Abstract
Mucopolysaccharidosis (MPS) is an inherited metabolic disorder of childhood, characterised by progressive multisystem involvement predominantly affecting the skeletal system leading to skeletal dysplasia. Mental retardation, neuropathy and cardiomyopathy may occur in the most severely affected patients, leading to progressive disability and death in their early third to fourth decades.
The purpose of this paper is to illustrate the typical imaging features of different types of MPS, in particular the MR features of the brain and spine in MPS, which are expected to be encountered by radiologists more frequently in their clinical practice as a result of prolonged life expectancy for those with MPS with recent advances in therapeutic interventions. The treatment options and outcomes for MPS patients are also briefly discussed.
Mucopolysaccharidosis (MPS) is an inherited disorder of metabolism characterised by enzyme deficiency and an inability to break down glycosaminoglycan (GAG), resulting in the accumulation of toxic intracellular substrate. All MPSs are inherited in an autosomal recessive fashion except Hunter syndrome (MPS Type II), which is X-linked. The cardinal clinical aspects were first described by Morquio and Brailsford in 1929 [1] and later by others [2,3].
Clinically, patients with MPS share a variety of cardinal skeletal manifestations, which include dwarfism (short trunk with proportionately long limbs), short neck, pigeon chest, lumbar kyphosis, genu valgum, prominent maxilla, broad mouth, macrocephaly, hypermobility of metacarpal joints and general osteoporosis. Extraskeletal manifestations include neurosensory deafness, aortic regurgitation, abdominal hernia and hepatosplenomegaly.
The inherited systemic disorders of acid mucopolysaccharide metabolism are currently classified into seven well-defined syndromes. Their genetic bases, biochemical characteristics and clinical features are summarised in Table 1.
Table 1. Types of mucopolysaccharidosis, their biochemical characteristics and their clinical features.
| Mucopolysaccharidosis (MPS) type | Syndrome name | Deficiency | Neurodegeneration | Somatic features | Corneal clouding | Bone/joint abnormality |
| MPS Type I-H | Hurler syndrome | Alpha-L-iduronidase | +++ | +++ | ++ | ++ |
| MPS Type I-S (formerly MPS Type V) | Scheie syndrome | Alpha-L-iduronidase | – | + | + | + |
| MPS Type I-H/S | Hurler-Scheie syndrome | Alpha-L-iduronidase | – | ++ | ++ | ++ |
| MPS Type II-A, mild | Hunter syndrome, mild form | L-sulfoiduronate sulfatase | + | + | – | + |
| MPS Type II-B, severe | Hunter syndrome, severe form | L-sulfoiduronate sulfatase | ++ | ++ | – | ++ |
| MPS Type III-A | Sanfilippo syndrome, Type A | Heparan sulfate sulfamidase | +++ | + | – | + |
| MPS Type III-B | Sanfilippo syndrome, Type B | N -acetyl-alpha-D-glucosaminidase | +++ | + | – | + |
| MPS Type III-C | Sanfilippo syndrome, Type C | Acetyl-coenzyme A (CoA): alpha-glucosamide N -acetyltransferase | +++ | + | – | + |
| MPS Type III-D | Sanfilippo syndrome, Type D | N -acetyl-alpha-D-glucosamine-6-sulfatase | +++ | + | – | + |
| MPS Type IV-A | Morquio syndrome, classic form | N -acetylgalactosamine-6-sulfatase (gal-6-sulfatase) | – | + | +/− | + / ++ |
| MPS Type IV-B | Morquio like syndrome | Beta-galactosidase | − | + | +/− | + / ++ / +++ |
| MPS Type VI | Maroteaux-Lamy syndrome, mild form | N -acetylgalactosamine-4-sulfatase (arylsulfatase B) | − | +/− | +/− | + |
| MPS Type VI | Maroteaux-Lamy syndrome, severe form | N -acetylgalactosamine-4-sulfatase (arylsulfatase B) | − | + | + | ++ |
| MPS Type VII | Sly syndrome | Beta-glucuronidase | − | ++ | ++ | ++ |
| MPS Type IX | Hyaluronidase deficiency | Hyaluronidase | − | − | − | + |
MPS, mucopolysaccharidosis; +, indicates present, increasing “+” means there is a stronger tendency of that feature to be present; −, absent.
Radiographic features of skeletal changes
The radiographic findings of MPS have been comprehensively described by Langer and Carry [4]. Characteristic dysplastic features are summarised in Table 2 (Figures 1–7). The characteristic radiographic features include paddle-shaped ribs, thick clavicles, wedge-shaped vertebral bodies with anterior beaking, odontoid hypoplasia, platyspondyly, lumbar gibbus, dorsal kyphosis, wide disc spaces and spinal canal stenosis.
Table 2. Summary table of radiological features.

Figure 1.
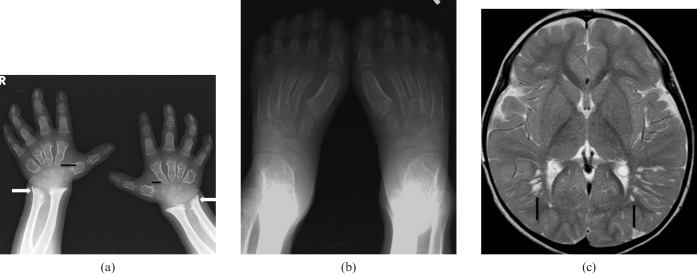
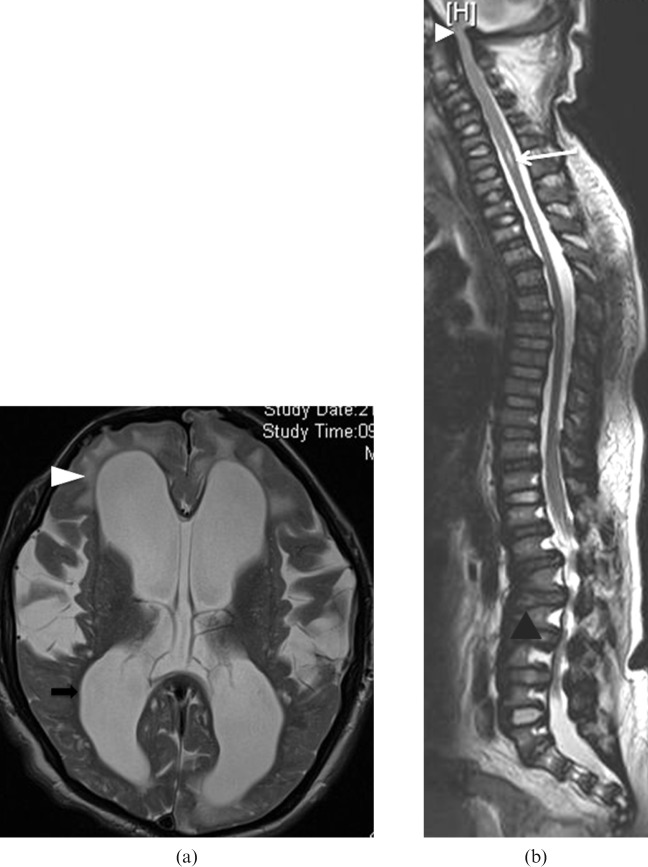
Mucopolysaccharidosis (MPS) Type I-S. A 5-year-old female has bilateral calcaneovalgus deformities of the feet, blue sclera and corneal clouding. She is neurologically normal. She has received bone marrow transplantation (BMT) from a human leukocyte antigen (HLA) identical sibling. Her post-BMT course is uneventful with rapid stable engraftment. (a) Anteroposterior (AP) view of wrist and forearm shows classic features of MPS. Note the short and wide metacarpals with proximal pointing (black arrows). Note the Madelung's deformity of the hands with tilting of distal radii and ulna towards each other (white arrows). (b) AP view of feet shows bilateral calcaneovalgus deformities of the feet. (c) MRI brain T2 weighted axial image shows prominent perivascular spaces with cribriform appearance (black arrows). She is neurologically normal with normal intelligence.
Figure 7.
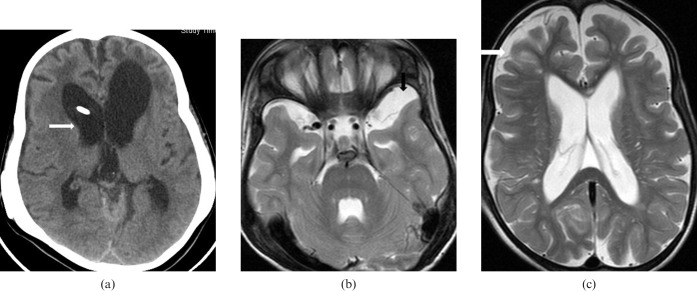
Mucopolysaccharide (MPS) Type VI. 7-year-old female, the younger sibling of the case in Figure 6, has short stature, coarse facial features, flat nasal bridge, severe lumbar kyphosis, bilateral corneal haziness, severe joint contractures and bilateral lower limb weakness. She has received peripheral blood stem cell transplant (PBSCT), followed by an uneventful clinical course. (a) CT brain axial image showing dolichocephaly and moderate hydrocephalus (white arrow). (b, c) MRI brain T2 weighted axial images showing left temporal arachnoid cyst (black arrow) and prominent cerebral sulci (white arrow) suggestive of brain atrophy.
Figure 3.
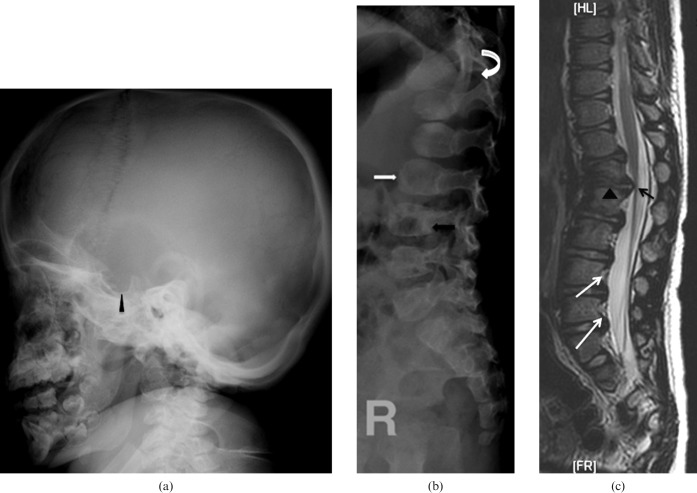
Mucopolysaccharide (MPS) Type II. A 2-year old male presented with bilateral lower limb weakness. He has mild developmental delay and moderate mental retardation. He has received physiotherapy and occupational therapy and is currently in a static course of disease. (a) Lateral radiograph of skull showing scaphocephaly and J-shaped sella turcica (arrowhead). (b) Lateral radiograph of the spine showing central beaking of the vertebrae (white arrow) and posterior vertebral scalloping (black arrow). (c) MRI spine T2 weighted sagittal image showing thoracolumbar kyphosis and gibbus at L1–2 level (arrowheads). Note the mild spinal canal narrowing at L1 and L2 without significant compression of conus medullaris (black arrow). There is also evidence of posterior vertebral scalloping (white arrows).
In the pelvis, characteristic features include long pelvis with narrowing at the acetabulae, widening of public symphysis and flaring of the ilia. In patients with MPS, the femoral head epiphyses appear normal in early life; however, dysplastic features develop in later life and include disappearance of the femoral head, widening of the femoral neck and a coxa valga deformity. Characteristic features also develop in the hands and include shortening of the metacarpals, small carpal bones (often with some absent) and the inclination of the distal portions of the radius and ulna toward each other (Madelung's deformity).
Magnetic resonance features of brain and spine
Neurological symptoms can be present at both the early and late course of MPS, although more common in the latter, and are caused by deposition of glucosaminoglycans (GAG) in the brain [5]. MRI is the prime imaging technique for detection of central nervous system (CNS) and spinal cord abnormalities [5]. White matter (WM) lesions, dilated perivascular spaces (Figure 1 and 2), hydrocephalus (Figure 6 and 7) and spinal canal stenosis (Figure 4–7) have been described in previous studies [6-10]. Typically, the perivascular spaces are dilated as the result of GAG accumulation, which gives rise to a cribriform appearance in the periventricular WM, corpus callosum and basal ganglia on T1 and T2 weighted images. Occasionally, arachnoid cysts (caused by meningeal GAG deposition) are seen (Figure 7). On T2 weighted and fluid-attenuated inversion-recovery (FLAIR) images, the dilated perivascular spaces are isointense to the cerebrospinal fluid (CSF). Sometimes, the surrounding white matter may show increased signal intensity on FLAIR sequence, representing gliosis, oedema, demyelination or dysmyelination, thus differentiating MPS-related brain changes from normal perivascular spaces. When MR spectroscopy is performed, a broad peak around 3.7 ppm (higher than the chemical shift of myoinositol) may be found, which is considered to represent signals from accumulated GAG [6,10].
Figure 2.
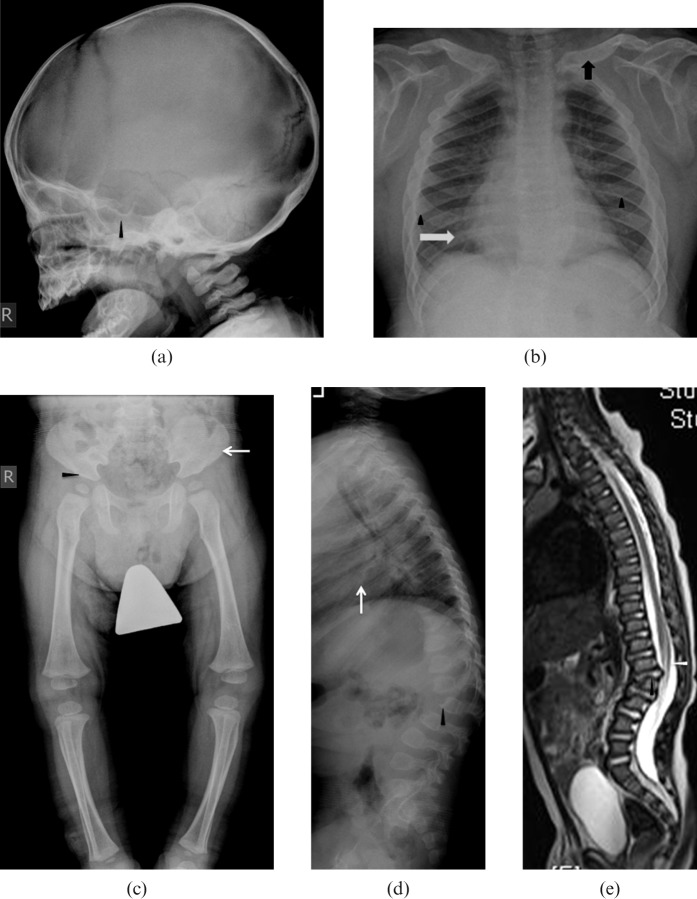
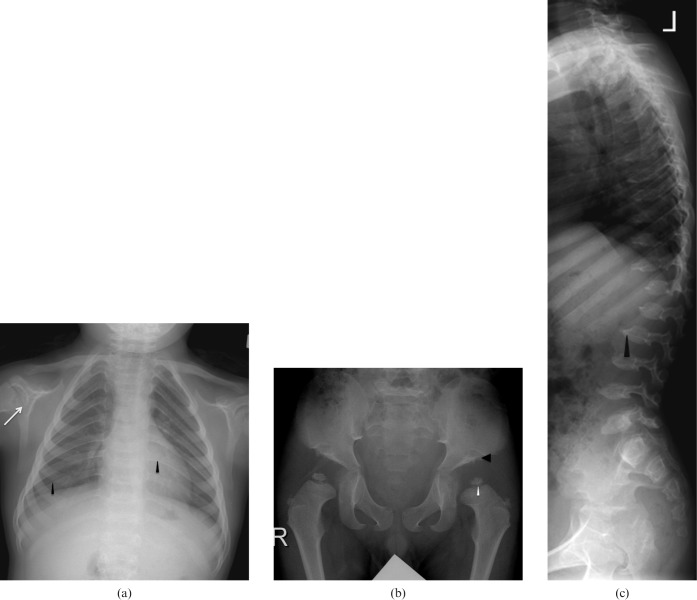
Mucopolysaccharide (MPS) Type I. A 2-year-old male has severe lumbar kyphosis but no neurological deficit on examination. He received double unit cord blood transplant. However, post-transplant, the patient developed severe graft vs host disease (GVHD) and died owing to severe immunosuppression and respiratory failure. (a) Lateral view of skull showing classical features of macrocephaly, J-shaped sella turcica (arrowhead) and platyspondyly (white arrows). (b) Frontal radiograph thorax showing cardiomegaly (white arrow), thick ribs (arrow heads) and short thick clavicles (black arrow). (c) Anteroposterior view of pelvis and thighs showing classical features with flattening of acetabuli (arrowhead) with squaring of iliac blades. (d) Lateral view of spine showing wide ribs (white arrow), thoracolumbar kyphosis, anterior beaking in L2 and L3 bodies (arrowhead). (e) Corresponding T2 weighted sagittal image of the spine showing the same acute kyphosis at the L1–2 level, associated with posterior disc herniation (black arrowhead). There is mild narrowing of spinal canal at this level with indentation onto the anterior thecal space but no significant compression of conus medullaris (white arrowhead).
Figure 6.
Mucopolysaccharide (MPS) Type VI. 12-year-old male suffering from repeated convulsions and on tegretol and epilim. Early in his life, the boy was noticed to have a big head, coarse facial features, a flat nasal bridge, protruding eyes, short stature, lumbar lordosis, hearing impairment and progressively increasing bilateral corneal haziness leading to blindness. He also has bilateral inguinal hernia and multiple joint stiffness including wrists, elbows, hips, knees and ankles. He is currently on enzyme replacement therapy. (a) T2 weighted axial image of brain showing moderate hydrocephalus (black arrow), bi-frontal white matter T2 hyperintensities suggestive of bifrontal demyelination (arrowhead). (b) MR spine T2 weighted sagittal image showing flattened vertebral bodies and disc protrusions at multiple levels (black arrowhead). Spinal canal narrowing and cord indentation is noted at the cervicomedullary junction (white arrowhead). Short segment syringomyelia is present at C4–6 levels (white arrow).
Figure 4.
Mucopolysaccharide (MPS) Type IV. A 6-year-old male has developmental delay and short stature. He has normal intelligence and no neurological deficit. He has received supportive physiotherapy and occupational therapy and is currently in a static course of disease. (a) Frontal chest radiograph shows thick ribs (black arrowhead) and hypoplastic glenoids fossae (white arrow). (b) Anteroposterior radiograph of the pelvis and thighs showing bilateral flared iliac wings, flattened acetabular roofs (black arrowhead) and dysplastic femoral epiphyses (white arrowhead). (c) Lateral radiograph of the spine showing platyspondyly and central beaking of the vertebrae (arrowhead).
Figure 5.
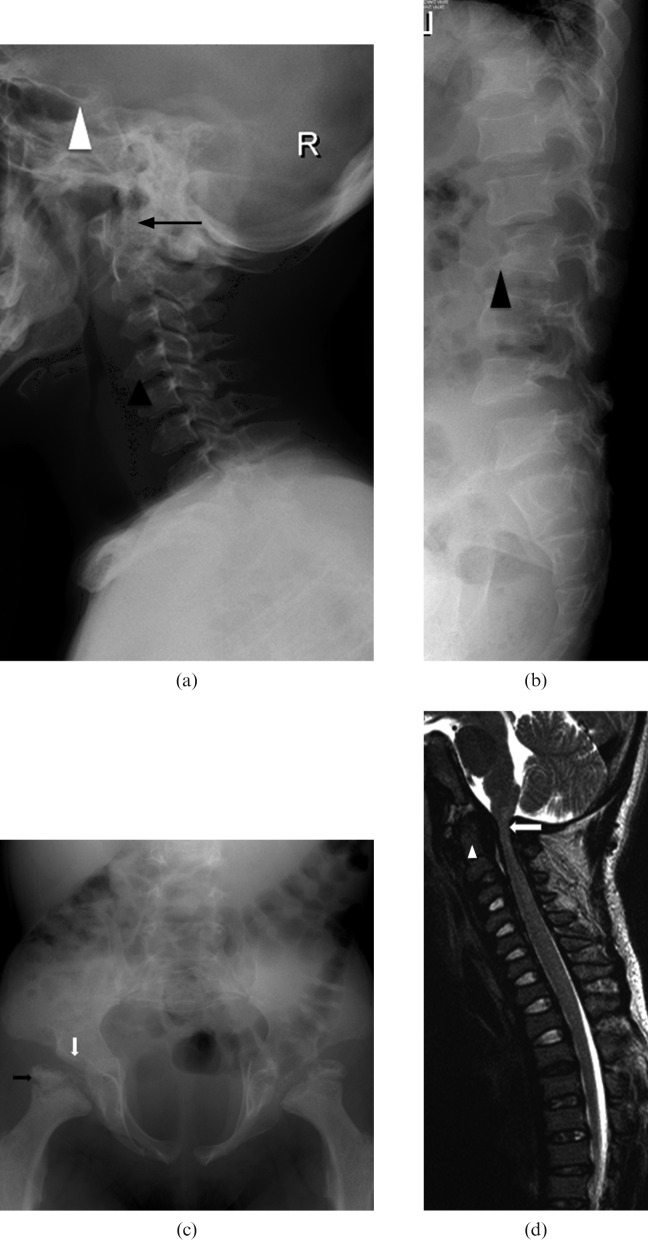
Mucopolysaccharide (MPS) Type VI. A 7-year-old boy has pectus excavatum, fixed flexion contractures of knees (with genu varum), elbows, wrists and the interphalangeal joints (with claw hands) and is suffering from progressive bilateral upper and lower limb weakness. He has bilateral corneal cloudiness with impaired vision. He also has recurrent bilateral otitis media with chronic suppurative middle ear effusion and conductive hearing impairment. He has received cord blood transplant (CBT) from a human leukocyte antigen (HLA)-identical younger brother. Subsequent bone marrow examination and DNA study showed satisfactory engraftment. (a) Lateral radiograph of cervical spine showing J-shaped sella (white arrowhead) dysplastic odontoid process (black arrow). Note the anterior beaking of cervical vertebrae (black arrowhead). (b) Lateral radiograph of lumbar spine showing posterior scalloping and hypoplastic L2 vertebral body (arrowhead). Note the Grade I retrolisthesis of L2 over L3. (c) Radiograph of pelvis showing hypoplastic acetabular roofs (white arrow), bilateral femoral subluxations, elongated pelvic inlet with flared iliac wings and fragmented femoral epiphyses (black arrow). (d) MRI spine T2 weighted sagittal image showing dysplastic odontoid process (arrow head), narrowing of the foramina magnum and cervical canal, which is most severe at the craniocervical junctions (white arrow); however, no myelomalacia or syringomyelia is noted.
Neurological manifestation vs MR features
The relationship between neuroimaging findings and mental dysfunction is controversial. Data from Gabrielli et al [12] showed a correlation between WM alterations and mental retardation. Matheus et al [13] did not find an association between neuroimaging findings and clinical status. In this pictorial review, most of the patients described had a normal IQ despite MR changes in the brain. Our observation was in agreement with the latter's findings.
MPS patients may have cervical myelopathy owing to spinal canal stenosis [5,14], which is more commonly seen in Hunter syndrome at young age. Unusual presentation of paraparesis has been reported [15]. Dysplasia of the odontoid process associated with soft-tissue mass, ligamentous laxity and invagination of the posterior arch of atlas leading to atlantoaxial subluxation are the other main causes of compromise of the spinal cord and cervical myelopathy [16]. In this pictorial review, the three cases of MPS Type VI showed a narrowing of the spinal canal at C1 and C2, two of which had severe spinal canal stenosis leading to compression of the cordomedullary junction accompanied by dysplastic odontoid process (Figure 5) and syringomyelia (Figure 6). These patients presented with progressive four limb weakness, multiple joint contractures and joint stiffness, respectively. The latter case had mild narrowing at C1 and C2 without significant cord compression. Instead, this patient presented with gibbus causing canal narrowing at L1 to L3 leading to compression of the cauda equina (Figure 7). No spinal canal stenosis was identified in the single case of Hunter's disease. In MPS patients with myelopathy, the signs and symptoms do not always correlate with the degree of cord compression. Instead, in most circumstances, the neurological deficits manifesting clinically are usually less severe than suggested by MRI [16].
Therapeutic interventions for MPS
Nowadays, therapeutic interventions in MPS aim at correction of enzyme activity, which can be achieved by bone marrow transplantation (BMT), peripheral blood haematopoietic cell transplantation (PBHCT) and umbilical cord blood transplantation (CBT). The transplantation options are mainly used in MPS Type I and VI [17-20]. BMT is an effective treatment that corrects the enzyme defect in white blood cells. The natural history of the disease is modified, and fatal complications of MPS are prevented, so that life expectancy is prolonged. However, the main limitation of allogeneic BMT is the availability of suitable human leukocyte antigen (HLA)-compatible donors.
In PBHCT, since the concentration of haematopoietic stem cells is very low in peripheral circulating blood, recombinant haematopoietic growth factors should be administrated to patients or donors to downregulate the adhesion molecules on the CD34 cells to release them into the peripheral blood. The major disadvantage of PBSCT is the increased incidence of graft vs host disease (GVHD) owing to higher T-cell load.
CBT is increasingly used as an alternative source of haematopoietic stem cells for transplantation. Cord blood has the advantage of the relative immaturity of cells and immune system at birth [21,22]. Haematopoietic progenitors from cord blood are enriched by the most primitive stem cells that have a naive phenotype [22,23]. These properties of haematopoietic stem cells from cord blood give them a proliferative advantage together with less frequent and less severe GVHD. The other advantages of CBT are the relative ease of procurement, availability for immediate use and absence of risks to the donors.
Enzyme-replacement therapy is an alternative treatment for MPS Type I, II and VI [25]. Kakkis et al [26] have shown clinical and biochemical improvements in MPS Type I following intravenous administration of recombinant human a-l-iduronidase. Although this is not the definitive treatment, the introduction of enzyme-replacement therapy has helped to prolong the life expectancy of MPS patients. This also has an impact on the imaging strategy for MPS patients as the incidence of myelopathy is expected to increase with prolonged survival, which also creates an increasing demand for pre-operative MR assessment and neurological assessment of MPS patients.
Conclusion
This pictorial review illustrates the typical imaging features of different types of MPS. With the recent advances in therapeutic interventions and prolonged life expectancy in MPS patients, the characteristic radiological features are expected to be more frequently encountered by radiologists during daily practice.
References
- 1.Morquio L. Sur une forme de dystrophie osseuse familiale. Archives de médicine des infants 1929;32:129–35 [Google Scholar]
- 2.Goidanich IF, Lenzi L. Morquio- Ullrich Disease. A new mucopolysaccharidosis. J Bone Joint Surg Am 1964;46:734–46 [PubMed] [Google Scholar]
- 3.Langer LO., Jr Spondyloepiphysial dysplasia tarda. Hereditary chondrodysplasia with characteristic vertebral configuration in the adult. Radiology 1964;82:833–9 [DOI] [PubMed] [Google Scholar]
- 4.Langer LO, Jr, Carey LS. The roentgenographic features of the KS mucopolysaccharidosis of Morquio (Morquio-Brailsford's disease). Am J Roentgenol Radium Ther Nucl Med 1966;97:1–20 [DOI] [PubMed] [Google Scholar]
- 5.Kaendler S, Bockenheimer S, Grafin Vitzthum H, Galow W. Cervical myelopathy in mucopolysaccharidosis type II (Hunter's syndrome). Neuroradiologic, clinical and histopathologic findings. Dtsch Med Wochenschr 1990;115:1348–52 [DOI] [PubMed] [Google Scholar]
- 6.Murata R, Nakajima S, Tanaka A, Miyagi N, Matsuoka O, Kogame S, et al. MR imaging of the brain in patients with mucopolysaccharidosis. AJNR Am J Neuroradiol 1989;10:1165–70 [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C, Dineen TE, Brack M, Kirsch JE, Runge VM. The mucopolysaccharidoses: characterization by cranial MR imaging. AJNR Am J Neuroradiol 1993;14:1285–92 [PMC free article] [PubMed] [Google Scholar]
- 8.Shimoda-Matsubayashi S, Kuru Y, Sumie H, Ito T, Hattori N, Okuma Y, et al. MRI findings in the mild type of mucopolysaccharidosis II (Hunter's syndrome). Neuroradiology 1990;32:328–30 [DOI] [PubMed] [Google Scholar]
- 9.Parsons VJ, Hughes DG, Wraith JE. Magnetic resonance imaging of the brain, neck and cervical spine in mild Hunter's syndrome (mucopolysaccharidoses type II). Clin Radiol 1996;51:719–23 [DOI] [PubMed] [Google Scholar]
- 10.Seto T, Kono K, Morimoto K, Inoue Y, Shintaku H, Hattori H, et al. Brain magnetic resonance imaging in 23 patients with mucopolysaccharidoses and the effect of bone marrow transplantation. Ann Neurol 2001;50:79–92 [DOI] [PubMed] [Google Scholar]
- 11.Takahashi Y, Sukegawa K, Aoki M, Ito A, Suzuki K, Sakaguchi H, et al. Evaluation of accumulated mucopolysaccharides in the brain of patients with mucopolysaccharidoses by (1)H-magnetic resonance spectroscopy before and after bone marrow transplantation. Pediatr Res 2001;49:349–55 [DOI] [PubMed] [Google Scholar]
- 12.Gabrielli O, Polonara G, Regnicolo L, Petroni V, Scarabino T, Coppa GV, et al. Correlation between cerebral MRI abnormalities and mental retardation in patients with mucopolysaccharidoses. Am J Med Genet A 2004;125A:224–31 [DOI] [PubMed] [Google Scholar]
- 13.Matheus MG, Castillo M, Smith JK, Armao D, Towle D, Muenzer J. Brain MRI findings in patients with mucopolysaccharidosis types I and II and mild clinical presentation. Neuroradiology 2004;46:666–72 [DOI] [PubMed] [Google Scholar]
- 14.Kopits SE. Orthopedic complications of dwarfism. Clin Orthop Relat Res 1976:153–79 [PubMed] [Google Scholar]
- 15.Kelly TE. The mucopolysaccharidoses and mucolipidoses. Clin Orthop Relat Res 1976:116–33 [PubMed] [Google Scholar]
- 16.Hughes DG, Chadderton RD, Cowie RA, Wraith JE, Jenkins JP. MRI of the brain and craniocervical junction in Morquio's disease. Neuroradiology 1997;39:381–5 [DOI] [PubMed] [Google Scholar]
- 17.Hobbs JR, Hugh-Jones K, Barrett AJ, Byrom N, Chambers D, Henry K, et al. Reversal of clinical features of Hurler's disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet 1981;2:709–12 [DOI] [PubMed] [Google Scholar]
- 18.Peters C, Balthazor M, Shapiro EG, King RJ, Kollman C, Hegland JD, et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood 1996;87:4894–902 [PubMed] [Google Scholar]
- 19.Peters C, Shapiro EG, Anderson J, Henslee-Downey PJ, Klemperer MR, Cowan MJ, et al. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group. Blood 1998;91:2601–8 [PubMed] [Google Scholar]
- 20.Krivit W, Pierpont ME, Ayaz K, Tsai M, Ramsay NK, Kersey JH, et al. Bone-marrow transplantation in the Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI). Biochemical and clinical status 24 months after transplantation. N Engl J Med 1984;311:1606–11 [DOI] [PubMed] [Google Scholar]
- 21.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A 1989;86:3828–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madrigal JA, Cohen SB, Gluckman E, Charron DJ. Does cord blood transplantation result in lower graft-vs-host disease? It takes more than two to tango. Hum Immunol 1997;56:1–5 [DOI] [PubMed] [Google Scholar]
- 23.Harris DT, Schumacher MJ, Locascio J, Besencon FJ, Olson GB, DeLuca D, et al. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A 1992;89:10006–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garban F, Ericson M, Roucard C, Rabian-Herzog C, Teisserenc H, Sauvanet E, et al. Detection of empty HLA class II molecules on cord blood B cells. Blood 1996;87:3970–6 [PubMed] [Google Scholar]
- 25.Crawley AC, Brooks DA, Muller VJ, Petersen BA, Isaac EL, Bielicki J, et al. Enzyme replacement therapy in a feline model of Maroteaux-Lamy syndrome. J Clin Invest 1996;97:1864–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med 2001;344:182–8 [DOI] [PubMed] [Google Scholar]