Abstract
Human herpesvirus 6 (HHV-6)-associated encephalitis or pneumonitis has been reported in immunocompetent and immunosuppressed individuals. Several MRI studies in patients with HHV-6-associated encephalitis have been presented. However, to the best of our knowledge, no studies describing thin-section CT imaging in patients with HHV-6-associated pneumonitis have been reported. Here we describe a case of HHV-6-associated encephalitis and pneumonitis that developed after bone marrow transplantation. Thin-section CT images of the chest revealed ground-glass attenuation, consolidation and centrilobular nodules in both lungs.
Human herpesvirus 6 (HHV-6) is one of the most prevalent human viruses, infecting over 80% of the world’s population. Primary infection usually occurs before 2 years of age, leaving the host with a lifelong infection. When the host’s defences are compromised HHV-6 can reactivate, like other herpes viruses [1]. Recently, HHV-6 has been associated with many clinical syndromes, including hepatitis, mononucleosis, chronic fatigue, bone marrow transplant failure, renal transplantation rejection and pneumonitis [1].
Immunocompromised patients are at increased risk of developing infections, such as Pneumocystis jirovecii pneumonia or cytomegalovirus infection, and several studies have reported characteristic features using CT and MR imaging [2–6].
HHV-6 has increasingly been recognised as an opportunistic and potentially life-threatening pathogen in patients who have received liver transplants or allogeneic stem cell transplantation [7]. Several cases of HHV-6-associated pneumonitis in immunocompetent and immunosuppressed individuals, as well as in bone marrow transplantation patients, have been reported [7, 8]. However, to the best of our knowledge, no radiological studies with CT findings have been reported.
We present the radiological features in a case with HHV-6-associated pneumonitis and encephalitis after bone marrow transplantation.
Case report
A 45-year-old man was admitted with general fatigue and had increased numbers of white blood cells (14 000 cells μl–1) as determined by blood examination. After further examinations, including bone marrow biopsies, the patient was diagnosed with acute lymphoblastic leukaemia. Although he responded completely to chemotherapy, the patient relapsed approximately 1 year after the initial treatment. He received an allogeneic marrow transplant from a human leukocyte antigen (HLA)-matched related donor (his brother). At 17 days after bone marrow transplantation, the patient experienced respiratory dysfunction and lost consciousness; thin-section CT examination of the chest was performed volumetrically using a multidetector CT system with one reconstruction from the apex of the lung to the diaphragm.
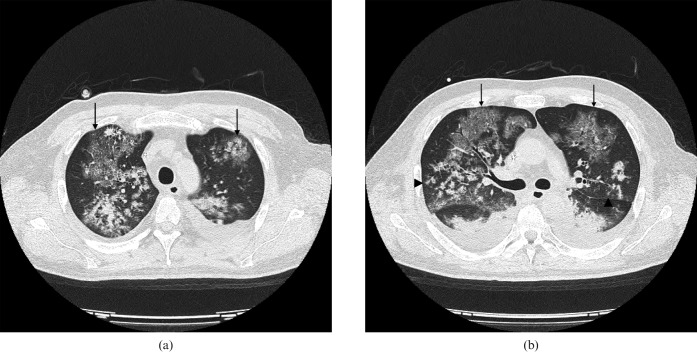
The thin-section chest CT images showed ground-glass attenuation, consolidation, interlobular reticular opacity and centrilobular nodules in both lungs, with peripheral lung sparing (Figure 1). Bilateral pleural effusions were also found. Mediastinal and hilar lymph node enlargement were not observed (not shown).
Figure 1.
Transverse thin-section CT images (1 mm thick sections) obtained at the level of (a) the upper lobes and (b) 1 cm below the division of the tracheal carina. The images show ground-glass attenuation and consolidation with interlobular reticular opacity (arrows) and centrilobular nodules with a tree-in-bud appearance (arrowheads) in both lungs with peripheral lung sparing. Bilateral pleural effusions were also observed.
On the same day, high copy numbers of HHV-6 DNA were detected in plasma specimens and in bronchoalveolar lavage fluid (BALF) (Table 1). Cytomegalovirus DNA, Aspergillus antigen, respiratory syncytial virus, adenovirus DNA, parainfluenza virus DNA and pneumocystis DNA were all negative. Moreover, no other viral DNAs were detected in cultures of plasma, BALF or sputum. Therefore, the patient was diagnosed with acute pneumonitis associated with HHV-6 infection. Treatment with ganciclovir and foscarnet was initiated and respiratory dysfunction and the bilateral infiltrations improved on the follow-up chest radiograph performed 4 days after treatment.
Table 1. Summarised results of virological studies.
| Type of material | Agent | Detection of nucleic acids (PCR) |
| Plasma | HHV-6 DNA | 138 673 copies ml–1 |
| Cytomegalovirus DNA | Negative | |
| Aspergillus antigen | Negative | |
| BALF | HHV-6 DNA | 6 840 990 copies ml–1 |
| Respiratory syncytial virus DNA | Negative | |
| Adenovirus DNA | Negative | |
| Parainfluenza virus DNA | Negative | |
| Culture | Negative | |
| Sputum | Pneumocystis DNA | Negative |
| Culture | Negative | |
| CSF | HHV-6 DNA | 18 215 copies ml–1 |
| Human simplex virus DNA | Negative | |
| Varicella–Zoster virus DNA | Negative |
BALF, bronchoalveolar lavage fluid; CSF, cerebrospinal fluid; HHV-6, human herpesvirus 6; PCR, polymerase chain reaction.
As the loss of consciousness continued despite treatment, a brain MRI was performed 24 days after the bone marrow transplant. The T2 weighted images (not shown), fluid-attenuated inversion recovery (FLAIR) images (not shown) and diffusion weighted images (DWI) (Figure 2) showed symmetrical regions of high signal intensity in the lateral lobes and the insular cortex. In addition, high copy numbers of HHV-6 DNA were detected in the cerebrospinal fluid, which was negative for other viral DNAs such as herpes simplex virus or varicella-zoster virus DNA. Therefore, the patient was diagnosed with encephalitis associated with HHV-6.
Figure 2.
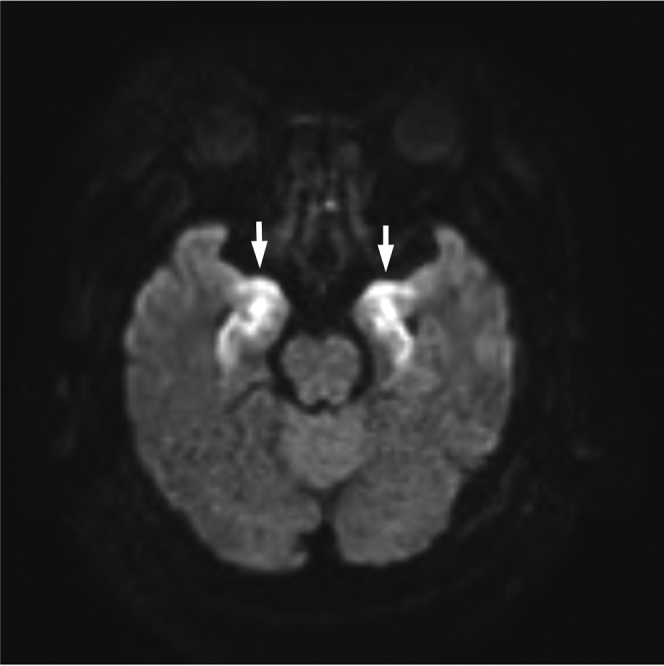
Diffusion weighted images showed symmetrical regions of high signal intensity in the lateral lobes and insular cortex (arrows).
Despite adequate antibiotic and antiviral therapy and intensive management, the patient died of multiple organ failure 47 days after bone marrow transplantation.
Discussion
HHV-6 might be one of the most important causes of pulmonary dysfunction in compromised hosts, such as bone marrow transplant recipients. Ogata et al [7] determined the incidence and clinical relevance of active HHV-6 infection among 50 allogeneic stem cell transplant recipients [7]. In their study, HHV-6 DNA was most frequently observed 14–27 days post transplantation; an increased risk of a positive finding of HHV-6 DNA was associated with transplantation from an allelic-mismatched donor. In addition, these authors reported that steroid therapy is associated with an increased incidence of infection and accelerated viral replication [7]. Although our case received an allogeneic bone marrow transplant from a related HLA-matched donor, the steroid medication might have triggered his pulmonary dysfunction in agreement with the findings of Ogata et al.
There are very few previous reports of HHV-6 infection after bone marrow transplantation with radiological findings.
Carrigan et al [8] reported two cases of severe interstitial pneumonitis associated with HHV-6 infection in bone marrow transplant recipients. The radiological findings were described only as progressive bilateral interstitial infiltrates on chest radiographs. Meanwhile, Merk et al [9] reported fatal pulmonary failure in a healthy 19-year-old woman. The chest radiographs showed developing pulmonary infiltration at the basal region of both lungs, with large amounts of pulmonary effusions; however, no previous reports have published CT images.
In our case, transverse thin-section CT images showed bilateral ground-glass attenuation and consolidation with peripheral lung sparing and interlobular reticular opacity. These CT findings are similar to those seen in patients with Pneumocystis jirovecii pneumonia [2, 3]. In addition, our CT images revealed centrilobular nodules in both lungs and bilateral pleural effusions, which are similar to findings in patients with cytomegalovirus pneumonia [4, 5]. Therefore, we initially diagnosed this case as concurrent Pneumocystis jirovecii pneumonia with cytomegalovirus. However, no pathogens other than HHV-6 were found in plasma specimens or in BALF. In our case, lung biopsy was not performed; therefore, a correlation between the CT findings and pathological findings could not be determined.
In 1993, Cone et al [10] reported that bone marrow transplant recipients with high levels of HHV-6 DNA in lung tissue tended to have idiopathic pneumonitis, a favourable outcome, severe graft vs host disease and changes in HHV-6 antibody titres, suggesting an association between HHV-6 infection and idiopathic pneumonitis. However, it would be difficult to differentiate HHV-6-pneumonitis from other opportunistic infections. Therefore, to better understand the characteristics of HHV-6 infection and the radiological findings, further studies with larger patient groups are needed. The final diagnosis is made by bacteriological and virological analysis using the plasma and BALF materials of patients.
In the present case, HHV-6 encephalitis developed 24 days after bone marrow transplantation, which was diagnosed by serological studies. The T2 weighted images, FLAIR images and DWI (b factor = 1000 s mm–2) showed symmetrical regions with high signal intensity in the lateral lobes and insular cortex. Provenzale et al [6] reported that the presence of MR signal intensity abnormalities in the medial temporal lobe should be recognised as HHV-6 encephalitis in immunosuppressed patients, especially when hyperintense lesions on FLAIR images and DWI are seen in the insular region and inferior frontal lobe. The MR images in our case were similar to those in the previous report.
In summary, we have presented a case of pneumonitis and encephalitis associated with HHV-6 infection after bone marrow transplantation with thin-section CT findings. In patients with immunosuppression after bone marrow transplantation who have acute respiratory symptoms, such as fever or cough, together with abnormal thin-section CT findings, HHV-6 pneumonitis should be considered in differential diagnosis.
References
- 1.Cone RW. Human herpesvirus 6 as a possible cause of pneumonia. Semin Respir Infect 1995;10:254–8 [PubMed] [Google Scholar]
- 2.Moskovic E, Miller R, Pearson M. High resolution computed tomography of Pneumocystis carinii pneumonia in AIDS. Clin Radiol 1990;42:239–43 [DOI] [PubMed] [Google Scholar]
- 3.Kuhlman JE, Kavuru M, Fishman EK, Siegelman SS. Pneumocystis carinii, pneumonia: spectrum of parenchymal CT findings. Radiology 1990;175:711–14 [DOI] [PubMed] [Google Scholar]
- 4.McGuinness G, Scholes JV, Garay SM, Leitman BS, McCauley DI, Naidich DP. Cytomegalovirus pneumonitis: spectrum of parenchymal CT findings with pathologic correlation in 21 AIDS patients. Radiology 1994;192:451–9 [DOI] [PubMed] [Google Scholar]
- 5.Vogel MN, Brodoefel H, Hierl T, Beck R, Bethge WA, Claussen CD, et al. Differences and similarities of cytomegalovirus and pneumocystis pneumonia in HIV-negative immunocompromised patients-thin section CT morphology in the early phase of the disease. Br J Radiol 2007;80:516–23 [DOI] [PubMed] [Google Scholar]
- 6.Provenzale JM, vanLandingham KE, Lewis DV, Mukundan S, Jr, White LE. Extrahippocampal involvement in human herpesvirus 6 encephalitis depicted at MR imaging. Radiology 2008;249:955–63 [DOI] [PubMed] [Google Scholar]
- 7.Ogata M, Kikuchi H, Satou T, Kawano R, Ikewaki J, Kohno K, et al. Human herpes virus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis 2006;193:68–79 [DOI] [PubMed] [Google Scholar]
- 8.Carrigan DR, Drobyski WR, Russler SK, Tapper MA, Knox KK, Ash RC. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet 1991;338:147–9 [DOI] [PubMed] [Google Scholar]
- 9.Merk J, Schmid FX, Fleck M, Schwarz S, Lehane C, Boehm S, et al. Fatal pulmonary failure attributable to viral pneumonia with human herpes virus 6 (HHV6) in a young immunocompetent woman. J Intensive Care Med 2005;20:302–6 [DOI] [PubMed] [Google Scholar]
- 10.Cone RW, Hackman RC, Huang ML, Bowden RA, Meyers JD, Metcalf M, et al. Human herpesvirus 6 in lung tissue from patients with pneumonitis after bone marrow transplantation. N Engl J Med 1993;329:156–61 [DOI] [PubMed] [Google Scholar]