Abstract
Objective
Klebsiella pneumoniae is one of the organisms most commonly isolated from pyogenic liver abscesses in Asian populations. We compared CT findings in liver abscesses caused by K. pneumoniae with those caused by other bacterial pathogens.
Methods
Of 214 patients with liver abscesses examined over a 5 year period, 129 patients with positive blood or aspirate cultures were enrolled. The patients were divided into two groups: the K. pneumoniae monomicrobial liver abscess (KLA) group (n = 59) and the non-K. pneumoniae monomicrobial or polymicrobial liver abscess (non-KLA) group (n = 70). Two radiologists blinded to the culture results evaluated the CT images, recording the number, size, location and configuration of abscesses, the thickness of the abscess wall, the pattern of rim enhancement, septal enhancement, the double target sign, internal necrotic debris, internal gas bubbles and underlying biliary disease. The presence of diabetes and metastatic infection was also compared between groups. Statistical analyses were performed using univariate (Student's t-test and χ2 test) and multivariate analyses.
Results
Multivariate analysis showed that a thin wall, necrotic debris, metastatic infection and the absence of underlying biliary disease were the most significant predictors of KLA. When three of the four criteria were used in combination, a specificity of 98.6% was achieved for the diagnosis of KLA.
Conclusion
A thin-walled abscess, internal necrotic debris, the presence of metastatic infection and the absence of underlying biliary disease may be useful CT findings in the early diagnosis of K. pneumoniae liver abscesses.
Pyogenic liver abscesses are caused by a wide range of bacteria. Escherichia coli was previously the most common causative pathogen of pyogenic liver abscesses. Recently, however, Klebsiella pneumoniae has become the leading cause of pyogenic liver abscesses in many Asian populations and in some Western populations [1-6].
There are several distinct clinical differences between K. pneumoniae liver abscesses (KLA) and non-K. pneumoniae liver abscesses (non-KLA). First, compared with other bacterial liver abscesses, KLA are associated with a higher frequency of bacteraemia and the potential for metastatic infection in other parts of the body. Although the mortality rate is generally lower for KLA than for non-KLA (4.1 vs 20.8%), the prognosis of KLA is often poor in patients with metastatic infection [6]. Second, ampicillin is ineffective against KLA because K. pneumoniae is intrinsically resistant to ampicillin. The preferred antibiotics for KLA are aminoglycoside and extended-spectrum β lactams. Thus, a high index of suspicion for KLA should be maintained when selecting antibiotic coverage. Third, non-KLA occur in patients with underlying biliary disease, whereas KLA frequently occur in the absence of any underlying biliary disease or predisposing medical condition [2-8].
Although the early recognition of KLA is important, the differentiation between KLA and non-KLA can be difficult. In fact, the clinical presentation and laboratory findings of patients with KLA are similar to those of patients with other pyogenic liver abscesses. Blood or pus culture is the standard method for the identification of bacterial pathogens, but these methods require several days to produce results, thus delaying treatment.
Imaging modalities, such as ultrasonography and CT, have been used to diagnose liver abscesses, to identify possible causes and to rule out other intra-abdominal conditions that cause similar symptoms [9,10]. However, only a few reports regarding the use of ultrasonography or CT in the differentiation of KLA and non-KLA have been published [11,12]. To our knowledge, there is only one published report on distinctive CT features in the differentiation of KLA and non-KLA [12], and no report has identified predictors that can be used to distinguish KLA from other bacterial liver abscesses using CT images. Thus, the purpose of our study was to retrospectively compare the clinical and CT features of pyogenic liver abscesses caused by K. pneumoniae and other bacterial pathogens, and to identify differences that may assist in differential diagnosis.
Methods and materials
Patients
This was a retrospective single-institution study approved by our institutional review board. Informed consent was routinely obtained from all patients before all CT examinations and all interventional procedures, such as the aspiration of abscess pus. A search of medical records was performed to identify all patients in whom a liver abscess was diagnosed between December 2001 and December 2006. This search yielded a total of 214 patients. After one radiologist, who did not participate as a reader, reviewed the medical records of these patients, 85 patients were excluded for one of following reasons: (a) no CT study had been performed (n = 3); (b) only a non-enhanced CT study had been performed (n = 7); (c) pus culture did not reveal a positive finding (n = 69); (d) lesions that had been depicted in imaging studies did not resolve after antibiotic treatment (n = 1); (e) the patient had a suspected amoebic or fungal abscess (n = 2); or (f) CT scans were obtained after interventional procedures or surgeries (n = 3). Finally, we identified 129 consecutive patients with clinically proven pyogenic liver abscesses. These included 60 men and 69 women, with a mean age of 67 years (range, 25–91 years). All patients underwent ultrasonography-guided needle aspiration, percutaneous abscess drainage or surgical drainage.
Patients were divided into two groups according to bacterial aetiology: the K. pneumoniae monomicrobial pyogenic liver abscess (KLA) group and the non-K. pneumoniae monomicrobial or polymicrobial pyogenic liver abscess (non-KLA) group. A similar classification was used by Hui et al [11]. A monomicrobial liver abscess is one in which only one organism was recovered from the abscess aspirate and/or blood culture. A polymicrobial liver abscess is one in which more than one organism (one of which could be K. pneumoniae) was recovered from the abscess aspirate and/or blood culture.
Medical data, including clinical, imaging and laboratory parameters for the 129 patients, were reviewed with particular attention to the presence of diabetes mellitus or metastatic infection in other parts of the body, such as meningitis, endophthalmitis, lung abscess, kidney abscess or fasciitis.
CT technique
CT scans were obtained on an average of 0.7 days before the procedures or surgeries. CT examinations were performed using a LightSpeed QX/i four-detector row scanner (GE Medical Systems, Milwaukee, WI; n = 105) or a Somatom Sensation 16 multidetector row scanner (Siemens Medical Systems, Erlangen, Germany; n = 24). The CT techniques varied because of the retrospective nature of this study. Most patients (n = 118) underwent dynamic CT and the remaining 11 patients underwent single-phase CT. Generally, unenhanced and dual-phase contrast-enhanced helical scans were obtained. After an unenhanced CT scan was obtained, 150 ml of iopromide (Ultravist 370; Schering Korea, Seoul, Korea) was administered at a flow rate of 3–4 ml s–1 using a mechanical injector. The scan delay time was determined using the bolus-tracking technique (Smart Prep; GE Medical Systems and CARE-Bolus Software; Siemens Medical Systems). Late arterial phase scanning was automatically initiated at 10 s after the contrast enhancement of the aorta reached the preferred point (100 HU). Portal venous phase scanning was obtained 20 s after the completion of the late arterial phase. CT parameters for the LightSpeed QX/i scanner included a detector configuration of 4 × 5 mm (unenhanced phase) or 4 × 2.5 mm (arterial and portal venous phase), a gantry rotation time of 0.6 s, 200 effective mAs, 120 kVp, 5 mm slice thickness and 5 mm interval. CT parameters for the Somatom Sensation 16 scanner included a detector configuration of 16 × 1.5 mm (unenhanced phase) or 16 × 0.75 mm (arterial phases), a table feed of 24 mm (unenhanced) or 12 mm (arterial and portal venous phases) per gantry rotation, 200 effective mAs, 120 kVp, 5 mm slice thickness and 5 mm interval. All contrast-enhanced CT image data were directly interfaced to our picture archiving and communications system (Marotech; Seoul, Korea), which displayed all image data on monitors (two monitors, 2048 × 2560 image matrices, 10-bit viewable grey-scale and 145.9-ft-lambert luminescence).
CT interpretation
Two experienced abdominal radiologists retrospectively reviewed the CT images and developed a consensus opinion. Both radiologists were aware that the patients had hepatic abscesses, but they were blinded to the results of the microbiological and clinical findings. During the analysis of the CT features, cases from the KLA and non-KLA group were randomly intermixed.
CT images of abscesses were analysed in terms of the number, location (right, left or both lobes of the liver), size and configuration (unilocular vs multilocular) of the abscesses, the thickness of the abscess wall, the pattern of rim enhancement, septal enhancement, the double target sign, internal necrotic debris and internal gas bubbles. Additionally, the radiologists recorded the presence of underlying biliary disease, including stones in the bile duct, air in the biliary tree, bile duct obstruction, cholecystitis and any previous hepatobiliary operation.
On contrast-enhanced CT images, the abscess wall was defined as one layer or two layers with varying degrees of enhancement between hypo-attenuating central necrotic areas and the surrounding hepatic parenchyma. The maximum thickness of the abscess wall was recorded, and the abscess wall was classified as thick (i.e. equal or greater than 2 mm) or thin (i.e. less than 2 mm). The pattern of rim enhancement was graded as increased enhancement or no enhancement. Increased enhancement was defined as the majority of the margin having higher attenuation than the surrounding liver. The presence of septal enhancement was defined as septal attenuation in the abscess cavity higher than that of the surrounding liver. The double-target sign consisted of a hypodense central abscess cavity surrounded by an inner hyperdense ring, and an outer hypodense zone on dynamic contrast-enhanced CT, as defined by Mathieu et al [10]. Necrotic debris was defined as a solid component with enhancement similar to or less than that of adjacent liver, larger than 1 cm in diameter, in a hypodense central necrotic area.
Statistical analysis
The χ2 test was used to assess differences in clinical and CT features between patients in the KLA and non-KLA groups with regard to the location and configuration of abscesses, the thickness of the wall, the pattern of rim enhancement, septal enhancement, the double target sign, internal necrotic debris, internal gas bubbles, underlying biliary disease, diabetes mellitus and metastatic infection. Differences in age and in the size and number of the abscesses between groups were analysed using Student's t-test. A multivariate stepwise logistic regression analysis used to test the univariate models demonstrated the best predictors of the KLA vs non-KLA groups. Using these data, the sensitivity, specificity and accuracy of the criteria for differentiating KLA from non-KLA were evaluated. A p-value of less than 0.05 was deemed to indicate a statistically significant difference in all analyses. All statistical analyses were performed using the SPSS software package (version 13.0; SPSS, Chicago, IL).
Results
Of the 129 patients with pyogenic liver abscesses, 59 patients had a monomicrobial infection caused by K. pnuemoniae (KLA group) and 70 patients had non-K. pnuemoniae monomicrobial or polymicrobial infections (non-KLA group) diagnosed by blood and/or abscess aspirate cultures. The mean age was 62.2 years (range, 35–86 years) in the KLA group and 65.8 years (range, 25–91 years) in the non-KLA group. The male-to-female ratio was 33:26 in the KLA group and 32:38 in the non-KLA group. No significant difference in age and gender was observed between the groups.
The species of organisms isolated from liver abscesses in the non-KLA group are summarised in Table 1. In the non-KLA group, 38 patients (54.3%) had monomicrobial and 32 patients (45.7%) had polymicrobial liver abscesses. The causative microorganism was predominantly E. coli in both monomicrobial and polymicrobial isolates (n = 36; 17/38 and 19/32, respectively) followed by Enterococcus spp. (n = 17; 8/38 and 9/32, respectively) and K. pneumoniae in polymicrobial infections (n = 12).
Table 1. Number of cases in which various organisms were isolated from liver abscesses in the non-KLA group (n = 70).
| Organisms | Blood culture | Abscess aspirate |
| Escherichia coli | 11 | 32 |
| Klebsiella pneumoniae | 5 | 10 |
| Klebsiella oxytoca | 0 | 1 |
| Pseudomonas aeruginosa | 1 | 2 |
| Morganella morganii | 2 | 7 |
| Proteus vulgaris | 1 | 2 |
| Enterobacter spp. | 1 | 4 |
| Acinetobacter spp. | 1 | 0 |
| Citrobacter freundii | 0 | 2 |
| Enterococcus spp. | 5 | 17 |
| Streptococcus spp. | 1 | 5 |
| Bacteroides fragillis | 1 | 2 |
| Stenotrophomonas maltophilia | 0 | 1 |
| Total isolates | 29 | 85 |
non-KLA, Klebsiella pneumoniae without monomicrobial liver abscess.
Table 2 summarises the distinguishing clinical and CT characteristics for patients in the KLA vs non-KLA group. Univariate analysis showed several significant differences between the KLA and non-KLA groups. The KLA group was significantly associated with a thin-walled abscess (p<0.001), no rim enhancement (p = 0.004) and necrotic debris (p<0.001), whereas the non-KLA group was significantly associated with a thick-walled abscess (p<0.001), increased rim enhancement (p = 0.004) and the double target sign (p = 0.007) (Figures 1–3). Underlying biliary disease was more common in the non-KLA group (n = 55, 78.6%) than in the KLA group (n = 16, 27.1%); this difference was statistically significant (p<0.001) (Figures 2 and 3). Metastatic infection concomitantly developed in 12 of 59 patients (20.3%) in the KLA group, of whom 10 were diabetics, and in 1 of the 70 patients (1.4%) in the non-KLA group; this was also significantly different (p<0.001) (Figure 1). Of the 12 patients with metastatic infection in the KLA group, there were 4 cases of endophthalmitis, four of lung abscess, two of renal abscess, 1 of brain abscess and 1 of endophthalmitis with lung and brain abscesses. A diabetic patient with a liver abscess caused by Stenotrophomonas maltophilia in the non-KLA group also had a lung abscess. Because images of the lower chest and kidney were usually obtained as part of the abdominal CT imaging, metastatic infections in the lung and kidney were readily identifiable during review of the CT images. In our study, abdominal CT demonstrated two septic renal complications and five lung abscesses in six patients in the KLA group and one patient in the non-KLA group. No differences were found between groups regarding the presence of diabetes mellitus, the number, size, location or configuration of the abscesses, the degree of septal enhancement or the presence of gas bubbles.
Table 2. Comparison of clinical and CT features in the KLA and non-KLA groups.
| Finding | KLA (n = 59) | non-KLA (n = 70) | p-value |
| Age (mean) | 62.2 | 65.8 | 0.741 |
| Sex (M:F) | 33:26 | 32:38 | 0.675 |
| Abscess feature (n (%)) | |||
| Location | 0.312 | ||
| Right | 41 (69.5%) | 49 (70.0%) | |
| Left | 14 (23.7%) | 16 (22.9%) | |
| Both | 4 (6.8%) | 5 (7.1%) | |
| Configuration | 0.103 | ||
| Unilocular | 9 (15.3%) | 19 (27.1%) | |
| Multilocular | 50 (84.7%) | 51 (72.9%) | |
| Abscess wall thickening | <0.001 | ||
| Thick wall (≥2 mm ) | 11 (18.6%) | 46 (65.7%) | |
| Thin wall (<2 mm ) | 48 (81.4%) | 24 (34.3%) | |
| Pattern of rim enhancement | 0.004 | ||
| Increased enhancement | 20 (33.9%) | 43 (61.4%) | |
| No enhancement | 39 (66.1%) | 27 (38.6%) | |
| Double target sign | 5 (8.5%) | 19 (27.1%) | 0.007 |
| Septal enhancement | 44 (74.6%) | 41 (58.6%) | 0.056 |
| Necrotic debris | 34 (57.6%) | 19 (27.1%) | <0.001 |
| Internal gas bubbles | 52 (89.7%) | 64 (91.4%) | 0.536 |
| Underlying biliary disease | 16 (27.1%) | 55 (78.6%) | <0.001 |
| Diabetes mellitus | 36 (61.0%) | 36 (51.4%) | 0.275 |
| Metastatic infection | 12 (20.3%) | 1 (1.4%) | <0.001 |
KLA, Klebsiella pneumoniae with monomicrobial liver abscess; non-KLA, non-Klebsiella pneumoniae monomicrobial liver abscesses; M, male; F, female.
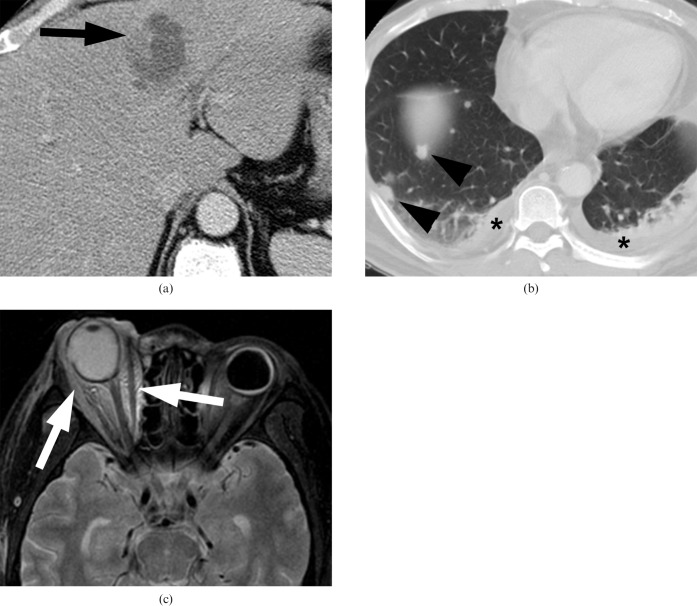
Figure 1.
Klebsiella pneumoniae liver abscess in a 59-year-old woman. (a) Contrast-enhanced abdominal CT scan shows a multiloculated thin-walled abscess containing necrotic debris (arrow) in the left lobe of the liver. (b) Lower chest scan obtained as part of the abdominal CT (lung window setting) scan shows multiple septic emboli (arrowheads) and pleural effusion (asterisks). (c) T2 weighted MRI shows diffuse high signal intensity in the right orbit (arrows), highly suggestive of endophthalmitis.
Figure 2.
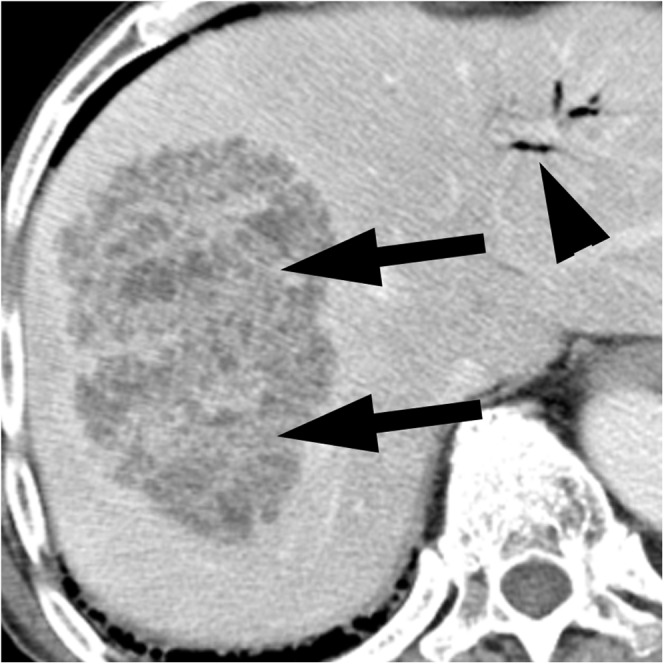
Klebsiella pneumoniae liver abscess in an 85-year-old man with fever and right upper quadrant pain who underwent metallic biliary stent insertion for bile duct cancer. The contrast-enhanced CT scan shows a multiloculated thin-walled abscess containing necrotic debris (arrows) in the right lobe of the liver. Air in the biliary tree (arrowhead) was observed secondary to the previous procedure.
Figure 3.
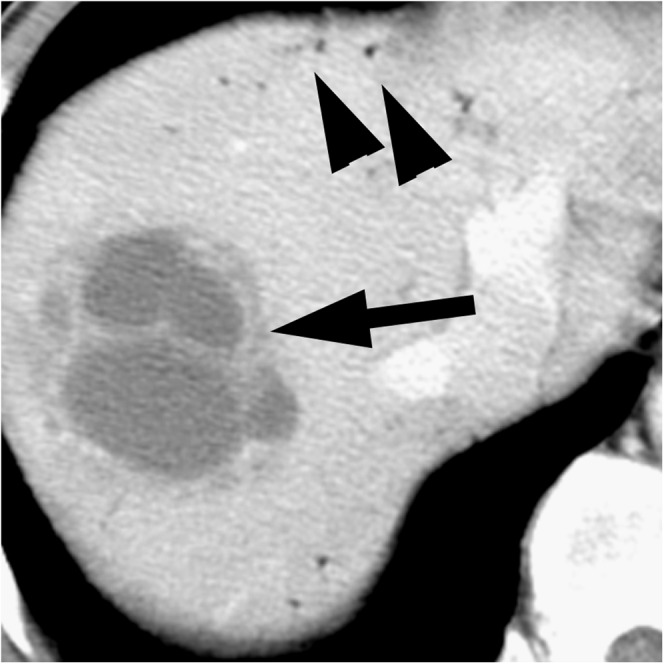
Non-Klebsiella liver abscess (caused by Enterococcus) in a 57-year-old man with fever and right upper quadrant pain who underwent plastic biliary stent insertion for bile duct cancer. The contrast-enhanced CT scan shows a multiloculated thick-walled abscess without evidence of necrotic debris (arrow) in the right lobe of the liver. Air in the biliary tree (arrowheads) was also observed.
Multivariable stepwise logistic regression analysis showed that a thin-walled abscess, internal necrotic debris, the absence of underlying biliary disease and the presence of metastatic infection were the most significant predictors of KLA. When included together in a model, the highest odds ratio was achieved for metastatic infection, followed by the absence of underlying biliary disease, necrotic debris and a thin-walled abscess (Table 3). When two or more of these four criteria were used in combination, we could identify 48 of 59 patients (81.4%) in the KLA group and 16 of 70 patients (22.8%) in the non-KLA group. When three or four of the four criteria were used, we could identify 28 of 59 patients (47.5%) in the KLA group, but only 1 of 70 patients (1.4%) in the non-KLA group. Thus, we achieved a high specificity of 98.6% despite a sensitivity of only 47.5% (Table 4).
Table 3. Sensitivity, specificity, accuracy values and odds ratio of clinical and CT features for predicting Klebsiella pneumoniae liver abscesses.
| Odds ratioa | Sensitivity (% (number of patients)) (n = 59) | Specificity (% (number of patients)) (n = 70) | Accuracy (% (number of patients)) (n = 129) | |
| Metastatic infection | 26.5 (2.2–311.9) | 20.3 (12) | 98.6 (69) | 62.8 (81) |
| Necrotic debris | 6.8 (2.2–20.8) | 57.6 (34) | 72.9 (51) | 65.9 (85) |
| Thin wall | 4.9 (1.7–13.7) | 81.4 (48) | 65.7 (46) | 72.9 (94) |
| Absence of underlying biliary disease | 11.7 (3.9–35.4) | 72.9 (43) | 78.6 (55) | 76.0 (98) |
aData in parentheses are the 95% confidence interval. The final statistical model included only those predictor variables that were found to be statistically significant in the multivariate analysis.
Table 4. Number of patients who would be identified by using combinations of clinical and CT findings to predict Klebsiella pneumoniae liver abscesses.
| No. of clinical and CT findingsa | KLA group (n = 59) | Non-KLA group (n = 70) |
| 2 | 48 (81.4%) | 16 (22.8%) |
| 3 | 28 (47.5%) | 1 (1.4%) |
| 4 | 1 (1.7%) | 0 (0%) |
KLA, Klebsiella pneumoniae with monomicrobial liver abscess; non-KLA, non-klebsiella pneumoniae monomicrobial liver abscesses.
aPredictions rely on one or more of the following findings: thin wall, necrotic debris, metastatic infection and the absence of underlying biliary disease.
Discussion
Klebsiella pneumoniae is clinically the most important member of the Klebsiella genus of Enterobacteriaceae and the most frequent aerobic Gram-negative bacillus present in normal human intestinal tract flora. However, this bacterium can produce infection at a variety of sites, with an increased risk in patients with impaired defences. In addition to pneumonia and urinary tract infections, an increase in the frequency of K. pneumoniae liver abscesses, especially in diabetic patients, has been observed in diverse nosocomial and community-acquired infections [13]. The frequency of KLA has increased markedly during the past two decades, and KLA now comprise the majority of pyogenic liver abscesses in Asian countries. Moreover, a shift from E. coli to K. pneumoniae abscesses has occurred gradually in some Western countries [1-6].
There are some important differences in the clinical significance and treatment of KLA and non-KLA. Thus, the differentiation between KLA and non-KLA before culture results is important for a favourable outcome [2-6]. Ultrasonography and CT play key roles in the diagnosis of pyogenic liver abscesses, but diagnostic modalities for differentiating KLA from non-KLA have received less attention in previous studies. To our knowledge, two reports have discussed distinctive imaging features for the differentiation of KLA and non-KLA [11,12]. Using ultrasonography, Hui et al [11] reported that masses that predominantly appeared to be solid and that had irregular or indistinct margins were associated with KLA. Using CT, Kim et al [12] showed that KLA had characteristic features, including septal breakage, hairball-like content, air-fluid level and no enhanced rim.
In our multivariate analysis, KLA were significantly associated with a thin walls and necrotic debris, whereas non-KLA were associated with a thick walls. Necrotic debris in the abscess cavity presents an internal solid structure in KLA, which is similar to the predominantly solid appearance of KLA upon ultrasonography described by Hui et al [11], and the hairball-like content of KLA on CT described by Kim et al [12]. It may be that KLA represent an immature form of liver abscess, with failure of liquefaction. The rapid invasion and destruction of K. pneumoniae, which does not permit enough time for the inflamed parenchyma to break down completely to homogeneous pus, may generate a mixture of immature pus and debris within the abscess. Our study found that KLA tend to have thin walls whereas non-KLA tend to have thick walls, this finding is similar to that of two previous reports in which KLA were associated with irregular or indistinct margins whereas non-KLA tended to have regular or smooth margis [11,12]. These findings may also be associated with different rates of invasion and destruction of the wall and hepatic parenchyma by inflammation and tissue necrosis by different microorganisms [12].
Generally, pyogenic liver abscesses may be caused by gastrointestinal infection through the portal vein, by disseminated sepsis via the hepatic artery, by ascending cholangitis or by superinfection of necrotic tissue. Recently, biliary disease has become the most common aetiology, although no cause is found in approximately half of cases [14]. K. pneumoniae first appears in the blood, causing bacteraemia. Liver abscesses form when the micro-organisms pass through the portal vein, resulting in sequestration by Kupffer cells in the liver. Thus, KLA are much more likely to be cryptogenic (64%) [15,16]. Previous reports have found that biliary disease was more common in non-KLA than in KLA [5,6,11,12]. In our study, underlying biliary disease was also lower in KLA than in non-KLA (27.1 vs 78.6%), and this was one of the CT criteria significant for differentiating KLA from non-KLA (p<0.001).
Patients with pyogenic liver abscesses may develop metastatic infection at other sites. In our study, metastatic infection is a more common complication for KLA than for non-KLA. For KLA, metastatic infection tends to occur beyond the abdominal region at more distant locations, whereas infection from non-KLA typically occurs in the abdominal region. In our study, the incidence of metastatic infection in KLA was higher than in non-KLA (20.3 vs 1.4%); this difference was statistically significant (p<0.001). Many previous studies have also reported that the rate of metastatic infection associated with KLA was higher than that associated with non-KLA [5,6,11,12]. In one of these studies [6], as in our study, this difference was statistically significant, whereas in other studies [5,11,12] this difference was not statistically significant. These different results might be attributable to a relatively small number of cases in the studies. Endophthalmitis is the most common and serious septic complication of KLA, leading to impaired vision or blindness. Other manifestations include meningitis, brain abscess, septic pulmonary embolism, lung abscess, splenic abscess, neck abscess, psoas abscess, necrotising fasciitis, spondylitis and osteomyelitis [2,15]. In our study, endophthalmitis was noted in five patients and was the most common septic complication. Thus, physicians should be alert to metastatic lesions when patients with KLA complain of ocular or central nervous system symptoms. In addition, patients with KLA should be routinely examined by an ophthalmologist. Because images of the lower chest and kidney were usually obtained as part of abdominal CT, metastatic infections involving the lung or kidney were noted during the interpretation of abdominal CT images. Thus, careful observation of other organs should be made during abdominal CT interpretation in patients with suspected KLA.
10 of 12 patients with metastatic infections from KLA were diabetics. Diabetes mellitus is a an important predisposing factor for metastatic infection from KLA [2,3]. Moreover, diabetes is a major risk factor for KLA. Diabetes has been reported in previous studies to be present in 70–75% of patients with KLA compared with 5–35% of patients with non-KLA [5,6,16]. A proposed mechanism suggests that poor glycaemic control selectively impairs neutrophil phagocytosis of the K1 and K2 capsular serotype in diabetic patients [3,13]. In contrast to many previous studies reporting an association between diabetes mellitus and KLA, we did not find a significant difference between the KLA and non-KLA groups with regard to the prevalence of diabetes mellitus (61.0% and 51.4%, respectively) [5,6,16]. The high prevalence of diabetes mellitus in the non-KLA group in our study is in contrast to previous studies, but the reasons for this difference remain unclear.
Several previous reports have mentioned a tendency for gas formation in KLA [12,16,17]. In Kim et al [12], the presence of an air-fluid level may indicate that the abscess was caused by K. pneumoniae. However, we did not find any difference between the KLA and non-KLA groups in terms of gas formation. In patients with diabetes mellitus, high blood glucose levels result in compromised immunity, neutrophil dysfunction and chemotaxis dysfunction, which may provide gas-forming micro-organisms with a more favourable environment for the production of gas by mixed acid fermentation of glucose [15,17]. Because the incidence of diabetes mellitus did not differ significantly between the KLA and non-KLA groups in our study, gas formation may not be a significant CT feature for differentiating KLA from non-KLA. In addition, apart from the Klebsiella species, other organisms such as E. coli, Salmonella and Clostridium infections could cause gas-forming pyogenic liver abscesses [18].
On the basis of the results of our multivariate analysis, we established reliable imaging criteria for the diagnosis of KLA: thin wall, necrotic debris, the presence of metastatic infection and the absence of underlying biliary disease. When at least three of the four signs were present, a diagnosis could be made with a specificity of 98.6%. Although further studies with a larger series of patients are required to determine whether these findings can be applied in a clinical setting, we believe that our results are meaningful.
Our study had some limitations. First, this was a retrospective study and included cases examined using different CT systems and slightly different CT imaging protocols. Second, we did not evaluate interobserver agreement because the two radiologists did not interpret the CT images independently. Third, we included polymicrobial K. pneumoniae liver abscesses in the non-KLA group, as did Hui et al [11]. In other previous reports comparing KLA and non-KLA groups, polymicrobial K. pneumoniae infection was included in the KLA group [6,12]. If the groups had been categorised according to Yang et al [6] or Kim et al [12], different results might have been observed. Fourth, metastatic infection was identified not only from abdominal CT scans but also from other CT or MR imaging and from other body parts, such as the orbit, brain and chest. If a physician overlooks the clinical manifestations of patients with metastatic infections, further imaging studies to examine other body parts may not be performed, which may lead to the underestimation of metastatic infection. In addition, we did not examine whether the prevalence of metastatic infection detected only during abdominal CT scanning was statistically different between groups.
Conclusion
Despite this study's limitations, a thin-walled abscess, necrotic debris in the abscess cavity, the presence of metastatic infection and the absence of underlying biliary disease were the most significant findings for discriminating KLA from non-KLA in patients with pyogenic liver abscesses in an Asian population. The combined presence of these findings is highly suggestive of KLA, and may therefore be helpful in differential diagnosis.
Acknowledgments
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea (A091047).
References
- 1.Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wang JH, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998;26:1434–8 [DOI] [PubMed] [Google Scholar]
- 2.Braiteh F, Golden MP. Cryptogenic invasive Klebsiella pneumoniae liver abscess syndrome. Int J Infect Dis 2007;11:16–22 [DOI] [PubMed] [Google Scholar]
- 3.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC.Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 2007;45:284–93 [DOI] [PubMed] [Google Scholar]
- 4.Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol 2005;100:322–31 [DOI] [PubMed] [Google Scholar]
- 5.Chen SC, Wu WY, Yeh CH, Lai KC, Cheng KS, Jeng LB, et al. Comparison of Escherichia coli and Klebsiella pneumoniae liver abscesses. Am J Med Sci 2007;334:97–105 [DOI] [PubMed] [Google Scholar]
- 6.Yang CC, Yen CH, Ho MW, Wang JH. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect 2004;37:176–84 [PubMed] [Google Scholar]
- 7.Lee CC, Chen CY, Chen FH, Zimmerman RA, Hsiao HS. Septic metastatic endophthalmitis from Klebsiella pneumoniae liver abscess: CT and MR imaging characteristics — report of three cases. Radiology 1998;207:411–16 [DOI] [PubMed] [Google Scholar]
- 8.Fang CT, Chen YC, Chang SC, Sau WY, Luh KT. Klebsiella pneumoniae, meningitis: timing of antimicrobial therapy and prognosis. QJM 2000;93:45–53 [DOI] [PubMed] [Google Scholar]
- 9.Mortele KJ, Segatto E, Ros PR. The infected liver: radiologic–pathologic correlation. Radiographics 2004;24:937–55 [DOI] [PubMed] [Google Scholar]
- 10.Mathieu D, Vasile N, Fagniez PL, Segui S, Grably D, Larde D. Dynamic CT features of hepatic abscesses. Radiology 1985;154:749–52 [DOI] [PubMed] [Google Scholar]
- 11.Hui JY, Yang MK, Cho DH, Li A, Loke TK, Chan JC, et al. Pyogenic liver abscesses caused by Klebsiella pneumoniae: US appearance and aspiration findings. Radiology 2007;242:769–76 [DOI] [PubMed] [Google Scholar]
- 12.Kim SB, Je BK, Lee KY, Lee SH, Chung HH, Cha SH. Computed tomographic differences of pyogenic liver abscesses caused by Klebsiella pneumoniae and non-Klebsiella pneumoniae. J Comput Assist Tomogr 2007;31:59–65 [DOI] [PubMed] [Google Scholar]
- 13.Wu JH, Tsai CG. Infectivity of hepatic strain Klebsiella pneumoniae in diabetic mice. Exp Biol Med (Maywood) 2005;230:757–61 [DOI] [PubMed] [Google Scholar]
- 14.Lee KT, Wong SR, Sheen PC. doi: 10.1159/000050194. Pyogenic liver abscess: an audit of 10 years' experience and analysis of risk factors. Dig Surg 2001;18:459–65; discussion 465–6. [DOI] [PubMed] [Google Scholar]
- 15.Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med 1991;151:1557–9 [PubMed] [Google Scholar]
- 16.Yang CC, Chen CY, Lin XZ, Chang TT, Shin JS, Lin CY. Pyogenic liver abscess in Taiwan: emphasis on gas-forming liver abscess in diabetics. Am J Gastroenterol 1993;88:1911–15 [PubMed] [Google Scholar]
- 17.Lee HL, Lee HC, Guo HR, Ko WC, Chen KW. Clinical significance and mechanism of gas formation of pyogenic liver abscess due to Klebsiella pneumoniae. J Clin Microbiol 2004;42:2783–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanazaki K, Kajikawa S, Horigome N, Shiohara E, Haba Y, Kuroda T, et al. Gas-forming liver abscess after transcatheter arterial embolization for hepatocellular carcinoma: report of a case. Surg Today 1993;23:747–9 [DOI] [PubMed] [Google Scholar]