, Sonoda A, Seko A, Tanaka T, Takahashi M, et al. Cisplatin-conjugated degradable gelatin microspheres: fundamental study in vitro. Br J Radiol 2009;82:380–5 [DOI] [PubMed] [Google Scholar]
- 8.Nitta N, Ohta S, Tanaka T, Takazakura R, Toyama T, Sonoda A, et al. doi: 10.1016/j.ejrad.2008.06.006. An initial clinical study on the efficacy of cisplatin-releasing gelatin microspheres for metastatic liver tumors. Eur J Radiol 2009;71:519–26. [DOI] [PubMed] [Google Scholar]
- 9.Tabata Y, Ikada Y. Synthesis of gelatin microspheres containing interferon. Pharm Res 1989;6:422–7 [DOI] [PubMed] [Google Scholar]
- 10.Ohta S, Nitta N, Takahashi M, Murata K, Tabata Y. Degradable gelatin microspheres as an embolic agent: an experimental study in a rabbit renal model. Korean I Radiol 2007;8:418–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez LH, Zhao Z, Rougier P, Bognel C, Dzodic R, Vassal G, et al. Pharmacokinetics and antitumor effects of mitoxantrone after intratumoral or intraarterial hepatic administration in rabbits. Cancer Chemother Pharmacol 1996;37:371–6 [DOI] [PubMed] [Google Scholar]
- 12.Senderowicz AM, Headlee D, Stinson SF, Lush RM, Kalil N, Villalba L, et al. Phase 1 trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. J Clin Oncol 1989;16:2986–99 [DOI] [PubMed] [Google Scholar]
- 13.Thomas JP, Tutsch KD, Cleary JF, Bailey HH, Arzoomanian R, Alberti D, et al. Phase 1 clinical and pharmacokinetic trial of the cyclin-dependent kinase inhibitor flavopiridol. Cancer Chemother Pharmacol 2002;50:465–72 [DOI] [PubMed] [Google Scholar]
- 14.Tan AR, Headlee D, Messmann R, Sausville EA, Arbuck SG, Murgo AJ, et al. Phase 1 clinical and pharmacokinetic study of flavopiridol administered as a daily 1-hour infusion in patients with advanced neoplasms. J Clin Oncol 2002;20:4074–82 [DOI] [PubMed] [Google Scholar]
- 15.Grendys EC, Jr, Blessing JA, Burger R, Hoffman J. A phase 2 evaluation of flavopiridol as second-line chemotherapy of endometrial carcinoma: agynecologic oncology group study. Gynecol Oncol 2005;98:249–53 [DOI] [PubMed] [Google Scholar]
- 16.Morris DG, Bramwell VHC, Turcotte R, Figueredo AT, Blackstein ME, Verma S, et al. doi: 10.1155/SRCM/2006/64374. A phase 2 study of flavopiridol in patients with previously untreated advanced soft tissue sarcoma. Sarcoma 2006;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dispenzieri A, Gertz MA, Lacy MQ, Geyer SM, Fitch TR, Fenton RG, et al. Flavopiridol in patients with relapsed or refractory multiple myeloma: a phase 2 clinical and pharmacodynamic end-points. Heamatologica 2006;91:390–3 [PubMed] [Google Scholar]
- 18.Van Veldhuizen PJ, Faulkner JR, Lana Jr PN, Gumerlock PH, Goodwin JW, Dakhil SR, et al. A phase 2 study of flavopiridol in patients with advanced renal cell carcinoma: results of Southwest Oncology Group Trial 0109. Cancer Chemother Pharmacol 2005;56:39–45 [DOI] [PubMed] [Google Scholar]
- 19.Aklilu M, Kindler H, Donehower RC, Mani S, Vokes EE. Phase 2 study of flavopiridol in patients with advanced colorectal cancer. Ann Oncol 2003;14:1270–3 [DOI] [PubMed] [Google Scholar]
- 20.Liu G, Gandara DR, Lara PN, Jr, Raghavan D, Doroshow JH, Twardowski P, et al. A phase 2 trial of flavopiridol (NSC #649890) in patients with previously untreated metastatic androgen-independent prostate cancer. Clin Cancer Res 2004;10:924–8 [DOI] [PubMed] [Google Scholar]
- 21.Bible K, Lensing JL, Nelson SA, Lee YK, Reid JM, Ames MM, et al. Phase 1 trial of flavopiridol combined with cisplatin or carboplatin in patients with advanced malignancies with the assessment of pharmacokinetic and pharmacodynamic end points. Clin Cancer Res 2005;11:5935–41 [DOI] [PubMed] [Google Scholar]
- 22.El-Rayes BF, Gadgeel S, Parchment R, Lorusso P, Philip PA. A phase 1 study of flavopiridol and docetaxel. Invest New Drugs 2006;24:305–10 [DOI] [PubMed] [Google Scholar]
- 23.Shah MA, Kortmansky J, Motwani M, Drobnjak M, Gonen M, Yi S, et al. A phase 1 clinical trial of the sequential combination of irrinotecan followed by flavopiridol. Clin Cancer Res 2005;11:3836–45 [DOI] [PubMed] [Google Scholar]
- 24.George S, Kasimis BS, Cogswell J, Schwarzenberger P, Shapiro GI, Fidias P, et al. Phase 1 study of flavopiridol in combination with paclitaxel and carboplatin in patients with non-small-cell lung cancer. Clin Lung Cancer 2008;9:160–5 [DOI] [PubMed] [Google Scholar]
- 25.Karp JE, Smith D, Levis MJ, Gore SD, Greer J, Hattenburg C, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase 2 trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res 2007;13:4467–73 [DOI] [PubMed] [Google Scholar]
- 26.Nitta N, Ohta S, Tanaka T, Takazakura R, Nagatani Y, Kono N, et al. Gelatin microspheres: initial clinical experience for the transcatheter arterial embolization. Eur J Radiol 2008;67:536–40 [DOI] [PubMed] [Google Scholar]