Abstract
The purpose of this study was to assess the potential of boron neutron capture therapy (BNCT), with a 6-h infusion of the boron carrier l-boronophenylalanine as a fructose preparation (BPA-f), as first-line radiotherapy for newly diagnosed glioblastoma multiforme (GBM). Patient survival data from a Phase II study using BNCT were compared with retrospective data from the two arms of a Phase III study using conventional radiotherapy (RT) in the reference arm and using RT plus concomitant and adjuvant medication with temozolomide (TMZ) in the experimental arm, and were also compared with small subgroups of these patients for whom the methylation status of the MGMT (O6-methylguanine–DNA methyltransferase) DNA repair gene was known. Differences in the baseline characteristics, salvage therapy after recurrence and levels of severe adverse events were also considered. The results indicate that BNCT offers a treatment that is at least as effective as conventional RT alone. For patients with an unmethylated MGMT DNA repair gene, a possible clinical advantage of BNCT over RT/TMZ was suggested. BNCT is a single-day treatment, which is of convenience to patients, with mild side effects, which would offer an initial 6 weeks of good-quality life during the time when patients would otherwise be undergoing daily treatments with RT and TMZ. It is suggested that the use of BNCT with a 6-h infusion of BPA-f should be explored in a stratified randomised Phase II trial in which patients with the unmethylated MGMT DNA repair gene are offered BNCT in the experimental arm and RT plus TMZ in the reference arm.
Boron neutron capture therapy (BNCT) is an experimental radiotherapy that, to date, has mostly been applied to treating patients with newly diagnosed glioblastoma multiforme (GBM). Several hundred GBM patients have been treated in Phase I and Phase II studies in Europe, the USA and Japan, and survival times similar to those obtained with standard radiotherapy (RT) have been reported from several of these studies [1]. No randomised trial in which BNCT is compared with standard therapies has yet been undertaken.
Proper assessment of the results from these Phase II trials has been hampered by the lack of information on baseline characteristics of the patient populations, both in reports of the BNCT studies and in reports on other therapies, notably standard fractionated photon therapy (RT), to which the results from BNCT should ultimately be compared. The recursive partitioning analysis (RPA) of prognostic factors by Curran et al [2] established a method to characterise patient status prior to treatment, useful both for the planning of clinical studies and for the assessment of the significance of the results from clinical studies. The reliability and usefulness of the RPA method has been verified by Mirimanoff et al [3] by applying it to data obtained from a randomised Phase III study [4], in which RT with concomitant and adjuvant temozolomide (RT/TMZ) was offered to 287 patients with newly diagnosed GBM in the experimental arm and RT only was offered to 286 patients in the reference arm. The median survival times (MSTs) from randomisation for patients in RPA Classes III, IV and V were 21, 16 and 10 months, respectively, in the experimental arm and 15, 13 and nine months, respectively, in the reference arm [3]. Thus, the survival advantage provided by TMZ medication decreases monotonically as the RPA class increases from III to V, and is only marginal for patients in RPA Class V. The value of prognostic factors in the prediction of outcome for newly diagnosed GBM has recently been reassessed and extended [5], particularly with respect to the importance of the methylation status of the MGMT (O6-methylguanine–DNA methyltransferase) DNA repair gene on patient survival when using RT alone or in combination with TMZ [6].
In view of the large difference in patient survival between different RPA classes and of the fact that extension of the MST by the order of two to three months is considered clinically significant [4], comparisons of survival times from BNCT studies in which the pre-treatment status of the patient population is not accounted for, as has been the case in the past [7, 8], are not very useful.
A detailed comparison of two BNCT studies, in which the baseline characteristics of the patient populations where available was reported recently [9], namely the Phase II study at Studsvik using a 6-h infusion of l-boronophenylalanine (l-BPA) as the fructose preparation BPA-f, and the Phase I/II study at Brookhaven National Laboratory (BNL) using a 2-h infusion of BPA-f. A significant survival benefit of the prolonged infusion was observed. The Studsvik study represents the state-of-the-art in the application of BNCT to newly diagnosed GBM and is therefore used in the present assessment of the efficacy of BNCT relative to present standard treatment.
A comparison of the results from the Phase II study at Studsvik with the results from the Stupp et al Phase III randomised trial with RT/TMZ [3, 4], for which the baseline characteristics of the patients are available in both cases and the eligibility criteria were comparable, makes it possible to derive useful information on the relative efficacy of BNCT. Although the results of such retrospective comparisons have to be viewed with caution, they are useful for the development of hypothesis for future research. This possibility is utilised in the present report.
Methods and materials
The updated European Organisation for Research and Treatment of Cancer (EORTC) data files from the original study by Stupp et al [4], reported previously in publications by Mirimanoff et al [3], Gorlia et al [5] and Hegi et al [6], and the updated data from the Studsvik BNCT study [9] were used in the present analysis. The clinical observations were compared using two approaches, namely univariate analyses based on the results for patient survival from Kaplan–Meier curves for BNCT vs either RT or RT/TMZ and the Cox proportional hazard model, which was used to adjust for factors such as the extent of initial surgery, age, World Health Organization (WHO) performance status and RPA class at the time of diagnosis. In a retrospective study of this type, caution is warranted, particularly in the case of parameters that are to some degree subjective, such as the designation of WHO performance status and RPA class. In the original study by Stupp et al [4], a total of 85 centres in 15 countries referred patients to the study. The WHO performance status of these patients was determined by the referring centres, and RPA class was computed. In the case of the BNCT study, patients were referred from six centres in Sweden. The WHO performance status was determined by the referring centre, and the RPA class was assessed centrally by the medical team at Studsvik prior to treatment. The WHO and the RPA rankings were consistent on a patient-by-patient basis, providing a degree of validation. In addition to the total patient populations, patients identified as RPA Class V were analysed separately. Also, patients for whom the methylation status of the MGMT DNA repair gene was known in the Phase III trial [6] were compared separately with the full cohort of patients in the BNCT study for whom the status of the MGMT DNA repair gene was not known.
In this study, survival was measured from the time of initial surgery. This is different from that reported in the original publications, where survival was measured either from the time of randomisation in the Phase III study [3, 5, 6] or from time of BNCT [8]. The statistical methods were the same as those originally used for the analysis of the Phase III study [3], where details may be found.
The patient characteristics and medical interventions in the two arms of the trial as published by Mirimanoff et al [3] and as published in the BNCT study [9] are given in Table 1. The median age of the patients in the BNCT study was slightly lower, and the fraction of patients with initial surgery was slightly higher, than indicated by Mirimanoff et al [3]. However, the pre-treatment performance status was much worse in the BNCT study, in terms of both RPA class and WHO grade. The fraction of patients offered salvage TMZ at recurrence was similar in the BNCT and RT groups and much higher than for patients initially offered RT with concomitant and adjuvant TMZ. Other forms of salvage chemotherapy were offered less frequently after BNCT, and the fraction of patients not offered any chemotherapy at all was similar in the three groups. Salvage surgery was offered less frequently in the BNCT study (22% vs 7% of cases). Cases of retreatment by radiotherapy were low in all groups.
Table 1. Baseline patient characteristics and salvage therapies for patients treated with radiotherapy (RT), RT/temozolomide (TMZ) and boron neutron capture therapy (BNCT).
| Parameter | RT | RT/TMZ | BNCT |
| Number of patients | 286 | 287 | 29 |
| Age, median (range) (years) | 57 (23–71) | 56 (19–70) | 53 (28–69) |
| Initial surgery (%) | |||
| Resection | 84 | 83 | 90 |
| Biopsy only | 16 | 17 | 10 |
| WHO performance status (%) | |||
| 0 | 39 | 40 | 10 |
| 1 | 49 | 47 | 66 |
| 2 | 12 | 13 | 24 |
| RPA class (%) | |||
| III | 14 | 15 | 14 |
| IV | 52 | 53 | 21 |
| V | 34 | 32 | 65 |
| Average RPA class | 3.8 | 3.7 | 4.5 |
| Salvage chemotherapy (%)* | |||
| Temozolomide | 56 | 23 | 59 |
| Other chemotherapy | 13 | 29 | 3 |
| No chemotherapy | 31 | 48 | 38 |
| Salvage surgery (%) | 22 | 22 | 7 |
| Salvage radiotherapy (%) | 4 | 5 | 7 |
RPA, recursive partitioning analysis; WHO, World Health Organization.
*Number of progressive patients: 282 RT and 272 TMZ/RT.
In a further analysis, patients treated in the BNCT study were compared with a subgroup of patients from the original paper by Stupp et al [4], for which the methylation status of the DNA repair gene, an independent prognostic indicator, had been established [6]. The patient characteristics and medical interventions for patients in the RT/TMZ arm from the publication by Hegi et al [6], with methylated and unmethylated MGMT DNA repair gene, are compared with those in the BNCT study in Table 2. The median age of patients was the same, 53 years, in the three groups. The pre-treatment performance status was much worse in the BNCT study than in the other two groups, in terms of both RPA class and WHO grade. Salvage TMZ was used more frequently and salvage surgery was used much less frequently for patients receiving BNCT. Salvage radiotherapy was used infrequently in all three groups.
Table 2. Baseline patient characteristics and salvage therapies for patients treated with boron neutron capture therapy (BNCT) or with radiotherapy/temozolomide for patients classified as either MGMT(O6-methylguanine–DNA methyltransferase) methylated or MTMG unmethylated.
| Parameter | MGMT methylated | MGMT unmethylated | BNCT |
| Number of patients | 46 | 60 | 29 |
| Age, median (range) (years) | 53 (19–69) | 53 (22–67) | 53 (28–69) |
| Initial surgery (%) | |||
| Resection | 98 | 97 | 90 |
| Biopsy only | 2 | 3 | 10 |
| WHO performance status (%) | |||
| 0 | 39 | 43 | 10 |
| 1 | 44 | 50 | 66 |
| 2 | 17 | 7 | 24 |
| RPA class (%) | |||
| III | 17 | 18 | 14 |
| IV | 59 | 62 | 21 |
| V | 24 | 20 | 65 |
| Average RPA class | 4.07 | 4.02 | 4.5 |
| Salvage chemotherapy (%) | |||
| Temozolomide | 20 | 18 | 59 |
| Other chemotherapy | 30 | 28 | 3 |
| No chemotherapy | 50 | 53 | 38 |
| Salvage surgery (%) | 26 | 22 | 7 |
| Salvage radiotherapy (%) | 7 | 3 | 7 |
RPA, recursive partitioning analysis.
Results
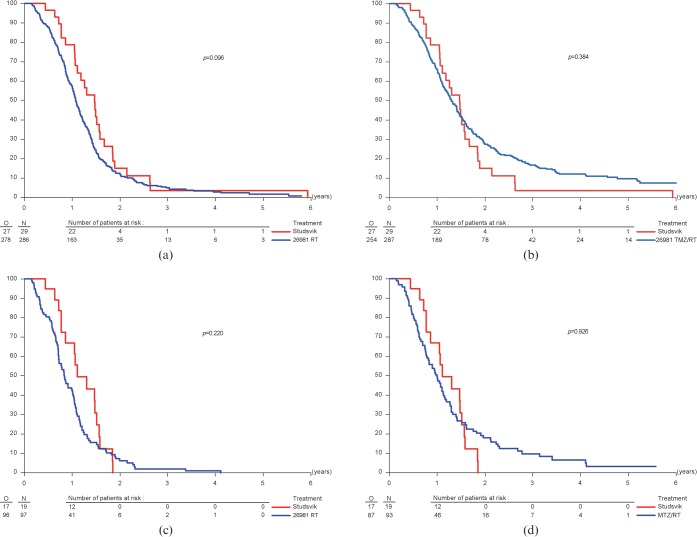
Kaplan–Meier plots comparing the survival for all patients from the two arms of the randomised trial by Stupp et al [4] with the Studsvik BNCT study [8, 9] are shown in Figure 1a,b. A similar analysis for RPA Class V patients alone [3] is given in Figure 1c,d. Corresponding survival parameters are reported in Table 3, together with the results for progression-free survival and for the frequency of severe adverse events.
Figure 1.
Time-related changes in the percentage of overall survival of patients with newly diagnosed glioblastoma multiforme from the time of initial surgery. All patients treated with boron neutron capture therapy at Studsvik are compared with all patients receiving conventional radiotherapy (RT) (a) or RT/temozolomide (TMZ) (b). Patients in recursive partitioning analysis Class V are compared on a similar basis with RT (c) or RT/TMZ (d).
Table 3. Summary of patient survival parameters and record of adverse events in patients receiving either boron neutron capture therapy (BNCT), radiotherapy (RT) only or radiotherapy with concomitant and adjuvant temozolomide (RT/TMZ).
| Parameter | RT (95% CI) | RT/TMZ (95% CI) | BNCT (95% CI) |
| Median PFS (months) | 5.8 (5.2–6.3) | 7.9 (6.7–9.2) | 5.8 (5.2–8.7) |
| Median OS (months) | |||
| All patients | 12.9 (12.2–14.0) | 15.5 (14.1–17.3) | 17.6 (13.3–20.1) |
| RPA Class V patients | 10.1 (8.5–12.2) | 12.0 (9.9–13.9) | 13.3 (10.3–18.2) |
| Survival at 1 year (%) | |||
| All patients | 57.2 (51.3–2.7) | 66.1 (60.3–71.3) | 78.7 (58.6–89.8) |
| RPA Class V patients | 42.8 (32.8–52.4) | 49.5 (39.0–59.1) | 66.9 (40.6–83.6) |
| Adverse events (WHO grade III–IV) | |||
| Number of patients (%) | 56 (21) | 77 (27) | 4 (14) |
CI, confidence interval; PFS, progression-free survival; OS, overall survival.
Univariate analysis, using the Kaplan–Meier method, suggested that a proportion of patients treated with BNCT did better than those receiving conventional fractionated radiotherapy, either alone or in combination with TMZ. However, only in the case of BNCT compared with RT, with all patients included (Figure 1a), did the advantage of BNCT approach an accepted level of significance (p = 0.096). This is perhaps not surprising given the small number of patients in the BNCT study. The survival curves for RT/TMZ have a larger proportion of patients showing longer term survival (≥2 years) than those for BNCT and for RT alone.
The median survival times for all patients offered BNCT were 17.6 months and 12.9 months for RT alone. The percentage survival at 1 year was approximately 20% higher for patients receiving BNCT (78.7% vs 57.2%). Comparable differences in patient survival at 1 year were seen in the separate analysis of RPA Class V patients. RT/TMZ patients showed values intermediate to those for RT and BNCT. Too few patients were alive at 2 years in the small cohort of patients treated with BNCT to make a meaningful comparison.
The percentage of patients showing possible treatment-related adverse events (WHO grade III–IV) was lowest in the BNCT group and highest in the RT/TMZ arm of the Phase III study. However, considering that a more stringent procedure may have been used in the recording of adverse events in the randomised study, these differences should be viewed with caution.
The Cox proportional hazard model was used to take account of differences in baseline characteristics. Hazard ratios for BNCT vs RT and vs RT/TMZ are shown in Table 4. Both unadjusted ratios and ratios adjusted for the extent of surgery, age and WHO performance status are shown. Ratios smaller than 1.0 indicate results in favour of BNCT. The data in Table 4 show a definite trend towards BNCT having better efficacy than RT alone, particularly when the analysis is stratified by RPA class (hazard ratio ∼0.6). For BNCT vs RT/TMZ the hazard ratios are in all cases close to 1.0. However, the median survival time and the percentage of patients alive at 1 year showed a tendency to be larger for patients treated with BNCT, both in the case of all patients and in the case of patients in RPA Class V.
Table 4. Cox proportional hazard ratios for boron neutron capture therapy (BNCT) vs either radiotherapy (RT) or RT/temozolomide (TMZ) for different subgroups of patients. Ratios are shown both for unadjusted data and for data adjusted by the extent of initial surgery, age and WHO performance status at the time of treatment.
| Hazard ratio |
||
| Unadjusted (95% CI) | Adjusted (95% CI) | |
| BNCT vs RT | ||
| All patients | 0.71 (0.48–1.07) | 0.72 (0.47–1.09) |
| Stratified by RPA class | 0.63 (0.42–0.95) | 0.64 (0.42–0.99) |
| Class V only | 0.72 (0.43–1.22) | 0.80 (0.46–1.4) |
| BNCT vs RT/TMZ | ||
| All patients | 1.19 (0.8–1.78) | 1.10 (0.73–1.67) |
| Stratified by RPA class | 1.01 (0.67–1.51) | 0.97 (0.63–1.48) |
| Class V only | 1.03 (0.6–1.74) | 1.02 (0.58–1.79) |
CI, confidence interval; RPA, recursive partitioning analysis.
The amount of repair of sublethal radiation damage is an important factor for the efficacy of conventional low linear energy transfer (LET) radiotherapy but will have little, if any, influence on the response to high LET radiation, such as that generated with BNCT. In the publication by Hegi et al [6], the methylation status of the MGMT DNA repair gene was shown to have a major impact on patient survival times for patients in the RT/TMZ arm of the study by Stupp et al [4].
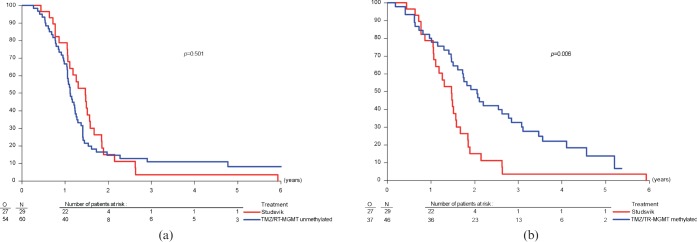
Kaplan–Meier plots showing the survival for patients from the two subgroups in the publication by Hegi et al [6] treated with RT/TMZ compared with the Studsvik BNCT study [8, 9] are shown in Figure 2a,b, and the corresponding survival parameters are reported in Table 5.
Figure 2.
Time-related changes in the percentage of overall survival of patients with newly diagnosed glioblastoma multiforme from the time of initial surgery. All patients treated with boron neutron capture therapy at Studsvik are compared with patients receiving conventional radiotherapy/temozolomide (RT/TMZ) and either unmethylated (a) or methylated (b) with respect to the MGMT(O6-methylguanine–DNA methyltransferase) DNA repair gene.
Table 5. Summary of patient survival parameters for patients receiving boron neutron capture therapy (BNCT) or radiotherapy/temozolomide with either the methylated or the unmethylated MGMT (O6-methylguanine–DNA methyltransferase) DNA repair gene.
| Parameter | MGMT methylated (95% CI) | MGMT unmethylated (95% CI) | BNCT all patients (95% CI) |
| Median OS (months) | 24.7 (19.3–34.1) | 13.4 (12.7–15.2) | 17.6 (13.3–20.1) |
| Survival at 1 year (%) | 80.0 (65.1–89.1) | 66.7 (53.2–77.0) | 78.7 (58.6–89.8) |
CI, confidence interval; OS, overall survival.
Univariate analysis, using the Kaplan–Meier method, indicated that patients with the methylated MGMT DNA repair gene treated with RT/TMZ did significantly better than those treated with BNCT (p = 0.006). However, for the unmethylated subgroup of patients there was an indication in favour of BNCT in the sense that the median overall survival was increased by approximately 4 months in the BNCT group (17.6 vs 13.4 months) and that survival at 12 months was 12% higher (78.7% vs 66.7%) (see Table 5). However, these differences were not statistically significant.
The Cox proportional hazard model was again used to take account of differences in baseline characteristics. Hazard ratios for BNCT vs RT/TMZ for patients classified as either MGMT methylated or unmethylated are shown in Table 6. Both unadjusted ratios and ratios adjusted for the extent of surgery, age and WHO performance status are shown. For the unmethylated MGMT subgroup of patients the adjusted hazard ratio of 0.68 suggests a possible clinically relevant advantage of BNCT over RT/TMZ. For patients in the methylated MGMT subgroup an advantage of RT/TMZ over BNCT is clearly indicated.
Table 6. Cox proportional hazard ratios for boron neutron capture therapy (BNCT) (all patients) vs radiotherapy/temozolomide (RT/TMZ) for subgroups of patients with methylated and unmethylated MGMT (O6-methylguanine–DNA methyltransferase) genes, respectively.
| Hazard ratio |
||
| Unadjusted (95% CI) | Adjusted (95% CI) | |
| BNCT vs RT/TMZ (unmethylated) | 0.85 (0.54–1.36) | 0.68 (0.4–1.17) |
| BNCT vs RT/TMZ (methylated) | 2.07 (1.23–3.5) | 1.95 (1.09–2.68) |
Ratios are shown both for unadjusted data and for data adjusted by the extent of initial surgery, age and WHO performance status at the time of treatment.
CI, confidence interval.
Discussion
BNCT vs RT for treatment of newly diagnosed GBM
BNCT with prolonged infusion resulted in a median survival time of 17.6 months compared with 12.9 months in the RT arm of the randomised Phase III trial by Stupp et al [4]. The 1-year survival was ∼20% higher with BNCT than with RT, despite the less favourable RPA profile of the BNCT patients. The Cox proportional hazard ratios reflected this when the analysis was stratified by RPA class and suggested a strong trend in favour of BNCT over RT alone. In addition to the trend to longer survival, the short treatment time with BNCT (1 day) offers a significant advantage to the patients. The fraction of patients undergoing surgery after progression was three times higher in the RT arm of the Stupp et al [4] trial than in the BNCT study (23% vs 7%) while the fraction of patients receiving temozolomide after disease progression was similar in the two studies (60% vs 59%). Thus, the salvage treatment was more extensive in the RT arm of the trial by Stupp et al [4] than in the BNCT study.
The frequency of WHO grade III–IV adverse events appeared to be lower after BNCT than after RT (Table 3). However, this finding should be viewed with some caution, since the reporting of adverse events in a small Phase II study may not be as reliable as that in a large Phase III trial.
BNCT vs RT/TMZ for treatment of newly diagnosed GBM
The median survival time in the RT/TMZ arm of the study by Stupp et al [4], which is now considered the treatment of choice for newly diagnosed GBM, was 15.5 months from surgery, which should be compared with 17.6 months in the BNCT study (Figure 1b and Table 3). The 1-year survival was 66.1% and 78.7% for RT/TMZ and BNCT, respectively, with a slightly larger difference for the Class V patients alone. The fraction of patients requiring second surgery after progression was three times higher with RT/TMZ than with BNCT (23% vs 7%).
Considering the above factors, it seems that BNCT with 6-h infusion achieved initial survival times that are similar to those achieved with RT/TMZ. The number of patients surviving at 2 years with BNCT was small and there was no suggestion of a high residual proportion of long-term survivors (≥2 years), as was achieved with RT/TMZ. A feature of the comparison between patients treated with BNCT and with RT/TMZ was that there was a distinct cross-over in the Kaplan–Meier survival curves, with a trend for patients to do slightly better with BNCT in the first year and with the reverse trend for long-term survival.
Potential advantage of BNCT for patients with the unmethylated MGMT promoter gene
As reported by Hegi et al [6], the methylation status of the MGMT DNA repair gene has a significant impact on the efficacy of both RT and RT/TMZ in patients with newly diagnosed GBM. Methylation of the MGMT promoter gene prolongs the survival of patients offered RT alone from 11.8 months to 15.3 months and has an even larger impact in the case of RT/TMZ, with an increase in the median overall survival from 12.7 months to 21.7 months, with survival as originally calculated from time of randomisation [6]. In the current analysis, using the updated EORTC data file, the median survival from time of surgery for the subpopulation of patients with the unmethylated MGMT promoter gene was 13.4 months compared with 24.7 months for the methylated subgroup of patients.
The mechanism by which the methylation of the MGMT promoter gene, particularly in the presence of TMZ, prolongs patient survival is believed to be related to the repair of DNA damage. The inhibition of the repair of DNA damage prolongs patient survival. The repair capacity, which is considerable after conventional photon irradiation, is thought to be inhibited when the promoter gene is methylated. In contrast, there is considerable evidence that DNA damage produced by high LET radiation, as is applicable to BNCT, is not repaired and, as a result, factors related to DNA repair will not be important. For patients treated with BNCT the methylation status is not known, but as the inhibition of DNA repair is not a factor for this treatment modality the methylation status of the MGMT promoter gene is very unlikely to be relevant. This working hypothesis, which is reasonable in the light of current radiobiological knowledge, still requires validation.
Data indicating a lack of repair of sublethal DNA damage being associated with radiation exposures similar to those used in the study at Studsvik are available for both normal tissue and tumour [10]. For BNCT irradiations of the rat spinal cord, with either a single dose or two and four daily dose fractions, there was no evidence for any sparing of dose as the number of fractions increased; the dose required to produce radiation myelopathy in 50% of animals (ED50) did not vary significantly (13.8±0.6, 14.9±0.9 and 14.3±0.5 Gy for one, two and four fractions, respectively); in contrast, with X-irradiation, there was a marked and significant nearly twofold increase in ED50 with escalating fraction number (19.0±0.2, 25.8±0.2 and 32±0.2 Gy for one, two and four fractions, respectively), in line with the established repair of sublethal damage. Irradiation of 9L gliosarcoma-bearing rats with BPA-f-mediated BNCT showed that the long-term tumour control probability was the same after a total dose of 10.4 Gy, whether given as a single dose or as two equal fractions of 5.2 Gy, separated by 48 h, again indicating that DNA repair is not influencing the efficacy of BNCT. The dose distribution in terms of fractional contributions from high and low LET radiation in the normal tissue study and the tumour study referred to above was similar to that in human applications of BNCT, i.e. in the Studsvik study [8, 9].
It is thus reasonable to expect that BNCT will be equally effective regardless of methylation status of the MGMT DNA repair gene. The median overall survival time of 17.6 months observed in the BNCT study [9] should therefore also be representative of patients with the unmethylated MGMT promoter gene and can thus be compared meaningfully with the median overall survival of 13.4 months for the corresponding subgroup of patients treated with RT/TMZ. The possible benefit of BNCT for this subpopulation of patients, which constitutes ∼55% of all GBM patients, could be even larger if the less favourable baseline characteristics of the patients in the Studsvik study [9] were taken into account. The limited numbers of patients in the groups analysed above did not allow a meaningful analysis based on stratification by RPA class using the Cox proportional hazard model. The proportion of patients in RPA Class V was higher in the BNCT study than in the group with the unmethylated MGMT DNA repair gene in the patients in the study by Stupp et al: 65% vs 20% of RPA Class V patients for BNCT and RT/TMZ, respectively. This alone might indicate that the possible clinical advantage of BNCT indicated by an adjusted hazard ratio of 0.68 may have been underestimated.
General considerations
BNCT, as carried out at Studsvik, is administered as a one-day treatment, with the infusion of the boron compound for 6 h followed by approximately 15 min of neutron irradiation, whereas conventional RT with TMZ involves six weeks with five days of radiation treatment per week and daily concomitant medication with TMZ, followed by six weeks of adjuvant TMZ. The low radiation doses used in recent BNCT treatments of recurrent GBM at Studsvik [11] were not associated with any treatment-related side effects. There was also no clear evidence of radiation damage detected in the post-mortem examination of seven patients offered BNCT as primary radiotherapy [12]. Thus, other advantages aside, BNCT could offer the patients an initial six weeks of good-quality life, during which time the patients would otherwise be receiving daily treatment with RT and concomitant TMZ. It could be argued that this should be considered in an overall assessment of BNCT as an alternative to RT or to RT/TMZ for newly diagnosed GBM. The need for a nuclear reactor as a neutron source has been a severe impediment to large-scale clinical use of BNCT in the past. However, it is also possible to use compact proton accelerators for neutron generation and several accelerator-based BNCT facilities are presently under development around the world [13–15]. Such facilities can be installed in hospital environments at a cost similar to that of the facilities presently used for standard RT. It can be anticipated that the cost of BNCT in routine use with large patient volumes will be similar to that for conventional fractionated RT, if the full cost to the health care system of daily treatment with conventional RT over periods of up to six weeks is considered [16, 17].
Conclusions
Comparison of the BNCT study at Studsvik [8, 9] with the results from publications based on the Phase III study by Stupp et al [3–6] indicated that BNCT with 6-h infusion of BPA-f is at least as effective as conventional RT for newly diagnosed GBM. The comparison also indicated that BNCT with the new infusion protocol could be at least as effective as RT/TMZ for patients with pre-treatment performance status in RPA Class V and that for patients with the unmethylated MGMT DNA repair gene there is possibly a clinically useful advantage with BNCT.
Owing to the short treatment time and the low risk of treatment-related side effects, BNCT could become the radiotherapy of choice for the group of patients with the unmethylated MGMT DNA repair gene, where the addition of concomitant and adjuvant TMZ to RT has little clinical advantage [3], if the present results are confirmed in a randomised Phase II trial stratified according to the range of recently validated prognostic factors for newly diagnosed GBM [5].
Acknowledgments
The authors are grateful to the EORTC Brain Tumour and Radiotherapy Groups and National Cancer Institute of Canada Clinical Trials Group participants for having allowed us to compare the data to the database of the Intergroup trial EORTC 26981-22081/NCIC CE.3. Authors KS, JH, VG and LP are all either employed by Hammercap Medical AB or engaged as consultants to the company. Hammercap Medical AB was sponsor of the Phase II study on newly diagnosed GBM at Studsvik.
For one of us (TG), this publication was supported by grants number 5U10CA11488-30 through 5U10 CA011488-40 from the National Cancer Institute (Bethesda, Maryland, USA) and by a donation from the Cancerfonden from Sweden through the EORTC Charitable Trust. Its content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Cancer Institute.
References
- 1.Barth RF, Coderre JA, Vicente MGH, Blue TE. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res 2005;11:3987–4002 [DOI] [PubMed] [Google Scholar]
- 2.Curran Jr WJ, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Nat Can Inst 1993;85:704–10 [DOI] [PubMed] [Google Scholar]
- 3.Mirimanoff R-O, Gorlia T, Mason W, van denBent MJ, Kortmann RD, Fisher , B , et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 Phase III randomized trial. J Clin Oncol 2006;24:2563–9 [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van denBent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96 [DOI] [PubMed] [Google Scholar]
- 5.Gorlia T, van denBent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981–22981/CE.3. Lancet Oncol 2008;9:29–38 [DOI] [PubMed] [Google Scholar]
- 6.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from Temozolomide in Glioblastoma. N Engl J Med 2005;352:997–1003 [DOI] [PubMed] [Google Scholar]
- 7.Barth RF, Joensuu H. Boron neutron capture therapy for the treatment of glioblastoma and extracranial tumours: As effective, more effective or less effective than photon irradiation? Radiother Oncol 2007;82:119–22 [DOI] [PubMed] [Google Scholar]
- 8.Henriksson R, Capala J, Michanek A, Lindahl Så, Salford LG, Franzén L, et al. Boron neutron capture therapy (BNCT) for glioblastoma multiforme: a phase II study evaluating a prolonged high dose of boronophenylalanine (BPA). Radiother Oncol 2008;88:183–91 [DOI] [PubMed] [Google Scholar]
- 9.Sköld K, H-Stenstam B, Diaz AZ, Giusti V, Pellettieri L, Hopewell JW. doi: 10.1111/j.1600-0404.2009.01267.x. Boron neutron capture therapy for glioblastoma multiforme: advantage of prolonged infusion of BPA-f. Acta Neurol Scand Doi: 10.111/i.1600-0404.2009.07.267. [DOI] [PubMed] [Google Scholar]
- 10.Coderre JA, Morris GM, Micca PL, Fisher CD, Ross GA. Comparative assessment of single-dose and fractionated boron neutron capture therapy. Radiat Res 1995;144:310–17 [PubMed] [Google Scholar]
- 11.Pellettieri L, H-Stenstam B, Rezaei A, Giusti V, Sköld K. An investigation of boron neutron capture therapy for recurrent glioblastoma multiforme. Acta Neurol Scand 2008;117:191–7 [DOI] [PubMed] [Google Scholar]
- 12.H-Stenstam B, Pellettieri L, Sköld K, Rezaei A, Brun A. Neuropathological postmortem evaluation of BNCT for GBM. Acta Neurol Scand 2007;116:169–76 [DOI] [PubMed] [Google Scholar]
- 13.Blue TE, Yanch JC. Accelerator-based epithermal neutron sources for boron neutron capture therapy of brain tumors. J Neurooncol 2003;62:19–31 [DOI] [PubMed] [Google Scholar]
- 14.Tanaka H, Sakurai Y, Suzuki M, Masunaga S, Kinashi Y, Kashino G, et al. Characteristics comparison between a cyclotron-based neutron source and KUR-HWNIF for boron neutron capture therapy. Nucl Instr and Meth B 2009;267:1970–7 [Google Scholar]
- 15.Kreiner AJ, Kwan JW, Burlon AA, Di Paolo H, Henestroza E, Minsky DM, et al. A Tandem-electrostatic-quadrupole for accelerator-based BNCT. Nucl Instrum Methods B 2007;261:751–4 [Google Scholar]
- 16.Johannesen TB, Norum J, Lote K, Scheie D, Hirschberg H. A cost-minimizing analysis of standard radiotherapy and two experimental therapies in glioblastoma. Radiother Oncol 2002;62:227–31 [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa Y, Yoshihara H, Kageji T, Matsuoka R, Nakagawa Y. Cost analysis of radiotherapy, carbon ion therapy, proton therapy and BNCT in Japan. Appl Radiat Iso 2009. (suppl) 67, 580–3 . [DOI] [PubMed] [Google Scholar]