Abstract
Objective
The aim of this study was to evaluate tumour vascularity and Kupffer cell imaging in hepatocellular carcinoma (HCC) using contrast-enhanced ultrasonography (CEUS) with Sonazoid (perfluorobutane) and to compare performance with dynamic CT.
Methods
We studied 118 nodules in 88 patients with HCC. HCC was diagnosed as a hyperenhancement lesion in the arterial phase with washout in the portal phase on dynamic CT or by percutaneous biopsy. We observed tumour vascularity at the early vascular phase (10–30 s after contrast injection) and Kupffer imaging at the post-vascular phase (after 10 min).
Results
Detection of vascularity at the early vascular phase was 88% in nodules that were found to be hypervascular on dynamic CT and 28% in hypo-/isovascular nodules; the detection of local recurrence nodules was 92%. The detection of vascularity was significantly lower in nodules >9 cm deep than in those ≤9 cm deep, but was not affected by tumour size. The detection of tumours at the post-vascular phase on CEUS was 83% in nodules with low density in the portal phase on dynamic CT and 82% in nodules with isodensity. The rate did not depend on the severity of underlying liver disease; rates decreased in nodules deeper than 9 cm, those smaller than 2 cm in diameter and in iso-enhancing nodules at the early vascular phase of CEUS.
Conclusion
CEUS with Sonazoid is a useful tool for assessing the vascularity of HCC and is equal to that of dynamic CT; however, the detectability of HCC vascularity is affected by location.
The development of imaging modalities has facilitated the detection and accurate diagnosis of hepatocellular carcinoma (HCC). Assessment of tumour vascularity and for the presence of Kupffer cells are important in differential diagnosis, the choice of treatment and for assessment of the therapeutic response. HCC tumour vascularity has been evaluated extensively using various imaging modalities, including colour or power Doppler ultrasonography [1,2], angiography, dynamic CT [3], CT during angiography [4,5] and MRI [3]. Dynamic helical CT is minimally invasive and provides information regarding arterial or portal supplies by scanning at different time intervals following an injection of contrast agent. Therefore, dynamic CT is the standard modality used in clinical assessment of tumour vascularity. Assessment of Kupffer cells is possible using superparamagnetic iron oxide (SPIO)-enhanced MRI [6,7]. The presence of Kupffer cells indicates normal or benign liver tissue, whereas the absence of Kupffer cells indicates non-liver tissue such as malignant neoplasms. Thus, evaluation of the presence of Kupffer cells is useful in the differential diagnosis of focal liver lesions.
Microbubble contrast agents are available for clinical use with ultrasound. Levovist (Schering AG, Berlin, Germany) is a first-generation contrast agent widely used to characterise focal liver lesions [8-12]. The advent of Sonazoid, a second-generation contrast agent (perfluorobutane; Diichi Sankyo, Tokyo, Japan), enables low mechanical index continuous real-time imaging and Kupffer imaging [13-15]. Therefore, contrast-enhanced ultrasound (CEUS) using Sonazoid could potentially offer high-quality, detailed vascular information and clearer Kupffer imaging. The aim of the present study was to compare CEUS using Sonazoid with dynamic CT in the assessment and characterisation of HCC.
Methods and materials
Patients
Between January 2007 and February 2008, 88 consecutive patients (57 men and 31 women; mean age 71 years) with HCC detected on screening ultrasound (92 newly developed tumours and 26 local recurrences) were enrolled in this study (Table 1). Of these, 21 patients had hepatitis B surface antigen, 55 had hepatitis C antibody and 1 patient had both. The remaining 11 patients were negative for both hepatitis B surface antigen and hepatitis C antibody. Diagnosis of HCC was based on histological findings from fine-needle (21 gauge (G)) aspiration biopsy in 42 nodules; diagnosis in the remaining tumours was based on imaging findings and tumour markers (the elevation of serum α-fetoprotein, normal range <20 ng ml–1, or serum des-γ-carboxy prothrombin, normal range <40 mAU ml–1). We defined HCC as a hyperenhancing lesion on the arterial phase and indicating washout on the portal phase in at least two imaging modalities (e.g. CT, MRI, CT under angiography). If a lesion did not have enhancement, diagnosis was obtained by liver biopsy. This study conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The nature of the study was fully explained to the patients and informed consent was obtained.
Table 1. Patient characteristics.
| Age (years) | 71±10 |
| Male:female ratio | 57:31 |
| Aetiology of liver disease | |
| Hepatitis B (n) | 21 |
| Hepatitis C (n) | 55 |
| Hepatitis B + C (n) | 1 |
| Alcoholics (n) | 2 |
| Cryptogenic (n) | 9 |
| Underlying liver disease | |
| Chronic hepatitis (n) | 16 |
| Cirrhosis (Child–Pugh Grade A/B/C) (n) | 72 (44/26/2) |
| No. of tumours | 118 |
| Tumour diameter (mm) | 20±17 |
Ultrasound examination
Ultrasound examinations were performed by two ultrasound experts (MK and MM who had 25 and 12 years of experience, respectively). Baseline B-mode ultrasound was performed using a 3–5 MHz curved array wide-band transducer before CEUS. Sonazoid contrast agent (perfluorobutane, microbubbles covered by a phosphatidyl serin natrium) was prepared by shaking with 2 ml of water. Approximately 0.5 ml of contrast agent suspension (0.0075 ml kg–1) was injected manually through a 21-G cannula inserted into the antecubital vein followed by an additional 2 ml physiological saline flush. CEUS was performed (Aplio XV and XG; Toshiba Medical System Corporation, Otawara, Japan) using a 3–5 MHz curved array wide-band transducer, which transmitted an ultrasound beam at 1.75 MHz and received 3.5 MHz in harmonic B-mode. The acoustic power of the harmonic ultrasound was set with a mechanical index (MI) of 0.2–0.3. We defined the vascular phases as follows: early vascular phase, 10–30 s after contrast injection; late vascular phase, 30–60 s after contrast injection; post-vascular phase, 10 min or more after contrast injection. We displayed both the tumour and surrounding hepatic parenchyma before injecting the contrast agent. After injection, the patient held his/her breath while vascular-phase ultrasound imaging was obtained in real time by slowly changing the scanning plane to observe the whole area of the nodule. Ultrasound images were obtained at the post-vascular phase in real time 10 min after injection. If the nodule could not be detected on baseline B-mode ultrasound before injection, we observed the post-vascular phase first and then observed the vascular phase after re-injection of Sonazoid (0.3 ml). There were no complications during or after the procedure. All ultrasound imaging data were stored digitally for subsequent analyses.
CT examination
Helical multiphasic CT examinations were performed with a 64-channel multidetector scanner (Aquilion 16; Toshiba Medical Systems, Tochigi, Japan) using a tube voltage of 120 kV, a tube current in the automatic milliampere exposure setting, a reconstruction section and interval thickness of 5 mm, a detector configuration of 32 × 1 mm, a pitch of 27 and a gantry speed of 0.5 s per rotation. Unenhanced CT images were acquired first and were followed by triple-phase contrast enhancement during the power injection of 100 ml of iopamidol (Iopamiron; Nihon-Schering, Osaka, Japan) at a rate of 2.7 ml s–1. The entire liver was scanned three times. Early arterial phase imaging was initiated at 10 s, late arterial phase imaging at 20 s and portal venous phase imaging at 120 s after initiation of the injection.
Tumour vascularity on CT was classified as “high density” or “low density” in the case of higher or lower density than that of surrounding liver parenchyma. “Isodensity” was classified as a tumour having the same density as that of the surrounding liver parenchyma.
Image analysis
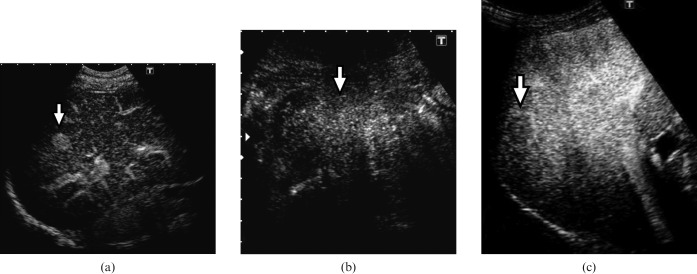
Vascular findings at the early vascular phase of CEUS were classified into three patterns depending on tumour vascularity relative to the surrounding liver parenchyma: hyperenhancement, iso-enhancement or hypo-enhancement (Figure 1). Hyperenhancement was defined as echogenicity of a higher degree than that of the surrounding liver parenchyma. Iso- or hypo-enhancement were defined as echogenicity of the same degree or of a lower degree, respectively, than that of the surrounding liver parenchyma. If different levels of enhancement were present in the early vascular phase, the greatest enhancement of the lesion was considered.
Figure 1.
Vascular findings at the early vascular phase of contrast-enhanced ultrasound. (a) Hyperenhancement (arrow), (b) iso-enhancement (arrow) and (c) hypo-enhancement (arrow).
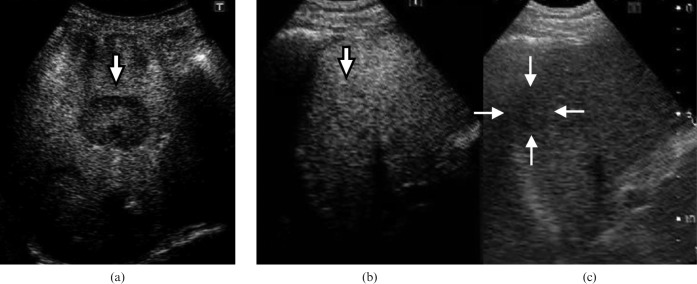
Findings of the post-vascular phase of CEUS were classified into two groups: iso-enhancement or hypo-enhancement relative to the surrounding liver parenchyma (Figure 2). Iso-enhancement was defined as echogenicity of the same degree as that of surrounding liver parenchyma; hypo-enhancement was defined as a lower degree of echogenicity than that of surrounding liver parenchyma.
Figure 2.
Kupffer images at the post-vascular phase on contrast-enhanced ultrasound. (a) Hypo-enhancement (arrow), (b) iso-enhancement (arrow) and (c) tumour (arrows) shown in monitor mode (ultrasound beam at 4.0 MHz and mechanical index of 0.1) in the same plane as image (b).
Statistical analysis
A χ2 or Fisher's exact test was used to compare differences in diagnostic performance among tumour size, between lesions located at a depth ≤9 cm and those at a depth >9 cm, between chronic hepatitis and cirrhosis and between hyperenhancement and iso-enhancement at the early vascular phase of CEUS. A p-value <0.05 was considered to be statistically significant. All statistical analyses were performed using StatView version 5.0 (SAS Institute Inc., Cary, NC).
Results
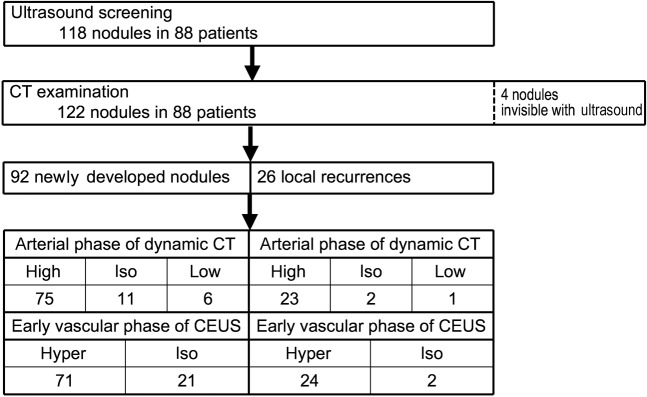
In 88 patients, 118 nodules were detected by ultrasound screening and 122 nodules were detected by dynamic CT (Figure 3). Four nodules (3.2%) could not be identified by baseline B-mode or any phases of CEUS. Among these four nodules, two were located in the subphrenic region; the remaining two nodules could not be visualised owing to gastrointestinal gas. Excluding these 4 nodules, 118 nodules were analysed. Of these, 92 nodules in 67 patients were newly developed HCC; the remaining 26 nodules in 21 patients were local recurrences from HCC treated by local ablation therapy (19 nodules treated by percutaneous ethanol injection, 7 nodules treated by radiofrequency ablation).
Figure 3.
Flow diagram of patient selection and imaging findings. CEUS, contrast enhanced ultrasound.
Among the 92 nodules in 67 patients with newly developed HCC, 75 nodules demonstrated high density at the arterial phase of dynamic CT. Hyperenhancement at the early vascular phase of CEUS was demonstrated in 66 (88%) of these 75 nodules (Figure 4), while iso-enhancement was demonstrated in 9 (12%) (Table 2). 3 (27%) of 11 nodules with isodensity at the arterial phase of dynamic CT demonstrated hyperenhancement at the early vascular phase of CEUS. Two (33%) of six nodules with low density at the arterial phase of dynamic CT demonstrated hyperenhancement at the early vascular phase of CEUS. When dynamic CT was used as a gold standard and the four nodules not detected by ultrasound were included, the sensitivity of CEUS with Sonazoid in detecting vascularity was 84% and the specificity was 71%.
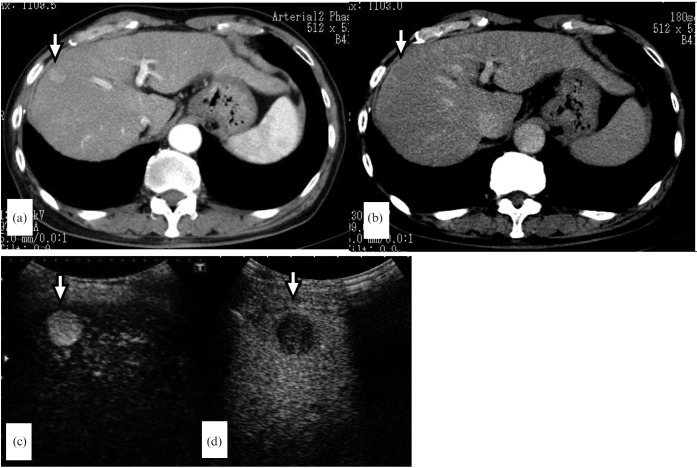
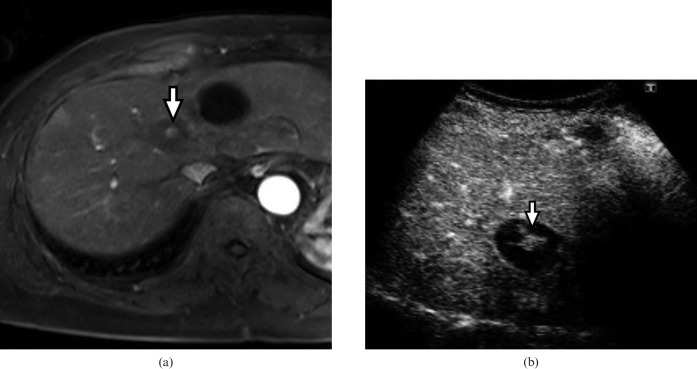
Figure 4.
A 69-year-old man with a 1.6 cm hepatocellular carcinoma in liver segment V. (a) The arterial phase of dynamic CT reveals a hypervascular tumour (arrow). (b) The portal phase of dynamic CT shows washout (arrow). (c) Contrast-enhanced ultrasound at the early vascular phase clearly shows hyperenhancement (arrow). (d) Contrast-enhanced ultrasound at the post-vascular phase shows hypo-enhancement (arrow).
Table 2. Comparison between the early vascular phase of contrast-enhanced ultrasound and the arterial phase of dynamic CT in the evaluation of vascularity.
| Early vascular phase of CEUS |
||||||
| Hyper | Iso | Total | ||||
| Arterial phase of dynamic CT | High density | 66 | (88%) | 9 | (12%) | 75 |
| Isodensity | 3 | (27%) | 8 | (73%) | 11 | |
| Low density | 2 | (33%) | 4 | (67%) | 6 | |
| Total | 71 | (77%) | 21 | (23%) | 92 | |
Hyper, hyperenhancement; iso, iso-enhancement; CEUS, contrast-enhanced ultrasound.
In the 24 nodules that were local recurrences with high density at the arterial phase of dynamic CT, 23 (96%) demonstrated hyperenhancement at the early vascular phase of CEUS (Table 3) (Figure 5). Two nodules with isodensity also demonstrated hyperenhancement at the early vascular phase of CEUS.
Table 3. Comparison between the early vascular phase of contrast-enhanced ultrasound and the arterial phase of dynamic CT in the evaluation of vascularity in local recurrence tumours.
| Early vascular phase of CEUS |
||||||
| Hyper | Iso | Total | ||||
| Arterial phase of dynamic CT | High density | 22 | (96%) | 1 | (4%) | 23 |
| Isodensity | 2 | (100%) | 0 | 2 | ||
| Low density | 0 | 1 | (100%) | 1 | ||
| Total | 24 | (92%) | 2 | (8%) | 26 | |
Hyper, hyperenhancement; iso, iso-enhancement; CEUS, contrast-enhanced ultrasound.
Figure 5.
A 75-year-old woman with a 1.0 cm local recurrence in liver segment IV after percutaneous ethanol injection. (a) Axial gadolinium-enhanced three-dimensional fat-saturated T1 weighted spoiled gradient-recalled echo image during the arterial phase reveals a hypervascular lesion (arrow) in the avascular tumour. (b) Contrast-enhanced ultrasound at the early vascular phase clearly shows a hyperenhancing lesion (arrow) within the hypo-enhancing tumour.
Tables 4 and 5 show the detection rates of vascularity by CEUS according to tumour size and depth in 118 nodules. In 98 nodules with high density at the arterial phase of dynamic CT, there was no significant difference in the detection rates of vascularity among the three groups (nodule size ≤1 cm, 87%; 1–2 cm, 88%; >2 cm, 97%). Of the eight nodules ≤1 cm with isodensity or low density at the arterial phase of dynamic CT, four (50%) showed hyperenhancement at the early vascular phase of CEUS.
Table 4. Detection of vascularity by contrast-enhanced ultrasound according to tumour size.
| Arterial phase of dynamic CT | Tumour size (cm) | Early vascular phase of CEUS |
|||||
| Hyper | Iso | Total | p-value | ||||
| High density | ≤1 | 13 | (87%) | 2 | (13%) | 15 | NS |
| 1–2 | 44 | (88%) | 6 | (12%) | 50 | ||
| >2 | 32 | (97%) | 1 | (3%) | 33 | ||
| Isodensity | ≤1 | 4 | (57%) | 3 | (43%) | 7 | NE |
| 1–2 | 0 | 6 | (100%) | 6 | |||
| >2 | 0 | 0 | 0 | ||||
| Low density | ≤1 | 1 | (100%) | 0 | 1 | NE | |
| 1–2 | 0 | 3 | (100%) | 3 | |||
| >2 | 1 | (33%) | 2 | (67%) | 3 | ||
Hyper, hyperenhancement; iso, iso-enhancement; CEUS, contrast-enhanced ultrasound; NS, not significant; NE, not evaluated owing to insufficient numbers.
Table 5. Detection of tumour vascularity by contrast-enhanced ultrasound according to tumour depth.
| Arterial phase of dynamic CT | Depth (cm) | Early vascular phase of CEUS |
|||||
| Hyper | Iso | Total | p-value | ||||
| High density | ≤9 | 79 | (95%) | 4 | (5%) | 83 | 0.0007 |
| >9 | 9 | (60%) | 6 | (40%) | 15 | ||
| Isodensity | ≤9 | 4 | (44%) | 5 | (56%) | 9 | NE |
| >9 | 1 | (25%) | 3 | (75%) | 4 | ||
| Low density | ≤9 | 2 | (33%) | 4 | (67%) | 6 | NE |
| >9 | 0 | 1 | (100%) | 1 | |||
Hyper, hyperenhancement; iso, iso-enhancement; CEUS, contrast-enhanced ultrasound; NE, not evaluated owing to insufficient numbers.
Of 83 nodules located within 9 cm in depth from the abdominal wall and with high density at the arterial phase of dynamic CT, 79 (95%) were demonstrated as hyperenhancement on CEUS; 9 (60%) of 15 nodules located more than 9 cm in depth from the abdominal wall showed hyperenhancement on CEUS (p = 0.0007). There was a significant difference in the detection rate of vascularity between nodules located within 9 cm and those deeper than 9 cm.
The 92 nodules of newly developed HCC were also observed at the post-vascular phase on CEUS examination. Comparing the portal phase of dynamic CT with the post-vascular phase of CEUS, 70 (82%) of the 85 nodules with low density on dynamic CT demonstrated hypo-enhancement at the post-vascular phase of CEUS. The other 15 (18%) nodules demonstrated low enhancement at the portal phase of dynamic CT (Table 6) and iso-enhancement at the post-vascular phase of CEUS. Six (86%) of seven nodules with isodensity at the portal phase of dynamic CT demonstrated hypo-enhancement at the post-vascular phase of CEUS.
Table 6. Detectability of tumours at the post-vascular phase of contrast-enhanced US compared with dynamic CT.
| Post-vascular phase of CEUS |
||||||
| Iso | Hypo | Total | ||||
| Portal phase of dynamic CT | Isodensity | 1 | (14%) | 6 | (86%) | 7 |
| Low density | 15 | (18%) | 70 | (82%) | 85 | |
| Total | 16 | (17%) | 76 | (83%) | 92 | |
Hypo, hypoenhancement; iso, iso-enhancement; CEUS, contrast-enhanced ultrasound.
We evaluated whether underlying liver disease, tumour depth, size and vascularity affected tumour detectability at the post-vascular phase of CEUS (Table 7). There were no significant differences in the detection rates at the post-vascular phase according to underlying liver disease. The detection rates at the post-vascular phase showed a tendency to be lower in nodules at a depth of >9 cm than in those at a depth of ≤9 cm. Detection rates were significantly higher in nodules >2 cm in diameter than in those <2 cm (p = 0.00076) and were higher in hypervascular nodules at the early vascular phase of CEUS than in isovascular nodules (p = 0.00005).
Table 7. Comparison of tumour detectability at the post-vascular phase of contrast-enhanced ultrasound according to underlying liver disease, tumour depth, size and vascularity.
| Post-vascular phase of CEUS |
||||||
| Iso | Hypo | p-value | ||||
| Underlying | Chronic hepatitis | 3 | (18%) | 14 | (82%) | NS |
| liver disease | Cirrhosis | 13 | (17%) | 62 | (83%) | |
| Pugh A | 6 | (12%) | 43 | (88%) | ||
| Hepatitis B | 7 | (29%) | 17 | (71%) | ||
| Hepatitis C | 0 | 2 | (100%) | |||
| Tumour depth | ≤9 cm | 13 | (15%) | 71 | (85%) | 0.12 |
| >9 cm | 3 | (37%) | 5 | (63%) | ||
| Tumour size | ≤1 cm | 5 | (25%) | 15 | (75%) | 0.00076 |
| 1–2 cm | 10 | (24%) | 31 | (76%) | ||
| >2 cm | 1 | (3%) | 30 | (97%) | ||
| Vascular phase of CEUS | Hyper | 6 | (8%) | 67 | (92%) | 0.00005 |
| Iso | 10 | (53%) | 9 | (47%) | ||
Hypo, hypo-enhancement; hyper, hyperenhancement; iso, iso-enhancement; CEUS, contrast-enhanced ultrasound; NS, not significant.
Discussion
First-generation contrast media such as Levovist have been used in the diagnosis of hepatic focal lesions [12,16-18]; however, this agent has some limitations [19]. Levovist must be insonated with high acoustic power, which destroys the microbubbles. The wide-band frequency signals from the destroyed microbubbles can then be imaged, resulting in intermittent images that have a marked mixture of tissue and microbubble harmonics. The advent of second-generation contrast media such as SonoVue (Bracco, Milan, Italy) has enabled real-time imaging using low-MI mode. Many studies report that CEUS with SonoVue has high performance in detecting the vascularity of HCC, even for small nodules <2 cm in diameter [20-24].
Sonazoid is a second-generation contrast agent composed of perfluorobutane microbubbles with a shell of phosphatidyl serine natrium; the microbubbles are 3 μm in mean diameter, similar to the size of SonoVue (2.5 μm in mean diameter) [13]. The optimal method for evaluating the vascular phase utilises low-MI imaging that minimises bubble destruction. As well as SonoVue, Sonazoid can provide real-time imaging at the vascular phase using low-MI modes. The enhancement pattern of HCC by CEUS with SonoVue is hyperenhancement at the early vascular phase (arterial dominant) and hypo-enhancement at the portal or parenchymal phase. Hypo-enhancement at the portal or parenchymal phase is caused by washout of contrast agent from the nodule, owing to perfusion of the surrounding liver parenchyma.
Gaiani et al [21] report that 91% of HCC nodules that are hypervascular on dynamic CT demonstrate hyperenhancement at the early vascular phase with SonoVue and that 75% demonstrate slight hypo-enhancement during the portal or parenchymal phase. Xu et al [24] reported that 87% of small HCC (≤2 cm) demonstrated hyperenhancement at the early vascular phase with SonoVue and that 54% demonstrated hypo-enhancement in the portal phase, with 46% demonstrating iso-enhancement. In the present study, 88% of HCC that were hypervascular on dynamic CT, regardless of tumour size, showed hyperenhancement at the early vascular phase of CEUS with Sonazoid. Therefore, the detection rate of hypervascular HCC by Sonazoid is equal to that by SonoVue.
Recent studies have shown that the detectability of tumour vascularity by CEUS with SonoVue is comparable to that by dynamic CT [25]. The present study demonstrated that Sonazoid has the same performance as dynamic CT, both for newly developed HCC and local recurrence, and that the detection rate of tumour vascularity by Sonazoid in hypervascular nodules was not affected by lesion size. We previously demonstrated that the detection rate of vascularity with Levovist is not affected by tumour size [12]. In addition, Xu et al [24] reported that SonoVue retains high detectability for small HCC nodules (≤2 cm). On the basis of these results, the detectability of vascularity by CEUS does not depend on tumour size, regardless of the contrast media used. Tumour location, however, is an important factor in tumour detection: the detection rate of vascularity using Sonazoid was less for nodules located deeper than 9 cm from the abdominal wall than for nodules located within 9 cm, as we previously demonstrated using Levovist [12].
Sonazoid can also provide Kupffer imaging at the post-vascular phase; this phase occurs more than 10 min after administration of the contrast agent, because most of the Sonazoid microbubbles (99%) are phagocytosed by Kupffer cells. By contrast, SonoVue is barely phagocytosed and cannot create a Kupffer image [15]. As only half of the Levovist microbubbles are phagocytosed by Kupffer cells, Levovist provides only intermittent images on high-MI, whereas Sonazoid can provide real-time continuous images on low-MI. Therefore, Kupffer imaging at the post-vascular phase using Sonazoid is more effective than that using Levovist and is easier to scan repeatedly. The number of Kupffer cells in cancerous tissues is markedly lower than that in non-cancerous liver tissues and numbers decrease with decreasing histological grade [26]. Thus, quantitative evaluation of Kupffer cells can be useful in the differential diagnosis of focal liver lesions and histological grade of HCC. Imai et al [7] studied SPIO-enhanced MRI in patients with HCC and showed that enhancement reflected Kupffer cell numbers. Although we did not evaluate the correlation between enhancement in the post-vascular phase of CEUS with Sonazoid and histological grade of HCC, Inoue et al [27] and Korenaga et al [28] reported that the post-vascular phase of CEUS with Sonazoid correlates closely with SPIO-enhanced MRI. In the present study, the detection rate of hypo-enhancing nodules at the post-vascular phase by Sonazoid was 83%, which is as high as that demonstrated by the previous study [27]. By contrast, the detection of hypo-enhancement at the portal or parenchymal phase by SonoVue is 54–75% [21,24]. These findings suggest that Sonazoid might be superior to SonoVue in tumour detection at the post-vascular and parenchymal phases.
The present study demonstrated that hypo-enhancement at the post-vascular phase was less frequent in small nodules (≤2 cm) than in large nodules (>2 cm) and less frequent in isovascular nodules than in hypervascular nodules. Liu et al [26] showed that the number of Kupffer cells in HCC decreases with increasing tumour size. Moreover, these authors reported that the number of Kupffer cells is reduced in moderately differentiated HCCs (which are hypervascular), as compared with dysplastic nodules or well-differentiated HCCs (which are iso- or hypovascular). Our results are in good agreement with these previous findings. Because well-differentiated HCCs have as many Kupffer cells as non-cancerous liver tissues, they have an isoechoic pattern at the post-vascular phase by Sonazoid [28].
CEUS with Sonazoid is useful not only for the assessment of HCC, but also for the differential diagnosis of benign hepatic nodules. Some benign hepatic focal nodules, mainly focal nodular hyperplasia (FNH) and haemangioma, have been shown to demonstrate hypervascularity [29,30]. FNHs show marked homogenous enhancement and a “central stellate” or “spoke-wheel” appearance at the early vascular phase; at the post-vascular phase these nodules show iso-enhancement. The sensitivity and specificity of the FNH pattern are both 100% [30]. By contrast, haemangiomas show a subtle non-specific enhancement at the early vascular phase and peripheral globular enhancement at the late vascular phase, together with a progressive centripetal fill-in pattern [31]. The sensitivity and specificity of the haemangioma pattern are reported to be 90% and 99.6%, respectively [30]. Sonazoid-enhanced ultrasound is therefore useful to differentiate HCCs from these benign nodules. Recent studies have reported that HCC nodules not detected by baseline B-mode ultrasound could be detected at the vascular phase and/or at the post-vascular phase of CEUS with Sonazoid [32]. Furthermore, Sonazoid-enhanced ultrasound has been shown to be a sensitive and accurate modality for evaluating the response of HCCs following arterial embolisation [33]. In the clinical setting, we routinely use CEUS with Sonazoid from diagnosis to therapy evaluation.
Conclusion
CEUS with Sonazoid is a useful tool for assessing vascularity and the presence and/or absence of Kupffer cells in HCC nodules.
References
- 1.Tanaka S, Kitamura T, Fujita M, Nakanishi K, Okuda S. Color Doppler flow imaging of liver tumors. Am J Roentgenol 1990;154:509–14 [DOI] [PubMed] [Google Scholar]
- 2.Lencioni R, Pinto F, Armillotta N, Bartolozzi C. Assessment of tumor vascularity in hepatocellular carcinoma: comparison of power Doppler US and color Doppler US. Radiology 1996;201:353–8 [DOI] [PubMed] [Google Scholar]
- 3.Winter TC, 3rd, Takayasu K, Muramatsu Y, Furukawa H, Wakao F, Koga H, et al. Early advanced hepatocellular carcinoma: evaluation of CT and MR appearance with pathologic correlation. Radiology 1994;192:379–87 [DOI] [PubMed] [Google Scholar]
- 4.Takayasu K, Muramatsu Y, Furukawa H, Wakao F, Moriyama N, Takayama T, et al. Early hepatocellular carcinoma: appearance at CT during arterial portography and CT arteriography with pathologic correlation. Radiology 1995;194:101–5 [DOI] [PubMed] [Google Scholar]
- 5.Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, et al. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. Am J Roentgenol 1999;172:969–76 [DOI] [PubMed] [Google Scholar]
- 6.Stark DD, Weissleder R, Elizondo G, Hahn PF, Saini S, Todd LE, et al. Superparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liver. Radiology 1998;168:297–301 [DOI] [PubMed] [Google Scholar]
- 7.Imai Y, Murakami T, Yoshida S, Nishikawa M, Ohsawa M, Tokunaga K, et al. Superparamagnetic iron oxide-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading. Hepatology 2000;32:205–12 [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto M, Moriyasu F, Nishikawa K, Nada T, Okuma M. Color Doppler sonography of hepatic tumors with a galactose-based contrast agent: correlation with angiographic findings. Am J Roentgenol 1994;163:1099–104 [DOI] [PubMed] [Google Scholar]
- 9.Choi BI, Kim TK, Han JK, Kim AY, Seong CK, Park SJ. Vascularity of hepatocellular carcinoma: assessment with contrast-enhanced second-harmonic versus conventional power Doppler US. Radiology 2000;214:381–6 [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Cioni D, Bartolozzi C. Tissue harmonic and contrast-specific imaging: back to gray scale in ultrasound. Eur Radiol 2002;12:151–65 [DOI] [PubMed] [Google Scholar]
- 11.Hirai T, Ohishi H, Tokuno E, Takahashi M, Sakaguchi H, Anai H, et al. Qualitative diagnosis of hepatocellular carcinoma by contrast enhanced ultrasonography using coded harmonic angio with Levovist. J Med Ultrasonics 2002;29:3–9 [DOI] [PubMed] [Google Scholar]
- 12.Koda M, Matsunaga Y, Ueki M, Maeda Y, Mimura K, Okamoto K, et al. Qualitative assessment of tumor vascularity in hepatocellular carcinoma by contrast-enhanced coded ultrasound: comparison with arterial phase of dynamic CT and conventional color/power Doppler ultrasound. Eur Radiol 2004;14:1100–8 [DOI] [PubMed] [Google Scholar]
- 13.Sontum PC. Physicochemical characteristics of sonazoid™, a new contrast agent for ultrasound imaging. Ultrasound Med Biol 2008;34:824–33 [DOI] [PubMed] [Google Scholar]
- 14.Waranabe R, Matsumura M, Chen C-J, Kaneda Y, Fujimaki M. Characterization of tumor imaging with microbubble-based ultrasound contrast agent, sonazoid, in rabbit liver. Biol Pharm Bull 2005;28:972–7 [DOI] [PubMed] [Google Scholar]
- 15.Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kuppfer cells. Ultrasound Med Biol 2007;33:318–5 [DOI] [PubMed] [Google Scholar]
- 16.Ding H, Kudo M, Maekawa K, Suetomi Y, Minami Y, Onda H. Detection of tumor parenchymal blood flow in hepatic tumors: value of second harmonic imaging with a galactose-based contrast agent. Hepatol Res 2001;21:242–51 [DOI] [PubMed] [Google Scholar]
- 17.Leen E. The role of contrast-enhanced ultrasound in the characterisation of focal liver lesions. Eur Radiol 2001;11:E27–34 [DOI] [PubMed] [Google Scholar]
- 18.Dill-Macky MJ, Burns PN, Khalili K, Wilson SR. Focal hepatic masses: enhancement patterns with SH U 508A and pulse-inversion US. Radiology 2002;222:95–102 [DOI] [PubMed] [Google Scholar]
- 19.Nicolau C, Brú C. Focal liver lesions: evaluation with contrast-enhanced ultrasonography. Abdom Imaging 2004;29:348–59 [DOI] [PubMed] [Google Scholar]
- 20.Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, et al. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology 2004;232:420–30 [DOI] [PubMed] [Google Scholar]
- 21.Gaiani S, Celli N, Piscaglia F, Cecilioni L, Losinno F, Giangregorio F, et al. Usefulness of contrast-enhanced perfusional sonography in the assessment of hepatocellular carcinoma hypervascular at spiral computed tomography. J Hepatol 2004;41:421–6 [DOI] [PubMed] [Google Scholar]
- 22.Xu H-X, Liu G-J, Lu M-D, Xie X-Y, Xu Z-F, Zheng Y-L, et al. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound 2006;34:261–72 [DOI] [PubMed] [Google Scholar]
- 23.Liu G-J, Xu H-X, Lu M-D, Xie X-Y, Xu Z-F, Zheng Y-L, et al. Enhancement pattern of hepatocellular carcinoma: comparison of real-time contrast-enhanced ultrasound and contrast-enhanced computed tomography. Clin Imaging 2006;30:315–21 [DOI] [PubMed] [Google Scholar]
- 24.Xu H-X, Xie X-Y, Lu M-D, Liu G-J, Xu Z-F, Zheng Y-L, et al. Contrast-enhanced sonography in the diagnosis of small hepatocellular carcinoma ≤2 cm. J Clin Ultrasound 2006;36:257–66 [DOI] [PubMed] [Google Scholar]
- 25.Giorgio A, Ferraioli G, Tarantino L, Stefano G, Scala V, Scarano F, et al. Contrast-enhanced sonographic appearance of hepatocellular carcinoma in patients with cirrhosis: comparison with contrast-enhanced helical CT appearance. AJR 2004;183:1319–26 [DOI] [PubMed] [Google Scholar]
- 26.Liu k, He X, Lei X-Z, Zhao L-S, Tang H, Liu L, et al. Pathomorphological study on location and distribution of Kupffer cells in hepatocellular carcinoma. World J Gastroenterol 2003;9:1946–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue T, Kudo M, Hatanaka K, Takahashi S, Kitai S, Ueda T, et al. Imaging of hepatocellular carcinoma: qualitative and quantitative analysis of postvascular phase contrast-enhanced ultrasonography with sonazoid. Oncology 2008;75:48–54 [DOI] [PubMed] [Google Scholar]
- 28.Korenaga K, Korenaga M, Furukawa M, Yamazaki T, Sakaida I. Usefulness of Sonazoid contrast-enhanced ultrasonography for hepatocellular carcinoma: comparison with pathological diagnosis and superparamagnetic iron oxide magnetic resonance images. J Gasroenterol 2009;44:733–41 [DOI] [PubMed] [Google Scholar]
- 29.Leen E. The role of contrast-enhanced ultrasound in the characterization of focal liver lesions. Eur Radiol;11:E27–E34 [DOI] [PubMed] [Google Scholar]
- 30.Hatanaka K, Kudo M, Minami Y, Ueda T, Tatsumi C, Kitai S, et al. Differential diagnosis of hepatic tumors: value of contrast-enhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology 2008;51:S61–S69 [DOI] [PubMed] [Google Scholar]
- 31.Quaia E, Bertolotto M, Dalla Palma L. Characterization of liver hemangiomas with pulse-inversion harmonic imaging. Eur Radiol 2002;12:537–44 [DOI] [PubMed] [Google Scholar]
- 32.Nunata K, Morimoto M, Ogura T, Sugimoto K, Takebayashi S, Okada M, et al. Ablation therapy guided by contrast-enhanced sonography with Sonazoid for hepatocellular carcinoma lesions not detected by conventional sonography. J Ultrasound Med 2008;27:395–406 [DOI] [PubMed] [Google Scholar]
- 33.Xia Y, Kudo M, Minami Y, Hatanaka K, Ueshima K, Chung H, et al. Responase evaluation of transcatheter arterial chemoembolisation in hepatocellular carcinoma: the usefulness of Sonazoid-enhanced harmonic sonography. Oncology 2008;75:S99–S105 [DOI] [PubMed] [Google Scholar]