Abstract
Acute haemobilia is an unusual and potentially catastrophic cause of gastrointestinal bleeding. We describe such a case presenting as a rare complication of a hepatic artery aneurysm following the development and successful treatment of subacute bacterial endocarditis during a radical downstaging chemoradiotherapy regime for locally advanced rectal cancer. We suggest that multiphase multidetector-row CT can have an important role in the diagnosis of acute haemobilia and discuss imaging findings associated with the condition. This case raises awareness of benign conditions mimicking malignancy in oncological patients and reinforces the importance of reviewing historical imaging.
Multidetector-row CT (MDCT) can have an important role in the diagnosis and management of acute haemobilia, an unusual and potentially catastrophic cause of gastrointestinal bleeding. We describe the rare complication of a hepatic artery aneurysm following the development and successful treatment of subacute bacterial endocarditis during a radical down-staging chemoradiotherapy regime for locally advanced rectal cancer. This case raises awareness of benign conditions mimicking malignancy in oncological patients and reinforces the importance of reviewing historical imaging.
Case report
A 51-year-old male presented with colicky abdominal pain, haematemasis and maleana with hypotension. He had been diagnosed 14 months before with locally advanced rectal cancer. He had chemoradiotherapy and was due to undergo a radical proctectomy and sacralectomy. Unfortunately, the chemoradiotherapy regimen was complicated by severe mitral valve regurgitation secondary to subacute bacterial endocarditis (SBE). This was initially treated with antibiotics, but he went on to require a metallic mitral valve replacement and commenced long-term warfarin therapy. Following recovery from cardiac surgery, a restaging CT and an 18FDG positron emission tomography (PET)-CT both only confirmed local disease spread and did not show evidence of metastatic disease.
He presented acutely 3 months later with shock and anaemia, with a haemoglobin level of 6 g dl−1 and an international normalised ratio of 3.8. He required resuscitation, blood transfusion and reversal of coagulation. An emergency upper gastrointestinal endoscopy demonstrated no active bleeding with altered blood seen within the stomach and duodenum, but no specific bleeding point could be established. He remained haemodynamically unstable and underwent an emergency laparotomy, but again no specific bleeding point was established.
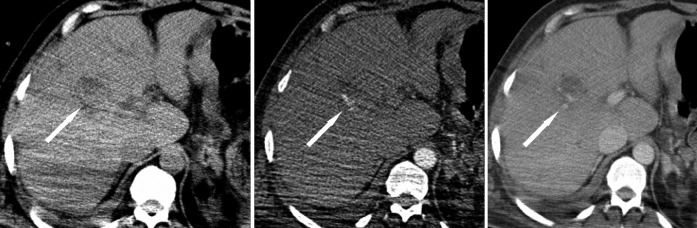
The patient then underwent a triple phase MDCT of the liver to identify the source of the haemorrhage (Figure 1 and 2). This showed high density within the biliary tree consistent with acute haemorrhage and a small elliptical area in the inferior aspect of segment eight of the liver of mixed attenuation with a contrast blush on arterial phase that persisted and was more prominent on portal venous phase images.
Figure 1.
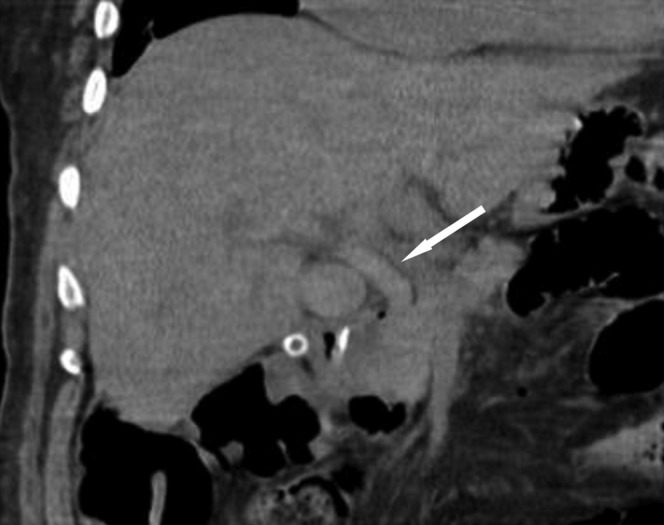
Coronal reconstruction of an unenhanced CT demonstrating a hyperdense common bile duct (arrow) representative of acute haemorrhage within the biliary tree. Note also the surgical drain placed at the time of laparotomy seen en face beneath the gallbladder.
Figure 2.
Acute multiphase axial CT at (a) pre-contrast, (b) arterial phase and (c) portal venous phase. This demonstrates initial hyperdensity at the site of the lesion, followed by contrast blush on arterial phase imaging and persistence of this on the portal venous phase scan (arrows). This represented a ruptured hepatic artery aneurysm.
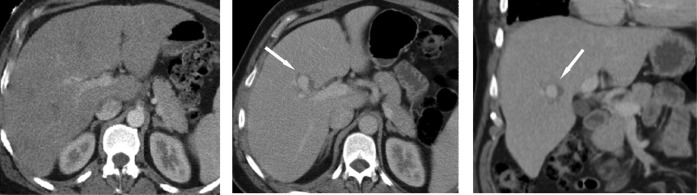
In view of the patient’s background history of locally advanced malignancy, bleeding relating to a focal hepatic metastatic lesion was considered within the differential. However, when the most recent previous staging CT was reviewed, a small (0.8 × 1.5 cm) contrast-filled density consistent with a hepatic artery aneurysm within the liver had been overlooked. It was located at the exact site of the current bleeding point (Figure 3) and represented the underlying cause for the haemobilia. When even earlier CT imaging was reviewed (obtained prior to a course of chemotherapy that was complicated by SBE), no such lesion was present (Figure 3), suggesting that aneurysm formation followed the development of endocarditis.
Figure 3.
Contrast-enhanced staging CT performed before (a) and after (b,c) the development of subacute bacterial endocarditis complicating chemotheradiotherapy. (b) Axial and (c) coronal reconstructions were performed 3 months prior to the acute presentation demonstrating an enhancing lesion consistent with a hepatic artery aneurysm (arrows).
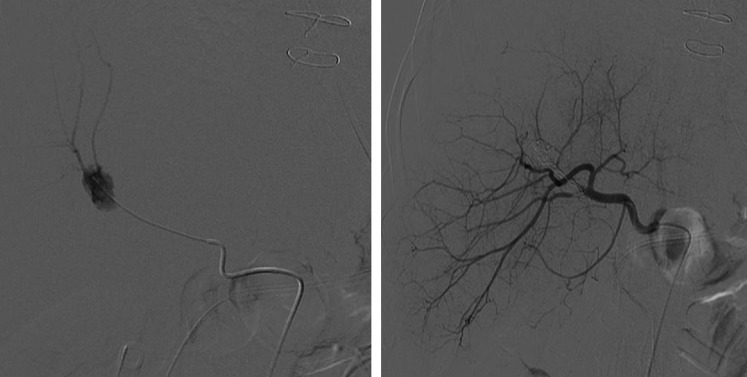
Coeliac angiography was performed using a right femoral 6-French sheath for access and Simmons catheter placed at the coeliac origin. This demonstrated a 2 cm pseudoaneurysm originating from a branch of the right hepatic artery. Eight fibred platinum coils were deployed within the lesion via a Progreat microcatheter (Terumo Medical Corporation, Somerset, NJ) (Figure 4). The bleeding stopped and the patient remained well with no further episodes of bleeding. He has gone on to have radical surgery for his rectal tumour.
Figure 4.
Coeliac angiography demonstrating the aneurysm (a) prior to and (b) following metallic coiling.
Discussion
The phenomenon of bleeding into the biliary tree was first recorded by Francis Glisson in 1654 [1,2]. Classically, the condition presents with colicky right upper quadrant pain, upper gastrointestinal bleeding and jaundice. However, only a minority of patients present in this way and often there is only one of these signs [2,3]. The commonest cause of haemobilia is usually in the acute setting of the liver or biliary tract trauma. This can be iatrogenic, with the acute presentation of haemobilia following a procedure [2]. Other causes include malignancy, gallstones, abscess formation and vascular lesions including aneurysms and arteriovenous malformations [2-4]; SBE is a well-recognised cause.
Endoscopy is the first investigation for upper gastro-intestinal bleeding. Haemobilia is rare and the diagnostic yield may be as low as 12% of cases [2,4]. Angiography is recognised as the gold-standard investigation for gastrointestinal bleeding [2,4]. The improvement in the speed and timing of multiphase MDCT has significantly enhanced the diagnostic accuracy of this study. Furthermore, MDCT is excellent in stratifying patients for therapeutic angiographic intervention. By comparison with angiography, Yoon et al [5] demonstrated a sensitivity of 90% and specificity of 99% for the detection of massive acute gastrointestinal bleeding using arterial phase MDCT. Demonstration of bleeding on MDCT is dependent on the rate of active haemorrhage, and patients with slow or intermittent gastrointestinal bleeding may have a negative CT or angiographic study. Although not well described, we believe the same principles of detecting acute gastrointestinal haemorrhage with MDCT can be used for the location and cause of haemorrhage into the biliary tract.
Surprisingly, although CT is well recognised as a useful tool in the setting of liver trauma, there have been few descriptions of the use of CT in the context of non-traumatic haemobilia. Lutter et al [6] recorded a case secondary to gallbladder ulceration and haemorrhage. CT findings in this case, as well as those described in traumatic cases [7,8], include an increase in the Hounsfield units (HUs) from approximately 0–20 HU in normal bile to approximately 50 HU representing acute haemorrhage on non-contrasted scans. Furthermore, pooling of intravascular contrast medium can be seen within the biliary tree. Imaging features on ultrasound and cholangiography have also been described [9,10]. Cholangiography shows cast-like filling defects in the common bile duct or string-like defects in the gallbladder representing clotted blood [9]. Sonographic features [10] vary with time and include diffuse echogenic gallbladder content during the initial stage, thought to be related to red cell aggregation before coagulation occurs. Later, irregular-shaped inhomogeneous non-shadowing masses are seen in the dependent parts of the lumen representing a blood clot. It has been suggested that the observation of a rapid evolution from diffuse hyper-reflectivity to less reflective masses is strongly suggestive of haemobilia [10].
Hepatic artery aneurysms account for approximately 7% of cases of haemobilia [2]. The majority of aneurysms are asymptomatic and are detected incidentally with routine imaging studies. Historically, the majority of these aneurysms were associated with endocarditis, but with improved antibiotic use, this is no longer the case, with a minority now having a mycotic aetiology [11]. To the best of our knowledge, there are only three reports describing hepatic artery aneurysms associated with endocarditis reported in the English language literature since 1950 [11-13]. Both blunt and penetrating trauma and atherosclerosis are implicated in the majority of cases, while other rarer associations include polyarteritis nodosa, Marfan's syndrome, prune belly syndrome, cholecystitis and pancreatitis [14,15].
Various reports describe the use of CT to visualise hepatic artery aneurysms [11,14]. Relative to the liver they are seen as a low-attenuation mass, often with peripheral calcification that show contrast enhancement. Extrahepatic aneurysms are four times as common as intrahepatic aneurysms. The majority of intrahepatic artery aneurysms (63%) are associated with the common hepatic artery, while 28% arise from the right hepatic artery [14]. In our case multiphase MDCT demonstrated a mixed attenuation lesion within the liver parenchyma on unenhanced images that showed a focus and then pooling of contrast medium on arterial and portal venous phase images, respectively, which is consistent with a ruptured hepatic artery aneurysm. Review of previous imaging further supported the diagnosis. This prompted angiography and our patient was successfully treated with transcatheter embolisation with metallic coils. This is often the first line of treatment for hepatic artery aneurysms, although covered stent grafts may be used depending on the morphology of the lesion [16]. Potential complications include hepatobiliary necrosis, abscess formation, re-bleeding and gallbladder fibrosis [4,14]. If stent grafts are used, stent graft fracture and recurrence have been reported as a late complication [17].
This case also highlights the need to consider benign and easily reversible conditions when assessing a patient with known underlying malignancy. Indeed, in one endoscopic series one-third of patients with a known underlying malignancy presenting with gastrointestinal haemorrhage were bleeding from a secondary benign source [18]. Furthermore, even in the acute setting, this case confirms that it is paramount to refer and compare with previous imaging.
Conclusion
This case illustrates the use of multiphase MDCT in the demonstration and diagnosis of acute haemobilia, and this should be considered prior to a laparotomy. Use of this technique is recommended for further investigation of patients with upper gastrointestinal haemorrhage or haemobilia when no focal bleeding point is identified on endoscopy. Multiphase MDCT features of a ruptured hepatic artery aneurysm, which is a rare complication of radical down-staging chemotherapy that developed following an episode of SBE, have been described. Finally, in oncology patients one must always consider and exclude benign conditions for new imaging findings.
Acknowledgments
The authors wish to thank Dr Mark Bratby, Interventional Radiologist, Oxford, who expertly and successfully deployed the aneurysm coils as an emergency referral and kindly provided us with images of his handiwork.
References
- 1.Sandblom P. Haemorrhage into the biliary tract following trauma: traumatic haemobilia. Surgery 1948;24:571–86 [PubMed] [Google Scholar]
- 2.Green MHA, Duell RM, Johnson RM, Jamieson NV. Haemobilia. Br J Surg 2001;88:773–86 [DOI] [PubMed] [Google Scholar]
- 3.Yoshida J, Donahue PE, Nyhus LM. Hemobilia: review of recent experience of a worldwide problem. Am J Gastroenterol 1987;82:448–52 [PubMed] [Google Scholar]
- 4.Merrell SM, Schneider PD. Hemobilia – evolution of current diagnosis and treatment. West J Med 1991;155:621–5 [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon W, Jeong YY, Shin SS, Lim HS, Song SG, Jang NG, et al. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi–detector row helical CT. Radiology 2006;239:160–7 [DOI] [PubMed] [Google Scholar]
- 6.Lutter DR, Berger DI. Diagnosis of nontraumatic haemobilia using computerised tomography of the abdomen. Am J Gastroenterol 1988;83:329–30 [PubMed] [Google Scholar]
- 7.Krudy AG, Doppman JL, Bissonette MB, Girton M. Hemobilia: computed tomographic diagnosis. Radiology 1983;148:785–9 [DOI] [PubMed] [Google Scholar]
- 8.Kambayashi M, Yong W, Watanabe K, Alam S. Hemobilia due to gallbladder contusion following blunt trauma – sonography and CT scanning for early detection:case report. J Trauma 1993;34:440–2 [DOI] [PubMed] [Google Scholar]
- 9.Osawa H, Mori Y, Inoue F. Malignant haemobilia detected in the gallbladder—retrograde cholangiographic findings. Br J Radiol 1996;69:79–81 [DOI] [PubMed] [Google Scholar]
- 10.Marchal G, Fevery J, Snowball S, van Holsbeeck M, Oyen R, Adisoeioso B, et al. The sonographic aspects of haemobilia. Clinical and experimental study Eur J Radiol 1985;5:211–15 [PubMed] [Google Scholar]
- 11.Kibbler CC, Cohen DC, Cruikshank JK, Kushwaha SS, Morgan MY, Dick RY. Use of CAT scanning in the diagnosis and management of hepatic artery aneurysm. Gut 1985;26:752–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stack BH, Rankin JT, Bentley RJ. Hepatic artery aneurysm after staphylococcal endocarditis. Br Med J 1968;3:659–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan M, Razvi S, Worthington M. Mycotic hepatic artery aneurysm complicating staphylococcus auresus endocarditis: successful diagnosis and treatment. Clin Infect Dis 2004;39:756–7 [DOI] [PubMed] [Google Scholar]
- 14.Song HY, Choi KC, Park JH, Choi BI, Chung YS. Radiological evaluation of hepatic artery aneurysms. Gastrointest Radiol 1989;14:329–33 [DOI] [PubMed] [Google Scholar]
- 15.Alhawsawi AM, Aljiffry M, Walsh MJ, Peltekian K, Molinari M. Hepatic artery aneurysm associated with prune belly syndrome: a case report and review of the literature. J Surg Educ 2009;66:43–7 [DOI] [PubMed] [Google Scholar]
- 16.O'Connor PJ, Chalmers AG, Chennells PM, Lintott DJ. The radiological treatment of hepatic artery aneurysms. Clin Rad 1995;50:792–6 [DOI] [PubMed] [Google Scholar]
- 17.Downer J, Choji K. Late recurrence of a hepatic artery aneurysm after treatment using an endovascular stent. Cardiovasc Intervent Radiol 2008;31:1236–8 [DOI] [PubMed] [Google Scholar]
- 18.Shivshanker K, Chu DZJ, Stroehlein JR, Nelson RS. Gastrointestinal hemorrhage in the cancer patient. Gastrointest Endosc 1983;29:273–5 [DOI] [PubMed] [Google Scholar]