Abstract
High-dose interleukin-2 (IL-2) therapy may cause acute myocarditis characterised by diffuse myocardial involvement and occasionally fulminant heart failure. Cardiac MRI (CMRI) provides a comprehensive assessment of myocardial function, inflammation and injury in a single examination and has shown value in the diagnosis of myocarditis. We report a case of a 54-year-old male with metastatic melanoma who developed acute severe myocarditis with fulminant heart failure after high-dose IL-2 therapy. CMRI using a combination of T2 weighted imaging and T1 weighted late post-gadolinium enhancement techniques played a key role in establishing the diagnosis. To our knowledge we present the first case report of the combined use of T1 and T2 weighted CMRI techniques to diagnose IL-2 induced myocarditis.
Myocarditis is the inflammation of the myocardium, which may present with a wide range of signs and symptoms [1]. While often viral, there are a wide range of causes [1]. The diagnosis of myocarditis is usually made on clinical grounds after exclusion of myocardial infarction [1]. Pathologically, myocarditis is defined by the presence of inflammatory cellular infiltrate with or without associated myocardial necrosis [2]. However, since routine myocardial biopsy for pathology is associated with inherent risks and has limitations, it is not readily performed [3]. Cardiac MRI (CMRI) has become the primary non-invasive tool for assessment of myocardial function, oedema, inflammation, fibrosis and other sequelae such as pericardial effusion in patients with suspected myocarditis [4].
High-dose interleukin-2 (IL-2) is one of the few drugs approved by the United States Food and Drug Administration for the treatment of metastatic melanoma. While high-dose IL-2 induces tumour response rates of approximately 15% in patients with metastatic melanoma, with a subset of these responses being extremely durable (>15 years), the therapy has significant toxicity [5]. Patients can develop capillary leak syndrome with significant third spacing of fluid and associated lymphopenia, thrombocytopenia and coagulopathy. Here, we describe a case of IL-2 induced myocarditis identified with CMRI.
Case report
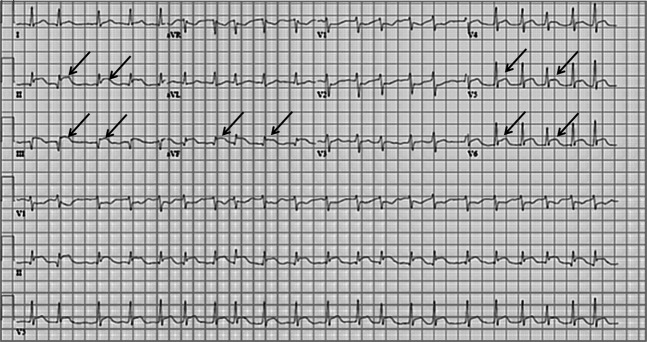
A 54-year-old male with biopsy-confirmed metastatic melanoma was admitted for high-dose IL-2 therapy. His medical history was otherwise unremarkable. He denied any recent infections or systemic symptoms. His cardiac investigations including a 12-lead electrocardiogram (ECG) and a transthoracic echocardiogram prior to initiation of IL-2 therapy were normal. On day 2 of IL-2 therapy he developed mild rigors and tachycardia, which responded to supportive care. On day 3 he developed a transient fever of 39.4°C, flushing, rigors and diarrhoea. Blood cultures during the time of fever were negative. On day 4 after receiving 11 IL-2 treatments, he refused further therapy because of chills, nausea, vomiting, diarrhoea and general fatigue. On day 5 he developed severe dyspnoea without chest pain. A 12-lead ECG showed atrial fibrillation with inferolateral ST segment elevation (Figure 1) and serological testing showed a markedly elevated troponin-I level of 58 ng ml–1 (normal, <0.11 ng ml−1), prompting cardiology consultation.
Figure 1.
Electrocardiogram on the day of transfer to the heart centre demonstrating diffuse ST changes and atrial fibrillation (ST segment elevation illustrated with arrows).
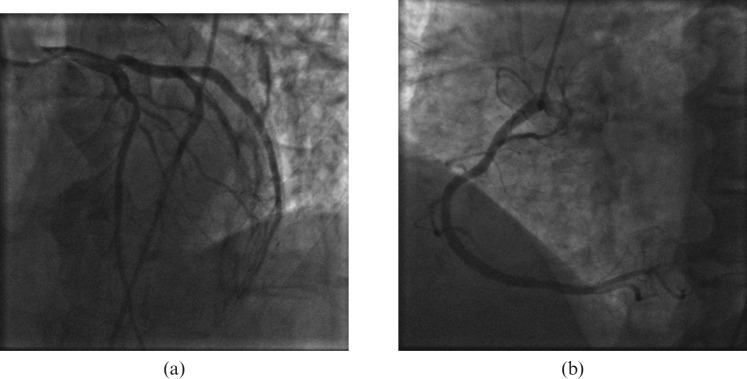
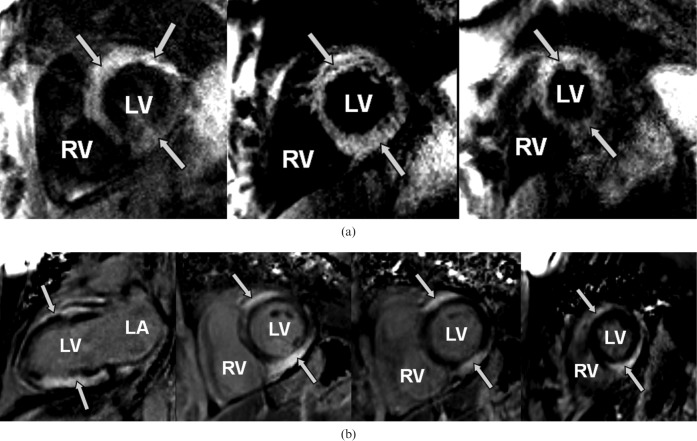
Based on the suspicion of possible acute coronary occlusion, urgent cardiac catheterisation was performed but showed no significant disease (Figure 2). Left ventricular (LV) end-diastolic pressures were elevated and ventriculography indicated globally depressed LV contractile function. Haematology showed mild anaemia, leukocytosis (haemoglobin 10.6 g l−1, white blood cell count 20.1 K μl−1, lymphocytes 6.9 K μL−1) and mildly elevated liver enzymes (alanine aminotransferase (ALT) 74 U l−1, alkaline phosphatase (ALP) 51 U l−1, aspartate aminotransferase (AST) 182 U l−1). An echocardiogram showed moderate global LV systolic dysfunction that was significantly different from the pre-treatment study. A CMRI examination was performed subsequently on a 1.5 Tesla clinical scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany) with a 12-channel phased array coil. The patient was in sinus rhythm at the time of the CMRI study. Quantitative analysis of steady-state free precession cine images showed a LV ejection fraction of 35%. Breath-hold, cardiac-triggered short tau inversion recovery (STIR) imaging illustrated regions of high T2 signal intensity indicating oedema in the anterior and inferior walls (Figure 3a, repetition time (TR) 1626.7 ms, echo time (TE) 64.0 ms, field of view (FOV) 313 × 400, matrix size 200 × 256). Late post-gadolinium T1 weighted (T1W) imaging was performed 10 min after intravenous administration of 0.2 mmol kg−1 of gadopentetate dimeglumine (Bayer Health Care Pharmaceuticals Inc, Wayne, NJ) using a fast-gradient recalled echo inversion recovery sequence and inversion times adjusted to null normal myocardium; scan parameters were as follows: TR, 736 ms; TE, 4.2 ms; FOV, 350 × 400; matrix size, 151 × 192; flip angle, 25°. This showed extensive, severe sub-epicardial hyperenhancement indicating myocardial injury (Figure 3b) in the anterior and inferior walls. Investigations for other causes of myocarditis were negative.
Figure 2.
(a) Left and (b) right coronary systems without obstructive disease.
Figure 3.
(a) High T2 signal intensity on short tau inversion recovery (STIR) imaging seen in anterior and inferior walls of the left ventricle (arrows) on short axis views (base, mid-ventricle and apical slices) extending from the base to the apex. (b) Late post-gadolinium T1 weighted imaging illustrates severe, diffuse sub-epicardial hyperenhancement (arrows) both in the vertical long axis and short axis views. Short axis views are matched to the STIR images. LV, left ventricle; RV, right ventricle; LA, left atrium.
The temporal relationship to IL-2 administration, normal pre-treatment echocardiogram and ECG, absence of other precipitating conditions, normal coronary angiogram and findings on CMRI helped confirm IL-2 therapy as the cause of the acute myocarditis. The patient received medical therapy including lisinopril, metoprolol and furosemide with significant clinical improvement. 6 weeks after discharge, dyspnoea on exertion had resolved and his B-type natriuretic peptide (BNP) was normal at 88 pg ml−1. At 8 months clinical follow-up a CT of the chest and abdomen showed excellent response of the malignancy to IL-2 therapy. He was asymptomatic from a cardiac point of view. On follow-up echocardiography the LV ejection fraction remained below 35%, resulting in the implantation of an implantable cardioverter-defibrillator (ICD). Follow-up CMRI was not performed owing to the ICD.
Discussion
This case demonstrates the value of CMRI in diagnosing and assessing the severity of myocarditis resulting from high-dose IL-2 therapy. Among the side effects associated with IL-2 therapy, cardiac side effects include arrhythmias, acute myocardial infarction (MI) and myocarditis. Myocarditis occurs in approximately 3–5% of patients receiving treatment [6]. As in our patient, initial presentation may mimic that of acute coronary syndrome. Thus, it is important for the clinician to consider IL-2 myocarditis in the differential diagnosis. The mechanism of IL-2-induced myocarditis is not well understood. One proposed hypothesis is the activation of lymphocytes by IL-2 immunotherapy, resulting in interaction with endothelial cells lining the cardiac capillaries and post-capillary venules, producing focal damage to the cardiac microcirculation [7]. This subsequently results in migration of lymphocytes and other inflammatory cells into the myocardial interstitium, culminating in cytotoxic damage and necrosis of myocytes [7].
In our patient, CMRI in conjunction with the clinical history played an essential role in the diagnosis of acute myocarditis. Therefore, CMRI including T2 weighted imaging, late-post gadolinium T1 weighted acquisitions and cines should be performed in symptomatic patients suspected of having IL-2 induced myocarditis. T2 weighted imaging can identify and quantify myocardial oedema [8]. The distribution of high T2 signal in myocarditis can be sub-epicardial, transmural, global or patchy. Early and late post-gadolinium T1 weighted imaging can provide information about acute hyperaemia, capillary leak and acute necrosis (as seen in myocarditis) as well as about scar (possible late consequence of myocarditis). In all these circumstances, the presence of interstitial expansion allows for adequate concentration of myocardial gadolinium that can subsequently be imaged using appropriate CMRI sequences [4,9]. Patterns of gadolinium enhancement in myocarditis consist of subepicardial, transmural or patchy enhancement. Other supportive CMRI findings include regional or global systolic dysfunction on cine imaging and pericardial effusion.
Based on a limited number of case reports [6] of IL-2-induced myocarditis, the myocardial dysfunction may be reversible with appropriate supportive and long-term targeted cardiovascular therapy. The prognosis in these patients appears to mainly be limited by the underlying malignancy and not the cardiac disease [6].
References
- 1.Cooper LT., Jr Myocarditis. N Engl J Med 2009;360:1526–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ, Jr, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol 1987;1:3–14 [PubMed] [Google Scholar]
- 3.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol 2007;50:1914–31 [DOI] [PubMed] [Google Scholar]
- 4.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009;53:1475–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 2000;6 (Suppl 1):S11–14 [PubMed] [Google Scholar]
- 6.Eisner RM, Husain A, Clark JI. Case report and brief review: IL-2-induced myocarditis. Cancer Invest 2004;22:401–4 [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Yu ZX, Hilbert SL, Yamaguchi M, Chadwick DP, Herman EH, et al. Cardiotoxicity of human recombinant interleukin-2 in rats. A morphological study. Circulation 1993;87:1340–53 [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Aty H, Simonetti O, Friedrich MG. T2-weighted cardiovascular magnetic resonance imaging. J Magn Reson Imaging 2007;26:452–9 [DOI] [PubMed] [Google Scholar]
- 9.Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction. Current and emerging applications. J Am Coll Cardiol 2010;55:1–16 [DOI] [PubMed] [Google Scholar]