Abstract
The purpose of this study was to evaluate the high-resolution computed tomographic (HRCT) findings of five adult patients (either immunocompromised or immunocompetent) with herpes simplex virus (HSV) pneumonia. We retrospectively assessed HRCT images of 5 patients (all male patients, age range 39–70 years; mean 62 years) with HSV pneumonia. The specific pathological findings that allowed for a definite diagnosis of HSV pneumonia included the presence of intranuclear inclusion bodies on haematoxylin and eosin staining, or positive immunohistochemical staining. High-resolution CT scans (HiSpeed Advantage or LightSpeed QX/i, GE Healthcare) using 1- or 1.25-mm collimation at 10-mm intervals without intravenous contrast medium injection were assessed, in particular for the presence and distribution of parenchymal abnormalities including ground-glass attenuation, airspace consolidation, nodules and interlobular septal thickening. In two patients, pathological specimens were obtained from open lung biopsy or bronchoscopic biopsy, and were correlated with HRCT findings. Three HRCT patterns of pulmonary abnormalities were identified in our series of HSV pneumonia: predominant areas of diffuse or multifocal ground-glass attenuation, predominant areas of multifocal peribronchial consolidations, and a mixed pattern of both. Histopathologically, areas of ground-glass attenuation seen on HRCT corresponded to diffuse alveolar damage in one patient who underwent open lung biopsy. No specific differences in HRCT findings were seen between the immunocompromised and the immunocompetent patients. In patients suspected of having an acute lower respiratory infection, whether immunocompromised or immunocompetent, a possibility of HSV pneumonia can be included in differential diagnoses when diffuse or multifocal areas of ground-glass attenuation and/or consolidations are seen on HRCT.
Herpes simplex virus (HSV) pneumonia can occur in patients with complications of immunosuppression, airway injury from intubation or smoke inhalation [1, 2]. Pathologically, HSV infection can have three main forms of pulmonary involvement: necrotising tracheobronchitis, necrotising pneumonia and interstitial pneumonitis [3]. The interstitial pneumonitis form of HSV pulmonary infection is characterised by diffuse alveolar damage consisting of interstitial lymphocytic infiltration, alveolar haemorrhage and hyaline membrane formation [4].
A few reports have previously described the high-resolution CT (HRCT) findings of HSV pneumonia in immunocompromised patients [1, 2], including areas of ground-glass attenuation, airspace consolidation and small centrilobular nodules.
The purpose of this study was to evaluate the HRCT findings of five adult patients (either immunocompromised or immunocompetent) with HSV pneumonia.
Methods and materials
We reviewed retrospectively HRCT images of five patients with HSV pneumonia. Patients (all male patients, age range 39–70 years; mean 62 years) were collected from the file archives of the department of pathology in our institute. Approval from the institutional review board was not needed for review of radiological images and pathological reports in our institute.
The specific pathological findings that allowed for a definite diagnosis of HSV pneumonia included the presence of intranuclear inclusion bodies on haematoxylin and eosin staining, or positive immunohistochemical staining. Immunohistochemical staining was accomplished by incubating the test material on a microscope slide with a 1:100 dilution of monoclonal rabbit anti-HSV solution recognising serotypes 1 and 2 (DAKO, Copenhagen, Denmark) for 30 min at 37°C [5].
In all patients, histopathological diagnoses were made using haematoxylin and eosin (n = 4) or immunohistochemical (n = 2) staining of the specimens obtained from sputum (n = 1), bronchoalveolar lavage (BAL) (n = 2), bronchoscopic biopsy (n = 1) and open lung biopsy (n = 1). Viral cultures of blood or BAL fluid for cytomegalovirus, adenovirus, influenza, parainfluenza, respiratory syncytial virus and HSV were negative in all patients. No bacterial or fungal organisms were isolated from the specimen.
Our study population comprised three immunocompromised patients and two immunocompetent patients. Of the three immunocompromised patients, one patient had received an allogeneic bone marrow transplant for myelodysplastic syndrome 15 months previously, the other had non-Hodgkin's lymphoma, and the remaining one had undergone steroid therapy for idiopathic pulmonary fibrosis before the onset of the HSV infection. The two immunocompetent patients had been previously healthy before the onset of HSV infection and were human immunodeficiency virus-negative with no history of smoking, surgery, malignancy, diabetes, steroid therapy, intravenous drug abuse or previous chest disease. Patients presented with fever (n = 3), dyspnoea (n = 3), cough (n = 2) and sputum (n = 1). At peak presentation, four of five patients had severe leukocytosis (mean 19 000 ± 3000 μl−1), and one had normal blood leukocyte count (7000 μl−1) (normal range for blood leukocyte count in our institute 3800–10580 μl−1). Despite antiviral therapy (ganciclovir or acyclovir) with conservative management including empirical antibiotic therapy and/or mechanical ventilation (n = 1), all five patients died of the infection (mean survival after pathological diagnosis 12 days; range 7–19 days).
High-resolution CT scans of the thorax were obtained in all five patients using helical CT scanners (HiSpeed Advantage or LightSpeed QX/i, GE Healthcare, Milwaukee, WI) using 1- or 1.25-mm collimation at 10-mm intervals without intravenous contrast medium injection. All CT data were reconstructed using a high spatial frequency algorithm. The time intervals between a CT examination and a pathological diagnosis were 2–15 days (median, 5 days).
Two thoracic radiologists, one with 8 years' experience and the other 3 years' experience in thoracic radiology, reviewed the chest CT scans, and decisions on findings were reached by consensus. High-resolution CT scans were assessed particularly for the presence and distribution of parenchymal abnormalities including ground-glass attenuation, airspace consolidation, nodules and interlobular septal thickening. Ground-glass attenuation was defined as a hazy increase in lung attenuation with no obscuration of underlying vessels. Airspace consolidation was defined as an area of opacification that obscured the underlying vessels. The anatomic distribution of parenchymal abnormalities was classified as subpleural, central or random; lobular, subsegmental/segmental or lobar in the axial plane; upper, lower or random in the longitudinal plane; peribronchial or random. Any other associated findings were also evaluated including the presence of pleural effusion and mediastinal or hilar lymphadenopathy.
In two patients, pathological specimens were obtained from open lung biopsy or bronchoscopic biopsy, and were evaluated by a lung pathologist with five years of experience in pulmonary pathology with CT–pathological correlation.
Results
The HRCT findings of HSV pneumonia included bilateral areas of ground-glass attenuation (n = 5), airspace consolidation (n = 5) and interlobular septal thickening (n = 3) (Table 1, Figures 1–4). Areas of ground-glass attenuation were either diffuse (n = 3) or multifocal (n = 2) in distribution, and showed mainly a random distribution in both the longitudinal (n = 5) and the axial (n = 4) planes. Airspace consolidation was predominantly lobular (n = 3) or subsegmental/segmental (n = 2) in distribution. Small, bilateral pleural effusions were seen in one patient. Right paratracheal lymphadenopathy (10–12 mm in short axis) was seen in one patient.
Table 1. High-resolution CT findings of five patients with herpes simplex virus pneumonia.
| Parenchymal abnormality | Laterality |
Lung zone |
Distribution |
|||||||
| Bilateral | Unilateral | Upper | Lower | Random | Central | Subpleural | Random | Peribronchial | Random | |
| Ground-glass attenuation (n = 5; 100%) | 5 | 0 | 0 | 0 | 5 | 0 | 1 | 4 | 1 | 4 |
| Consolidation (n = 5; 100%) | 5 | 0 | 2 | 1 | 2 | 0 | 1 | 4 | 2 | 3 |
| Interlobular septal thickening (n = 3; 60%) | 3 | 0 | 1 | 0 | 2 | 0 | 2 | 1 | 0 | 3 |
Small, bilateral pleural effusions were seen in one patient.
Figure 1.
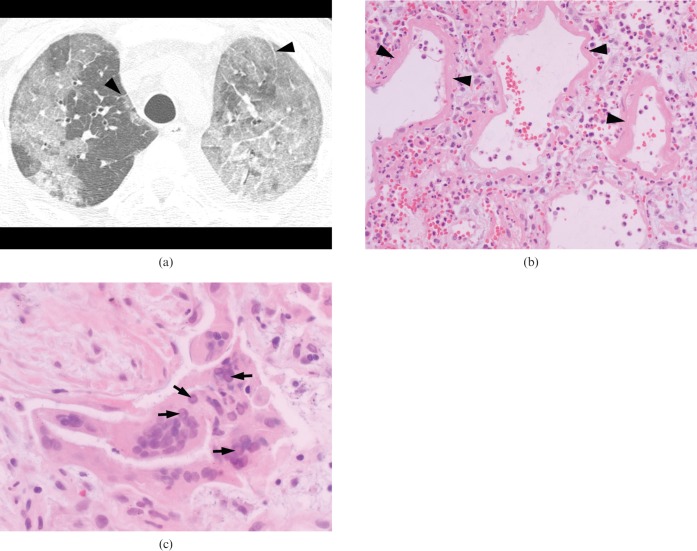
A 39-year-old immunocompromised man with myelodysplastic syndrome who developed herpes simplex virus pneumonia 15 months after allogeneic bone marrow transplantation. (a) High-resolution (1.0-mm collimation) CT scans obtained at levels of the aortic arch shows predominant areas of diffuse ground-glass attenuation in both lungs. Note associated interlobular septal thickening (arrowheads). (b) Photomicrograph of pathological specimen obtained by open lung biopsy shows interstitial fibroblastic proliferation and hyaline membrane formation (arrowheads), suggesting a late exudative or proliferative phase of diffuse alveolar damage (haematoxylin and eosin, ×200). (c) Higher magnification photomicrograph of pathological specimen demonstrates alveolar lining cells containing an intranuclear inclusion (arrows), consistent with herpes viral infection (haematoxylin and eosin, ×400).
Figure 4.
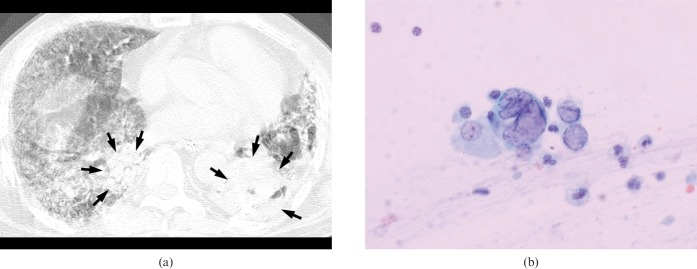
A 65-year-old immunocompromised man on steroid therapy for idiopathic pulmonary fibrosis who developed herpes simplex virus pneumonia. (a) High-resolution (1.0-mm collimation) CT scan obtained at the level of the inferior pulmonary vein shows a mixed pattern of diffuse ground-glass attenuation and subsegmental/segmental peribronchial consolidations (arrows). Motion artefact is seen because the patient was dyspnoeic and mechanically ventilated during CT scanning. (b) Photomicrograph of sputum smear cytological specimen demonstrates mononuclear and multinuclear epithelial cells with intranuclear inclusions, consistent with herpes viral infection (haematoxylin and eosin, ×400).
Overall, three patterns of pulmonary abnormalities were identified on HRCT: predominant areas of diffuse or multifocal ground-glass attenuation (n = 3) (Figure 1, 2), predominant areas of peribronchial consolidation (n = 1) (Figure 3) and a mixed pattern of both (n = 1) (Figure 4). In three immunocompromised patients, patterns of pulmonary abnormalities were either predominant areas of ground-glass attenuation (n = 2) or a mixed pattern of ground-glass attenuation and peribronchial consolidation (n = 1). In two immunocompetent patients, there were either predominant areas of ground-glass attenuation (n = 1) or predominant areas of peribronchial consolidation (n = 1).
Figure 2.
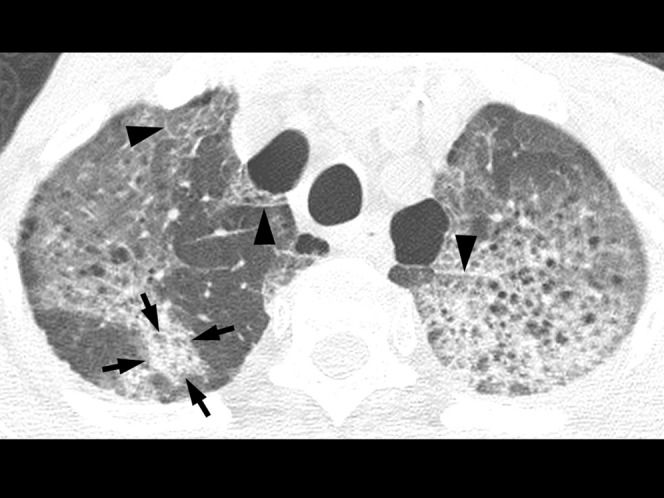
A 67-year-old immunocompetent man with herpes simplex virus pneumonia. High-resolution (1.0-mm collimation) CT scans obtained at levels of great vessels shows predominant areas of diffuse ground-glass attenuation. Note an area of lobular consolidation (arrows) and interlobular septal thickening (arrowheads). Centrilobular emphysema is seen in the underlying lungs.
Figure 3.
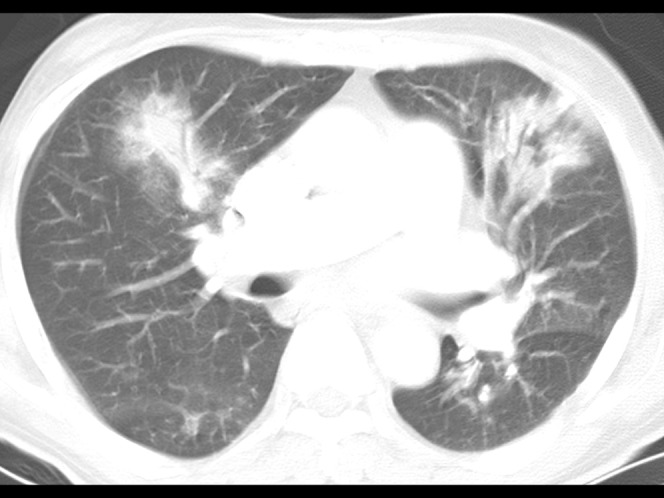
A 67-year-old immunocompetent man with herpes simplex virus pneumonia. Conventional (5.0-mm collimation) and high-resolution (1.0-mm collimation) CT scans obtained at levels of the bronchus intermedius shows a pattern of multifocal peribronchial consolidations surrounded by small areas of ground-glass attenuation in both lungs. Air bronchograms are seen within the consolidations.
On CT–pathological correlation of the specimen obtained from open lung biopsy in one patient, areas of ground-glass attenuation observed on HRCT corresponded histopathologically to areas of interstitial fibroblastic proliferation with inflammatory cell infiltration, intra-alveolar fibrinous exudate and hyaline membrane formation on haematoxylin and eosin staining. This suggested a late exudative or proliferative phase of diffuse alveolar damage (Figure 1b). Histopathological examination also demonstrated type II pneumocyte hyperplasia, giant cell formation and alveolar lining cells containing intranuclear eosinophilic inclusions, which were characteristic cytopathological changes of herpes viral infection (Figure 1c). Immunohistochemical staining of the pathological specimen also showed HSV-positive cells. In the other patient, a bronchoscopic examination showed a focal necrotic mucosal erosion in the mid-portion of the left main bronchus, and the mucosal biopsy specimen revealed acute suppurative and necrotising inflammation on haematoxylin and eosin staining. Immunohistochemical staining of the pathological specimen confirmed HSV-positive cells. In the remaining three patients, cytological examinations of specimens obtained from BAL (n = 2) or sputum (n = 1) demonstrated mononuclear and multinuclear epithelial cells containing an intranuclear inclusion on haematoxylin and eosin staining, which were consistent with herpes viral infection (Figure 4b).
Discussion
HSV pneumonia has been known to be mostly caused by HSV type 1, and rarely by HSV type 2 [1, 2]. In a CT study of isolated HSV pneumonia in four patients, Aquino et al [1] reported that the CT findings of HSV type 1 pneumonia were predominant areas of ground-glass attenuation with multifocal, segmental or subsegmental, or non-dependent distribution accompanied with some areas of consolidation. In a case report of HSV type 2 pneumonia after bone marrow transplantation, Gasparetto et al [2] reported that the HRCT findings mainly consisted of focal areas of consolidation, small centrilobular nodules, and areas of ground-glass attenuation.
From the review of the two previous reports [1, 2], the HRCT findings of HSV type 1 pneumonia were similar to those of HSV type 2 pneumonia, mainly comprising areas of ground-glass attenuation and consolidation. In our study of HSV pneumonia in five patients, although the organism was not specifically subtyped, the HRCT finding was predominant areas of diffuse or multifocal ground-glass attenuation (n = 3), or predominant areas of peribronchial consolidation (n = 1), or a mixed pattern of both (n = 1). These HRCT findings were similar to those of the previous studies.
According to Aquino [1] and Gasparetto [2], small and larger nodules were seen in one patient and two patients, respectively. They thought that those nodules corresponded to a coexisting fungal pneumonia or multiple haemorrhagic nodules. In our study, however, no small or larger nodules were seen at HRCT.
HSV pneumonia has been seen most commonly in patients with organ transplantation or cell-mediated immunity deficiency, but can also affect immunocompetent patients whose airways have been traumatised from intubation or smoke inhalation [1, 2]. To our knowledge, the HRCT findings of HSV pneumonia have not been compared between immunocompromised and immunocompetent patients. Although the number of patients was small in our series, consisting of three immunocompromised and two immunocompetent patients, the two groups did not show specific differences in HRCT findings, both manifesting as predominant areas of ground-glass attenuation and/or consolidations.
For diffuse or multifocal areas of ground-glass attenuation seen in patients with HSV pneumonia, many other diseases can be included in the differential diagnoses based on the patient's immune status. An acute process of ground-glass attenuation in an organ transplant recipient or in a patient with acquired immunodeficiency syndrome can suggest acute rejection from lung transplantation, drug reaction, Pneumocystis jiroveci pneumonia or cytomegalovirus pneumonia [6], as well as HSV pneumonia. In an immunocompetent patient, on the other hand, acute lung disease with ground-glass attenuation can represent diffuse pulmonary haemorrhage, pulmonary oedema, acute eosinophilic pneumonia and atypical pneumonias caused by Mycoplasma pneumoniae, influenza or varicella-zoster virus [7]. Areas of ground-glass attenuation are the most common HRCT finding in adult viral pneumonia including cytomegalovirus, influenza virus, varicella-zoster virus and adenovirus, and correspond histopathologically to areas of diffuse alveolar damage [8, 9]. These HRCT and histopathological results were also applicable to HSV pneumonias in our series.
Our study has several limitations. Its first and major limitation was that the sample size was small, which was mainly due to a difficulty in a specific confirmative diagnosis of viral pneumonia. Second, we failed to get a positive viral culture result for HSV and to identify a type-specific HSV. However, HSV pneumonia can be confidently diagnosed based on the presence of positive intranuclear inclusion bodies on haematoxylin and eosin staining or positive immunohistochemical staining for HSV [5].
In conclusion, three HRCT patterns of pulmonary abnormalities were identified in our series of HSV pneumonia: predominant areas of diffuse or multifocal ground-glass attenuation, predominant areas of peribronchial consolidation and a mixed pattern of both. Histopathologically, areas of ground-glass attenuation seen on HRCT corresponded to diffuse alveolar damage in one patient who underwent open lung biopsy. No specific differences in HRCT findings were seen between the immunocompromised and immunocompetent patients. In patients suspected of having an acute lower respiratory infection, whether immunocompromised or immunocompetent, a possibility of HSV pneumonia can be included in differential diagnoses when diffuse or multifocal areas of ground-glass attenuation and/or consolidations are seen on HRCT.
References
- 1.Aquino SL, Dunagan DP, Chiles C, Haponik EF. Herpes simplex virus 1 pneumonia: patterns on CT scans and conventional chest radiographs. J Comput Assist Tomogr 1998;22:795–800 [DOI] [PubMed] [Google Scholar]
- 2.Gasparetto EL, Escuissato DL, Inoue C, Marchiori E, Muller NL. Herpes simplex virus type 2 pneumonia after bone marrow transplantation: high-resolution CT findings in 3 patients. J Thorac Imaging 2005;20:71–3 [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Colby TV, Koss MN, Rosado-de-Christenson ML, Muller NL, King TE., Jr Non-neoplastic disorders of the lower respiratory tract. Washington DC, USA: American Registry of Pathology, 2002 [Google Scholar]
- 4.Ramsey PG, Fife KH, Hackman RC, Meyers JD, Corey L. Herpes simplex virus pneumonia: clinical, virologic, and pathologic features in 20 patients. Ann Intern Med 1982;97:813–20 [DOI] [PubMed] [Google Scholar]
- 5.Oda Y, Okada Y, Katsuda S, Nakanishi I. Immunohistochemical study on the infection of herpes simplex virus, human cytomegalovirus, and Epstein-Barr virus in secondary diffuse interstitial pneumonia. Hum Pathol 1994;25:1057–62 [DOI] [PubMed] [Google Scholar]
- 6.Fraser RS, Muller NL, Colman N, Pare PD. Diagnosis of diseases of the chest. Philadelphia, PA: W.B.Saunders, 1999 [Google Scholar]
- 7.Tanaka N, Matsumoto T, Kuramitsu T, Nakaki H, Ito K, Uchisako H, et al. High resolution CT findings in community-acquired pneumonia. J Comput Assist Tomogr 1996;20:600–8 [DOI] [PubMed] [Google Scholar]
- 8.Kim EA, Lee KS, Primack SL, Yoon HK, Byun HS, Kim TS, et al. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics 2002;22:S137–49 [DOI] [PubMed] [Google Scholar]
- 9.Chong S, Lee KS, Kim TS, Chung MJ, Chung MP, Han J. Adenovirus pneumonia in adults: radiographic and high-resolution CT findings in five patients. AJR Am J Roentgenol 2006;186:1288–93 [DOI] [PubMed] [Google Scholar]