Abstract
Delay in diagnosis of pregnancy-associated breast cancer (PABC) is common, often attributed to the difficulty in evaluating tumours in the gravid breast, low awareness among physicians and reluctance of patients and physicians to perform imaging or invasive procedures during pregnancy. Familiarity with imaging features of PABC is crucial for prompt diagnosis. This article illustrates imaging findings of PABC and provides an approach for the evaluation of pregnant and lactating women with palpable abnormalities.
Pregnancy-associated breast cancer (PABC) is defined as cancer diagnosed during pregnancy or in the first 12 months post partum, or at any time while the patient is lactating [1]. PABC is a rare but serious occurrence with an incidence of approximately 0.3/1000 pregnancies [2]. As more and more women choose to delay childbearing until their 30s or 40s, there may be an increase in the incidence of PABC, as the proportion of pregnant women at an advanced age who are already at a higher risk of breast carcinoma increases. Around two-thirds of all PABC cases are diagnosed in the post-partum period, mostly in the first six months following delivery.
The diagnosis of PABC is often delayed and remains challenging because of underlying hormone-induced anatomical and physiological changes occurring in breast tissue. The lack of awareness of PABC may preclude timely imaging or biopsy, leading to larger and more advanced neoplasms at diagnosis compared with age-matched non-PABC cases [3].
The aim of this pictorial review is to increase awareness of PABC by illustrating its imaging spectrum. This article also emphasises the importance of mammographic and sonographic evaluation of pregnant and lactating women with palpable abnormalities.
Imaging approach in the pregnant or lactating women with a palpable abnormality
Patients with PABC almost always present with a palpable mass [4]. Breast sonography is commonly the initial imaging modality used for evaluating breast disorders in women during pregnancy and lactation [4]. Mammography and ultrasound are essential complementary modalities in imaging patients with palpable abnormalities. Immediate image-guided biopsy of suspicious or indeterminate breast masses is necessary for prompt and accurate diagnosis. Theoretically, fine-needle aspiration (FNA) during pregnancy may be associated with both increased false-positive and increased false-negative rates [2]. Ultrasound guided core biopsy is the accepted cost-effective standard procedure for assessing breast masses during pregnancy and lactation [4]. However, the decision to use core biopsy requires caution because of increased risk of bleeding, milk fistula formation and infection. These risks can be minimised by cessation of breastfeeding before core biopsy, paying close attention to haemostasis, and maintaining strict asepsis.
Imaging findings in women with PABC
Ultrasound
Ultrasound constitutes the most appropriate radiological method for evaluating breast disorders in women during pregnancy and lactation [4]. Breast sonography helps to determine whether the palpable area represents a true mass or normal parenchyma. The reported sensitivity of sonography in the diagnosis of PABC is 100% [5, 6]. Sonographic appearance can be useful in differentiating benign from malignant breast masses. In non-pregnant patients, sonographic Breast Imaging Reporting and Data System (BI-RADS) descriptors such as spiculated margin, irregular shape and non-parallel orientation has high predictive value for malignancy, whereas circumscribed margin, oval shape and parallel orientation are generally considered predictive of a benign diagnosis.
Physiological changes associated with the breast and lactation may alter typical sonographic features of breast carcinoma. Parallel orientation (Figure 1b) has been reported in 58% of PABC [5]. Posterior acoustic enhancement (Figures 2a and 3a) commonly seen in benign breast lesions has been reported in 63% of PABC cases [5].
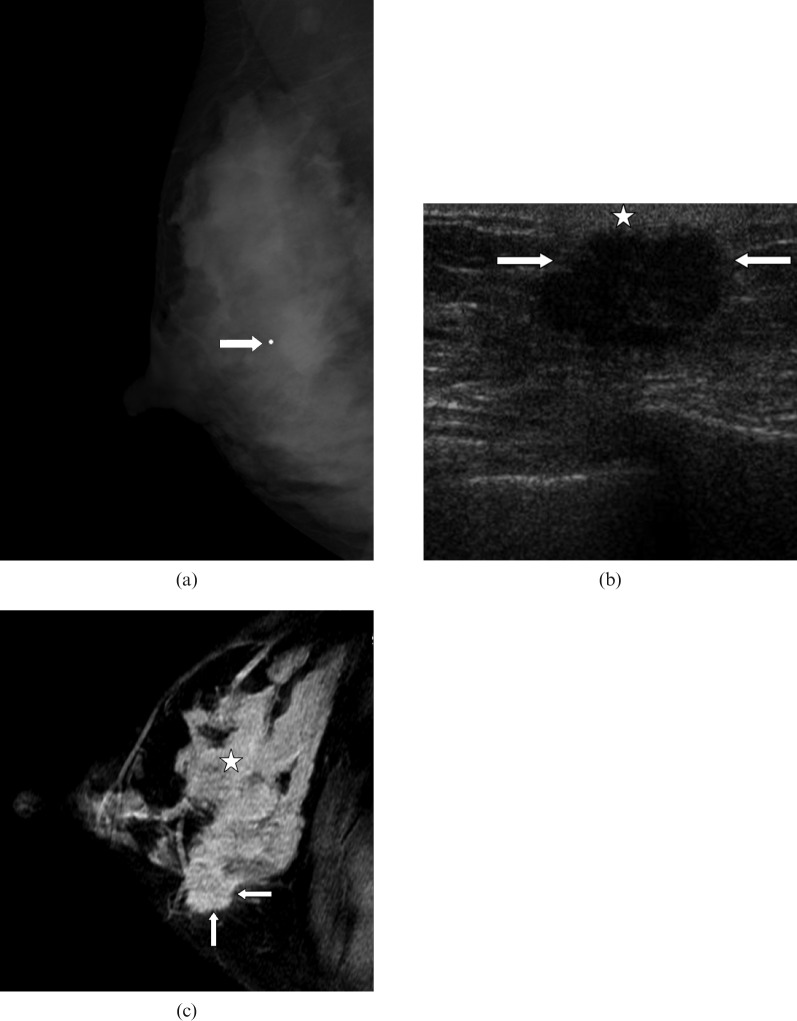
Figure 1.
40-year-old lactating woman, 6 months post partum, who presented with a palpable mass. Mastectomy revealed a 4 cm triple-negative high-grade invasive ductal carcinoma with extensive high-grade ductal carcinoma in situ (DCIS). The patient died 4 years after diagnosis. (a) Right mediolateral oblique mammogram shows a mass (arrow) with irregular margins. (b) Ultrasound image demonstrates a hypoechoic mass (arrows) with microlobulated margins, parallel orientation and adjacent skin thickening (star). (c) Post-contrast T1 weighted image shows strong enhancement of breast parenchyma (star) likely to hamper identification of breast carcinoma (arrows).
Figure 2.
39-year-old lactating woman, 2 months post partum, who presented with a clinical history of breast abscess. Ultrasound-guided biopsy revealed high-grade invasive ductal carcinoma and high-grade ductal carcinoma in situ (DCIS). The patient underwent mastectomy after neoadjuvant chemotherapy. The size of the largest focus of residual invasive carcinoma in the mastectomy specimen was 5 mm. (a) Sonography reveals hypoechoic solid mass (star) with angular margins and posterior acoustic enhancement (arrows). (b) Left mediolateral oblique mammogram was performed after placing a clip (curved arrow) before commencing neoadjuvant therapy. Carcinoma is mammographically occult. (c) Sagittal T1 weighted contrast-enhanced MRI shows heterogeneously enhancing mass (star) and susceptibility artefact (arrow) secondary to the clip. (d) Sagittal T1 weighted post-contrast MR image following completion of neoadjuvant therapy shows no residual mass.
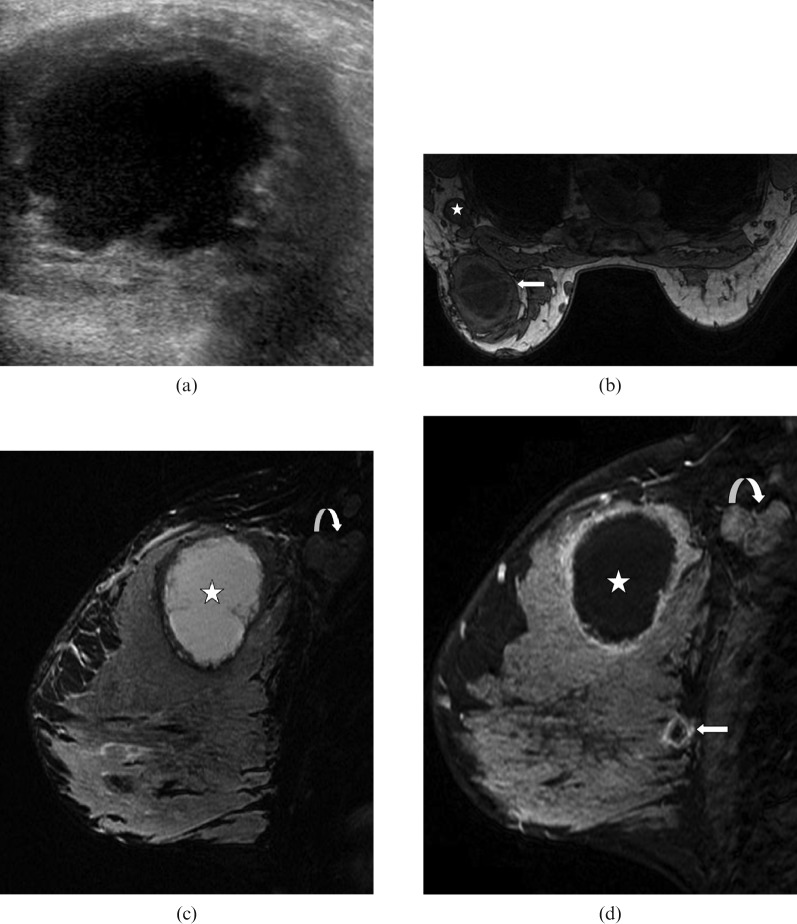
Figure 3.
35-year-old lactating woman, 4 months post partum, who presented with a palpable mass. Ultrasound-guided biopsy revealed oestrogen receptor-negative and human epidermal growth factor 2 (HER2)-negative invasive ductal carcinoma. The patient underwent mastectomy after neoadjuvant chemotherapy. Only microscopic residual disease was found in the mastectomy specimen. The patient died 1 year after diagnosis. (a) Ultrasound image shows complex cystic mass with a thick irregular wall. (b) Axial T1 weighted MRI image demonstrates large cystic mass with axillary lymphadenopathy (star). (c) Sagittal T2 weighted image shows a hyperintense mass with a thick irregular wall. (d) Post-contrast sagittal T1 weighted image shows otherwise unsupected small focus of malignancy (arrow) in addition to the index necrotic malignant mass (star) and axillary lymphadenopathy (curved arrow).
PABCs are biologically aggressive neoplasms that may outgrow their vascular supply, leading to necrosis or cystic degeneration (Figure 3a). Therefore, new palpable complex cystic masses identified during pregnancy and lactation warrant tissue sampling and should not be disregarded as a galactocele or an abscess unless these are clinically obvious.
Mammography
Bilateral mammography is recommended in pregnant and lactating women with clinical abnormalities. Most authorities believe that with the proper abdominal shielding two-view mammography poses very little risk of radiation exposure to the fetus, as the radiation dose to the foetus is negligible [4]. There is no concern about radiation dose in performing mammography on lactating women.
The mammographic features of PABC do not differ from those of non-PABC and include masses, microcalcifications, asymmetrical density, architectural distortion and skin thickening (Figures 1a and 4a). The sensitivity of mammography is that the diagnosis of PABC has been reported to be 78–90% despite dense breast parenchyma [5, 6]. Although a mass may not be discernible by mammography because of increased density of breast tissue, demonstration of microcalcifications, asymmetrical density, axillary lymphadenopathy or skin thickening might help in diagnosing PABC.
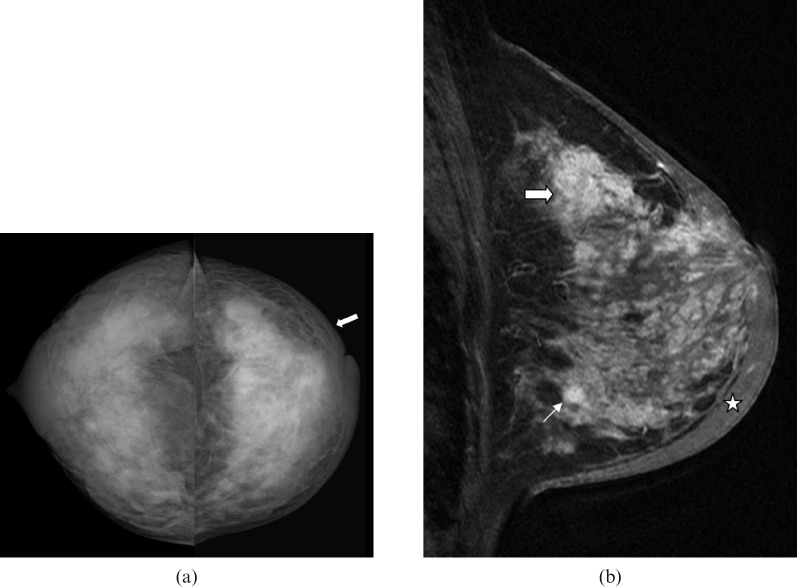
Figure 4.
40-year-old lactating woman, 9 months post partum, who presented with an enlarged, firm breast, inverted nipple and peau d'orange. Percutaneous ultrasound-guided biopsy revealed invasive ductal carcinoma and ductal carcinoma in situ (DCIS). Pre-operative imaging revealed bone and liver metastases. (a) Bilateral craniocaudal mammogram reveals extremely dense breast tissue. There is diffuse skin thickening (arrow) in the left breast. (b) Sagittal T1 weighted contrast-enhanced MRI demonstrates multiple masses and diffuse breast enhancement along with pronounced skin thickening (star) – findings are consistent with inflammatory breast carcinoma.
MRI
The routine use of contrast-enhanced MRI in the evaluation of pregnant patients is not appropriate and is recommended only in situations where the risk–benefit ratio is clear. Contrast-enhanced MRI can be safely performed during lactation. There is a paucity of data on MRI features of PABC. Espinosa et al [7] reported MRI findings of five cases of breast carcinoma in lactating women. The MRI features of cancer in lactating breast tissue are similar to those of non-PABC cases and include homogeneously and heterogeneously enhancing masses (Figure 2c), mass with rim enhancement (Figure 3d), non-mass segmental enhancement and diffuse unilateral enhancement.
Unlike non-lactating breast tissue (Figure 5a), lactating parenchyma (Figure 5b) shows rapid enhancement following intravenous administration of contrast material, followed by an early plateau of enhancement, a feature attributed to increased vascular permeability similar to that seen in invasive malignancy [7, 8]. Rapid enhancement of lactating parenchyma may hamper MRI detection of malignancy (Figure 1c). Usually PABCs demonstrate a faster and greater degree of enhancement than lactating tissue [7, 8]. MRI may be more reliable in depicting disease extent and multicentricity (Figures 2c, 3d and 4b).
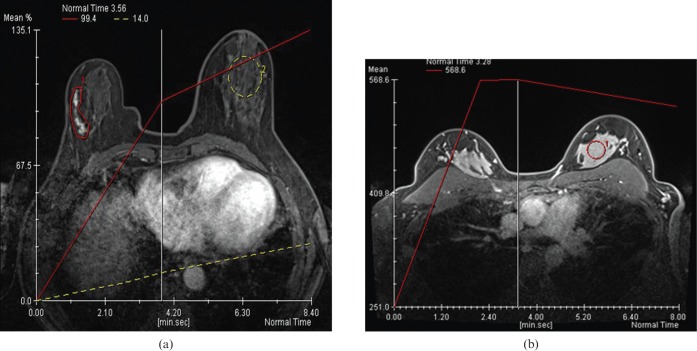
Figure 5.
(a) Computer-generated kinetic curve (yellow dotted line) demonstrates minimal progressive enhancement of benign parenchyma in the left breast in a non-pregnant, non-lactating woman. The patient was diagnosed with progressively enhancing right breast ductal carcinoma in situ (red area of segmental enhancement with corresponding red line). (b) Computer-generated kinetic curve (red line) shows rapid enhancement of lactating breast parenchyma.
Conclusion
The incidence of PABC may increase in the future as more women postpone childbearing to middle age. Ultrasound is the initial imaging of choice for the assessment of palpable abnormality in pregnant and lactating women. Palpable solid and complex cystic masses identified during pregnancy and lactation warrant biopsy. Mammography and ultrasound are essential complementary tests in women with palpable abnormalities and suspected PABC, as mammography detects otherwise occult malignant microcalcifications. If a focal lesion is not detected with mammography or sonography, a palpable suspicious mass should be biopsied.
References
- 1.Keinan-Boker L, Lerner-Geva L, Kaufman B, Meirow D. Pregnancy-associated breast cancer. Isr Med Assoc J 2008;10:722–7 [PubMed] [Google Scholar]
- 2.Keleher AJ, Theriault RL, Gwyn KM, Hunt KK, Stelling CB, Singletary SE, et al. Multidisciplinary management of breast cancer concurrent with pregnancy. J Am Coll Surg 2002;194:54–64 [DOI] [PubMed] [Google Scholar]
- 3.Beadle BM, Woodward WA, Middleton LP, Tereffe W, Strom EA, Litton JK, et al. The impact of pregnancy on breast cancer outcomes in women </ = 35 years. Cancer 2009;115:1174–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabate JM, Clotet M, Torrubia S, Gomez A, Guerrero R, de lasHeras P, et al. Radiological evaluation of breast disorders related to pregnancy and lactation. Radiographics 2007;27:S101–24 [DOI] [PubMed] [Google Scholar]
- 5.Ahn BY, Kim HH, Moon WK, Pisano ED, Kim HS, Cha ES, et al. Pregnancy- and lactation-associated breast cancer: mammographic and sonographic findings. J Ultrasound Med 2003;22:491–7 [DOI] [PubMed] [Google Scholar]
- 6.Liberman L, Gliess CS, Dershaw DD, Deutch BM, Petrek JA. Imaging of pregnancy-associated breast cancer. Radiology 1994;191:245–8 [DOI] [PubMed] [Google Scholar]
- 7.Espinosa LA, Daniel BL, Vidarsson L, Zakhour M, Ikeda DM, Herfkens RJ. The lactating breast: contrast-enhanced MR imaging of normal tissue and cancer. Radiology 2005;237:429–36 [DOI] [PubMed] [Google Scholar]
- 8.Talele AC, Slanetz PJ, Edmister WB, Yeh ED, Kopans DB. The lactating breast: MRI findings and literature review. Breast J 2003;9:237–40 [DOI] [PubMed] [Google Scholar]