Abstract
We report three cases of eosinophilic cystitis. Contrast-enhanced computed tomography (CT) revealed characteristic bladder wall thickening exceeding 10 mm, with preservation of the mucosal lining and intense, progressive contrast enhancement on sequential arterial and delayed scans. Eosinophilic cystitis might have been associated with eosinophilic infiltration in other organs, such as the gastrointestinal tracts and liver.
Eosinophilic cystitis is a relatively rare form of bladder inflammation that affects both children and adults. Since Palubinskas [1] and Brown [2] first reported this condition independently in 1960, many additional cases in both adults and children have been described [3–5]. To our knowledge, however, eosinophilic cystitis that is associated with eosinophilic disease of the gastrointestinal tract is rare [1, 6]. We report three cases of eosinophilic cystitis associated with eosinophilic enterocolitis in whom no specific cause could be found and review the literature.
Case reports
Case one
A 81-year-old man was referred to our institution with three-week history of suprapubic tenderness, dysuria and abdominal pain with distension. Blood counts revealed an elevated white blood cell (WBC) count of 20,400 cells/mL and an elevated eosinophil count (11,700 cells/mL, 57.2%). The urine specimen was positive (+) for protein and contained two red cells and one white cell per high-power field. Stool examination was negative for parasites or ova. There was no history of allergy to food or medicine. On the second day of admission, ascitic fluid analysis obtained by paracentesis showed 10–19 WBC (differential count: polymorphonuclear leukocyte (PMN) cells 1%, mononuclear cells 11% and eosinophil count 88%). On the third day of admission, a peripheral blood smear revealed markedly elevated WBC and eosinophil counts, although morphological configuration was normal.
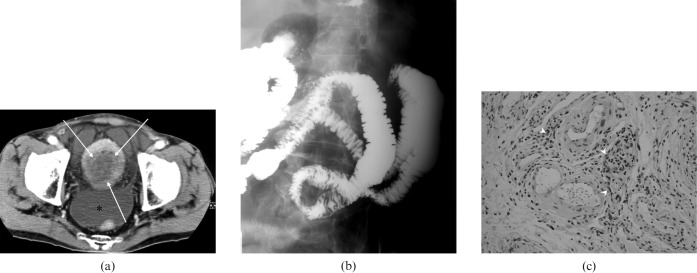
Contrast-enhanced computed tomography (CT) showed marked and diffuse bladder wall thickening with preservation of the mucosa (Figure 1a), and generalised oedema in the jejunum and ileum that was also seen in a small bowel meal (Figure 1b). Cystoscopy revealed diffuse erythema and inflammation with no evidence of a mass. A biopsy showed numerous eosinophilic infiltrations in the submucosa (Figure 1c).
Figure 1.
Case one: (a) Contrast-enhanced CT scan shows marked and diffuse bladder wall thickening with preservation of the mucosal lines (arrows); * = ascites. (b) Small bowel meal demonstrates thickening of the mucosal folds in the jejunum. (c) Cystoscopic biopsy in the urinary bladder revealed numerous eosinophilic infiltrations in the submucosa (arrowheads) (hematoxylin-eosin stain, ×20).
The patient was managed conservatively with antibiotics and steroid therapy. The symptoms subsided and the peripheral blood eosinophilia disappeared.
Case two
A 40-year-old man was admitted with intermittent diffuse abdominal pain for 7 days. There were no urinary symptoms. The routine blood counts revealed an elevated WBC count of 14,700 cells/mL and an elevated eosinophil count (2,820 cells/mL, 19.1%).
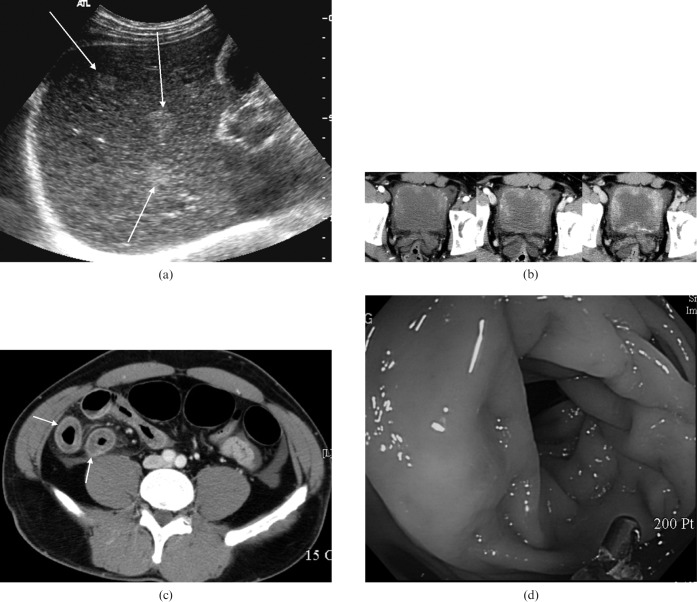
Urinalysis, urine culture and stool examination for parasites or ova were negative. On ultrasonography, the liver showed multiple ill-defined echogenic nodules (Figure 2a), and diffuse wall thickening of the distal ileum and urinary bladder was observed.
Figure 2.
Case two. (a) Axial ultrasonographic image of the liver reveals multiple ill-defined echogenic nodules (long arrows). (b) Contrast-enhanced CT in the arterial (left), portal (middle), and delayed (right) phases shows marked bladder wall thickening, which exceeded 10 mm, with progressive enhancement in the sequential time. (c) On contrast-enhanced CT scan, small intestine reveals uniform thickening with target sign (short arrows). (d) Colonoscopy shows oedema in the ascending colon.
Contrast-enhanced CT also revealed marked bladder wall thickening, which exceeded 10 mm, with progressive enhancement on the arterial, portal, and venous phase scans and diffuse wall thickening of the distal ileum (Figure 2b,c). Colonoscopy revealed oedema in the ascending colon (Figure 2d), and a colonoscopic biopsy indicated numerous infiltrating eosinophils in the mucosa and submucosa.
Cystoscopy revealed an oedematous, friable mucosa, and mucosal biopsies showed extensive submucosal eosinophilic infiltration.
The patient was started on intravenous antibiotics and steroid therapy, and the symptoms subsided. Ultrasonography carried out five days later showed that the hepatic nodules had disappeared, and that the wall thickening of the distal ileum and urinary bladder had improved markedly.
Case three
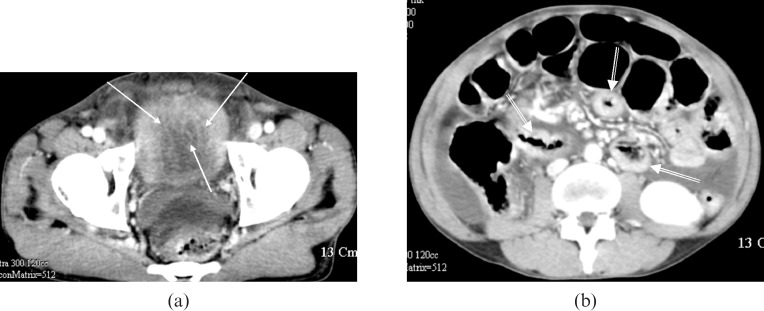
A 38-year-old man was referred to our institution with diffuse abdominal pain with distension and urinary frequency for 10 days. The routine laboratory data revealed an elevated WBC count of 11,100 cells/mL and elevated eosinophil count (960 cells/mL, 8.7%). The urine analysis was negative. Stool examination was negative for parasites or ova. There was no history of allergy to food or medicine. A contrast-enhanced CT scan showed diffuse, marked bladder wall thickening of up to 10 mm, and multifocal segmental thickening of the small intestine (Figure 3). Eosinophilic cystitis and eosinophilic colitis were confirmed by cystoscopic and colonoscopic biopsies.
Figure 3.
Case three. (a) A contrast-enhanced CT scan shows that the bladder wall thickening is diffuse and marked, exceeding 10 mm. Note the preservation of the mucosal line (arrows). Eosinophilic cystitis was confirmed by cystoscopic biopsy. (b) Contrast enhanced CT of the mid-abdomen demonstrates multifocal segmental thickening of the small intestine (arrows).
Discussion
Eosinophilic gastrointestinal disorder is associated with eosinophilic cystitis in 4.5% of cases [7]. All three of our patients had eosinophilic cystitis associated with eosinophilic gastrointestinal disorder. In Case two, hepatic nodules were seen on the initial ultrasound examination but had disappeared in the follow-up examination five days later. Although not confirmed by biopsy, we suspected that they were eosinophilic nodules, such as eosinophilic granulomas or eosinophilic abscesses. In reported cases [1, 6], the eosinophilic enteritis preceded or was concurrent with development of the bladder lesion. In our patients, the lesions of the urinary bladder and intestine, including the liver, were detected concurrently.
The cause of eosinophilic cystitis is unclear, but it is believed to be associated with injury; medications (e.g. methicillin, warfarin, anthranilic acid, intravesical mitomycin, and thiotepa); bacterial, viral, and infections; and reactions to food (e.g. vegetables, spices, and chocolate) and other allergens. Males are affected more commonly than females. Eosinophilic cystitis may present with dysuria, haematuria, proteinuria, urinary frequency, suprapubic pain and urinary retention. In cases of eosinophilic cystitis, physical examination may reveal suprapubic tenderness or a mass anterior to the rectum. Urinalysis may indicate proteinuria, microscopic haematuria, pyuria, and in rare cases, eosinophiluria. It is unusual for an adult to have a peripheral blood eosinophilia with eosinophilic cystitis alone, although the presence of peripheral blood eosinophilia is helpful diagnostically. It has been reported in 43% of cases [7] and is seen in approximately 50% of patients with a history of allergy or atopy. All of our patients had peripheral blood eosinophilia with no history of allergy or atopy. Eosinophiluria is rare in eosinophilic cystitis, as eosinophils are either degraded rapidly or little mucosal shedding from the urothelium occurs [8]. Ascites is sometimes noted [9]. In Cases one and two, a large amount of ascites was noted together with elevated eosinophil counts.
Radiographic studies may show variable thickening of the bladder wall, ranging from diffuse thickening to mass formation [10]. The pseudotumourous form of eosinophilic cystitis is characterized by an extensive infiltration of the bladder wall that may resemble an invasive neoplasm [11]. In our patients, contrast-enhanced CT showed diffuse thickening of the bladder wall exceeding 10 mm, with intense, progressive enhancement on sequential arterial, arterial, portal and delayed scans, and preservation of the mucosal line, which is a characteristic finding.
The diagnosis of eosinophilic cystitis is confirmed by cystoscopy with biopsy of the lesion. Histological examination in cases of eosinophilic cystitis usually demonstrated focal necrosis, fibrosis and the presence of inflammatory cells [12]. The lamina propria, and sometimes the lamina muscularis, are infiltrated with eosinophils and plasma cells together with other acute and chronic inflammatory cells. Eosinophilic infiltrations may be the final pathway by which endogenous and exogenous allergens cause IgE-mediated degranulation of mast cells and the release of eosinophilic chemotactic factors. Studies suggest that activated eosinophils release cystotoxic cationic proteins that can induce tissue damage [13].
Therapy for eosinophilic cystitis has not been standardised. Treatment begins with the removal of the antigenic stimulus, if present. Treatments have included the use of steroidal and nonsteroidal medications, anti-inflammatory drugs, antibiotics, antihistamines, cyclophosphamide and doxorubicin, as well as transurethral resection, hydrodilation of the bladder, radiation, intravesical silver nitrate irrigation, partial cystectomy, and cystectomy with urinary diversion.
Eosinophilic cystitis is a rare condition of unknown cause that mimics many other urological conditions. Eosinophilic cystitis can be overlooked clinically and microscopically, and a biopsy is essential to establish the diagnosis. However, eosinophilic cystitis should be considered when encountering a patient with bladder wall thickening exceeding 10 mm and with preserved mucosal lines on CT, with or without peripheral blood eosinophilia. The patient might also have associated diffuse or segmental bowel wall thickenings, ascites or hepatic nodules.
References
- 1.Palubinskas AJ. Eosinophilic cystitis: case report of eosinophilic infiltration of the urinary bladder. Radiology 1960;75:589–91 [DOI] [PubMed] [Google Scholar]
- 2.Brown EW. Eosinophilic granuloma of the bladder. J Urol 1960;83:665–8 [DOI] [PubMed] [Google Scholar]
- 3.Sutphin M, Middleton AW., Jr Eosinophilic cystitis in children: a self limited process. J Urol 1984;132:117–9 [DOI] [PubMed] [Google Scholar]
- 4.Nkposong EO, Attah EB. Eosinophilic cystitis. Eur Urol 1978;4:274–8 [DOI] [PubMed] [Google Scholar]
- 5.Harris NL, McNeely WF, Shepard J, Ebeling SH, Ellender SM, Peters CC. Case records of the Massachusetts General Hospital. Case 27-1998. A 10-year-old girl with urinary retention and a filling defect in the bladder. N Engl J Med 1998;27:616–22 [DOI] [PubMed] [Google Scholar]
- 6.Gregg JA, Utz DC. Eosinophilic cystitis associated with eosinophilic gastroenteritis. Mayo Clin Proc 1974;49:185–7 [PubMed] [Google Scholar]
- 7.Ouden DV. Diagnosis and management of eosinophilic cystitis: a pooled analysis of 135 cases. Eur Urol 2000;37:386–94 [DOI] [PubMed] [Google Scholar]
- 8.Itano NM, Malek RS. Eosinophilic cystitis in adults. J Urol 2001;165:805–7 [PubMed] [Google Scholar]
- 9.Peterson NE. Eosinophilic cystitis. Urol 1985;26:167–9 [DOI] [PubMed] [Google Scholar]
- 10.Leibovitch I, Heyman Z, Ben Chaim J, Goldwasser B. Ultrasonographic detection and control of eosinophilic cystitis. Abdom Imaging 1994;19:270–1 [DOI] [PubMed] [Google Scholar]
- 11.Barry KA, Jafri SZ. Eosinophilic cystitis: CT findings. Abdom Imaging 1994;19:272–3 [DOI] [PubMed] [Google Scholar]
- 12.Hellstrom HR, Davis BK, Shonnard JW. Eosinophilic cystitis: a study of 16 cases. Am J Clin Pathol 1979;72:777–84 [DOI] [PubMed] [Google Scholar]
- 13.Weller PF. The immunology of eosinophils. N Engl J Med 1991;324:1110–8 [DOI] [PubMed] [Google Scholar]