Abstract
The mediastinum is an uncommon location for vascular malformations. We describe an unusual case of a young patient with a large, extensive mediastinal vascular malformation and draining vein to the left atrium who presented with recurrent ischaemic episodes.
Vascular anomalies such as malformations and haemangiomas are commonly seen in the extremities, head and neck, and visceral organs but are rarely seen in the mediastinum. CT and MRI play important roles in the diagnosis of mediastinal vascular malformations and in determining their extent. We present a rare case of a young man who presented with ischaemic lesions in the cerebellum from recurrent emboli originating in an extensive middle mediastinal vascular malformation that had a large vein draining into the left atrium.
Case report
A 31-year-old man presented with sudden onset of diplopia and unsteadiness. He had recently begun noticing numbness in his arms, episodic fatigue, weakness, dizziness and headaches. 5 years earlier, he had an episode of central vision loss with headache. The patient also reported multiple episodes of transient peripheral vision loss and transient blurry vision. He suffered from mild impairment of memory and insomnia. The patient denied fever, chills, weight loss, rashes, joint aches and hearing loss.
On physical examination, he was alert and co-operative, with normal cognition and affect. Vital signs and cardiovascular examination were normal. Visual acuity and optic discs were normal. Cranial nerves were normal, except for slight left-beating gaze-evoked nystagmus. Strength, reflexes, co-ordination, and pain and temperature sensation were normal.
MRI of the brain showed patchy, high T2 signal foci in the white matter of the cerebellar hemispheres, consistent with remote infarcts in the distribution of posteroinferior cerebellar artery. Diffusion imaging and magnetic resonance angiography (MRA) of the circle of Willis and neck vessels were normal. The stroke was considered cryptogenic, with vasculitis and inflammatory disorders as the leading causes.
Transoesophageal echocardiogram for evaluation of a cardiac source of emboli showed normal ventricular sizes and systolic function. The interatrial and interventricular septa were intact. A vague soft-tissue mass was noted posterior to the left atrium.
MRI and MRA of the thoracic aorta performed for evaluation of vasculitis showed normal appearance of the aorta and its branch vessels. However, a large soft-tissue mass centred in the middle mediastinum was present, extending from the level of the thoracic inlet to the level of the distal oesophagus. The mass was diffusely infiltrative, with intermediate signal intensity on T1 and steady-state free precession (SSFP) images and intensely high signal on short tau inversion recovery (STIR) images (Figure 1). The mass demonstrated mild enhancement on T1 weighted images following the intravenous administration of gadolinium chelate. A large vein extended from the mass into the left inferior pulmonary vein (Figure 2).
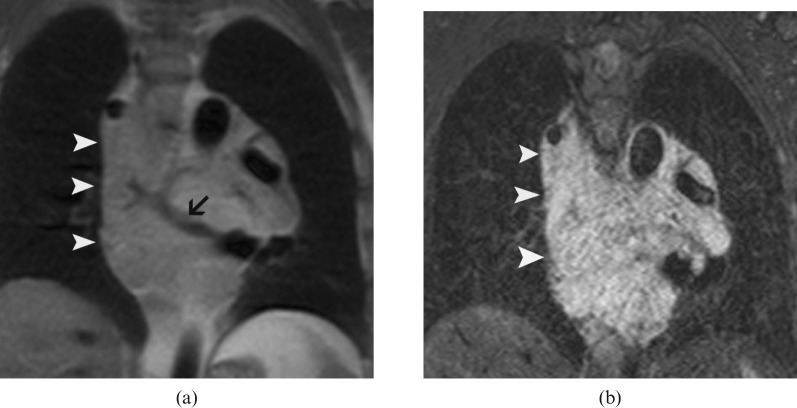
Figure 1.
Coronal MR images of the mediastinum show the heterogeneous, infiltrative malformation in the middle mediastinum. The mass (arrowheads) is isointense on T1 weighted black blood images (a) and hyperintense on short tau inversion recovery images (b). A vascular structure is seen as flow void within the mass (arrow) (a).
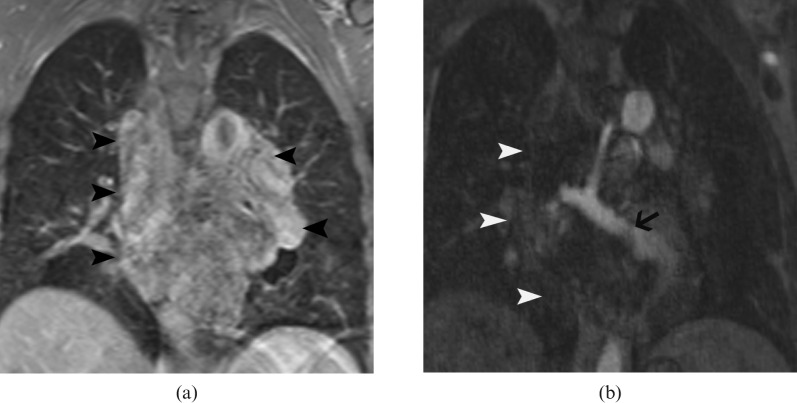
Figure 2.
Post-contrast T1 weighted MR image (a) shows heterogeneous enhancement of the mass (arrowheads). MR angiography (b) shows a large draining vein (arrow) within the mass (arrowheads) that communicates with the left inferior pulmonary vein.
A contrast-enhanced CT scan of the chest showed multiple calcified phleboliths in the mediastinal mass (Figure 3) and showed extension into the left hilum (Figure 4) with involvement of the wall of the upper oesophagus (Figure 4), and extension into the inferior wall of the left main bronchus (Figure 4). Angiography did not demonstrate any feeding artery. The imaging findings were consistent with a low-flow venous malformation with a vein draining into the left atrium. Biopsy was not performed because of the location and vascular nature.
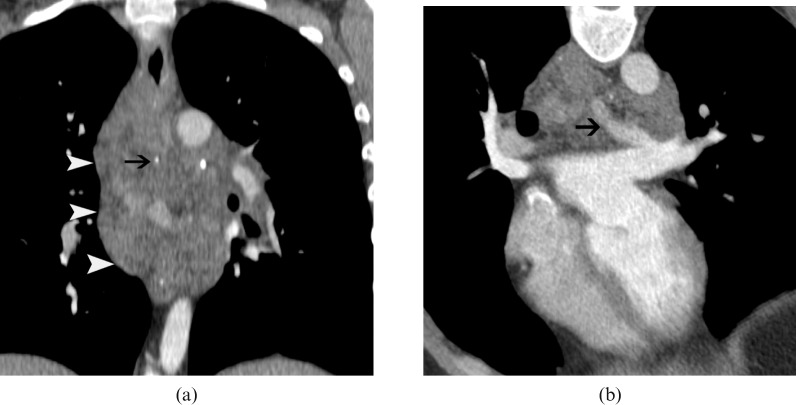
Figure 3.
(a) Contrast-enhanced CT scan shows the extensive, infiltrative mediastinal vascular malformation (arrowheads) which is heterogeneous, with areas of dense, calcified phleboliths (arrow). Note also the large draining vein inside the tumour (arrow in b), which communicates with the left pulmonary vein and left atrium.
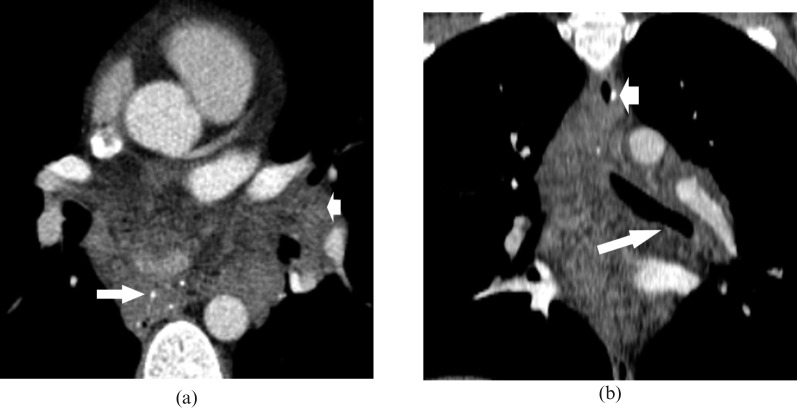
Figure 4.
(a) Axial CT scan shows the mass with phleboliths (long arrow), extending to the left hilum (short arrow). (b) Coronal CT scan reconstruction shows infiltration of the upper oesophagus (short arrow) and inferior portion of left main bronchus (long arrow).
Owing to the absence of effective surgical or interventional options to either remove the mass or exclude the draining vein, indefinite warfarin therapy was initiated for prevention of recurrent ischaemic strokes.
Discussion
Vascular anomalies are broadly classified into neoplasms and vascular malformations (Table 1) [1]. Haemangiomas are benign congenital vascular neoplasms, which can be subdivided into superficial, deep and compound types. Haemangiomas show cellular proliferation, are small or absent at birth, grow rapidly during infancy and often involute during childhood. Vascular malformations are abnormalities of vascular embryological development that are composed of dysplastic vessels, are present a birth, grow with physical development and show no spontaneous regression [2]. They can have any combination of capillary, venous, arterial and lymphatic components, with or without fistula [1]. Vascular malformations with arterial components are termed high flow and those without are termed low flow. Haemangiomas are more common than vascular malformations [1].
Table 1. Classification of vascular anomalies.
| Vascular neoplasms | Haemangioma |
| Vascular malformation | Low flow |
| Venous malformation | |
| Lymphatic malformation | |
| Lymphatic venous malformation | |
| Capillary malformation (port-wine stain) | |
| High flow | |
| Arteriovenous malformation | |
| Arteriovenous fistula | |
| Overgrowth syndromes | |
| Low flow | |
| Klippel–Trenauney syndrome | |
| Sturge–Weber syndrome | |
| Mafucci syndrome | |
| Proteus syndrome | |
| High flow | |
| Parkes–Weber syndrome |
Imaging plays a vital role not only in the diagnosis and characterisation of vascular malformations (Table 2), but also in determining the anatomical extent of the lesion and involvement of adjacent structures such as the neurovascular bundles. This information is essential in determining the optimal therapeutic option. MRI is the optimal imaging modality as it provides the morphological and vascular features necessary for treatment. However, the superior spatial resolution of CT can show subtle organ involvement, as in this case.
Table 2. Salient features of the common vascular anomalies.
| Type | Characteristics | CT/MRI | Angiography |
| Haemangioma | Benign vascular neoplasm | Discrete mass | No draining vein |
| Small/absent at birth | High signal in T2 | ||
| Rapid growth in infancy | Phleboliths | ||
| Involution during childhood | Slow enhancement | ||
| Low flow | |||
| Venous malformation | Dysplastic venous channels | Phleboliths ± | Draining vein |
| Present at birth | Serpentine structures of slow flowing blood | ||
| Grow with physical development | T1 intermediate, T2 high | ||
| No regression | Slow enhancement | ||
| Lymphatic malformation | Dysplastic lymph channels | Cystic signal | No draining vein |
| Present at birth | |||
| Grow with physical development | T1 low, T2 high | ||
| No regression | |||
| Mixed malformation | Composed of different tissues | Signal heterogeneity depending on composition | Draining vein ± |
| Present at birth | |||
| Grow with physical development | T2 heterogeneously high | ||
| No regression | |||
| High flow | |||
| AV malformation | Present at birth | Serpentine tangle of vessels | Direct communication between artery and vein |
| Grow with physical development | Vascular nidus | ||
| No discrete mass | |||
| No regression | Flow voids in MRI | ||
| AV fistula | Post traumatic | Tangle of blood vessels | Direct communication between artery and vein |
| No discrete mass | |||
| Flow voids in MRI |
AV, arteriovenous.
High-flow vascular malformations are rare and include arteriovenous malformations (AVMs) and arteriovenous fistulas. AVMs are direct connections between the arterial and the venous systems through a vascular nidus. Arteriovenous fistulas are usually post-traumatic. In MRI, no discrete mass is seen, but a tangle of multiple flow voids indicating high flow is seen [2]. Dynamic MRI can be useful in differentiating a high-flow malformation from a low-flow malformation, based on contrast rise time [3]. Doppler ultrasound shows the direct communication and low resistance flow.
A venous malformation is a type of low-flow malformation that is pathologically characterised by dysplastic venous channels, which communicate with adjacent veins, interspersed with a hamartomatous stroma. Lymphatic malformations are composed of chyle-filled dilated lymph channels lined by endothelium. In mixed vascular malformations, different tissues are found. They usually become symptomatic in the second and third decades of life.
MRI findings of low-flow malformations depend on the tissue composition. Venous malformations are composed of serpentine structures of slow-flowing blood, separated by septations, which usually show intermediate signal intensity in T1 weighted images and very high signal intensity on T2 weighted images. Phleboliths are calcified thrombi and manifest as low-signal, round or ovoid foci within the malformation. Following contrast administration, slow enhancement usually occurs. The differential diagnosis includes lymphatic malformation, haemangioma, angiolipoma, solid tumours and extralobar pulmonary sequestration.
Lymphatic malformations have cystic components, which have low signal on T1 and high signal on T2 weighted images and lack central enhancement. Fluid levels may be present. Although exact tissue characterisation of vascular malformations by imaging is not essential [2], determining whether a malformation is slow or high flow is important.
Haemangiomas are congenital, benign vascular neoplasms characterised by proliferation of normal vascular elements interspersed with various stromal elements (fat, fibrous tissue and myxoid material). Phleboliths are seen in haemangiomas has been reported [4, 5]. Only 0.8% of haemangiomas are extra-cutaneous, and they constitute 5% of all mediastinal tumours. In the mediastinum, they most commonly occur in the superior portion [5]. The presence of fat is common in peripheral soft-tissue haemangiomas but is uncommon in mediastinal haemangiomas. Intense high-signal intensity on T2 weighted images, especially with fat suppression, is a characteristic feature [6] and is related to the presence of numerous vascular channels and myxoid changes. Haemangiomas demonstrate no uptake on fluorodeoxyglucose positron emission tomography [7], differentiating them from malignancies. In our case, the presence of a draining vein is helpful in excluding haemangioma and diagnosing a slow-flow vascular malformation.
Soft-tissue tumours such as lymphoma, germ-cell tumours and sarcomas are differential diagnosis of mediastinal lesions. However, involvement of multiple tissue types, lack of respect for fascial planes, infiltration and presence of prominent vascular elements are features that can differentiate these from solid tumours. Posterior mediastinal angiomyolipomas are extremely rare and present on CT scans or MR images as fat-containing masses. However, these lack a draining vein. Extra-lobar sequestration is a mass of non-functioning lung tissue without normal tracheobronchial connection and rarely occurs in the mediastinum [8]. It typically has a systemic arterial supply (in 95% of cases) and systemic venous drainage.
Mediastinal vascular malformations are rare, and are either asymptomatic, or when large present with signs and symptoms related to mass effect on adjacent mediastinal structures [9]. Venous malformations usually drain into a systemic vein. Large high-flow lesions may lead to high-output cardiac failure. Spontaneous rupture of a mediastinal arteriovenous malformation is a rare complication. Diagnosis of these lesions depends on careful evaluation of the mass and the vascular supply [10].
Pulmonary arteriovenous malformation is a well-recognised cause of stroke from paradoxical embolisation of a peripheral venous thrombus through the malformation into the pulmonary veins and ultimately into the systemic arterial circulation. However, a mediastinal vascular malformation with a vein directly draining into the left atrium is an extremely rare cause of emboli causing stroke, with only one case reported to our knowledge [11]. In our case, the direct communication between the malformation and the left atrium resulted in the stroke, and the patient remains at risk for future cerebrovascular accidents unless the communication is closed. Moreover, his previous neurological episodes presumably are the result of embolus from this vascular malformation.
Low-flow vascular malformations are difficult to treat. Asymptomatic patients can be managed conservatively with regular follow-up. Surgical resection, medical therapy and embolisation have limited success in symptomatic patients who present with cardiac or embolic complications. Ligation and division of the anomalous draining vein is essential to prevent recurrent systemic emboli. However, surgical resection was technically difficult in our patient, and percutaneous embolisation was not advisable owing to connection with the left atrium. Indefinite anticoagulation therapy is necessary to prevent recurrent embolic episodes.
Conclusion
Knowledge of the CT and MRI features of vascular malformations is essential to differentiate them from solid tumours. A mediastinal vascular malformation with a draining vein to the left atrium is an extremely uncommon cause of thromboembolic stroke. CT and MRI are very useful in characterisation of the malformation and demonstrating the vascular connection and involvement of adjacent structures. In a young patient presenting with recurrent cerebral ischaemia, any possible source of emboli should be considered and investigated.
References
- 1.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982;69:412–22 [DOI] [PubMed] [Google Scholar]
- 2.Donnelly LF, Adams DM, Bisset GS. Vascular malformations and Hemangiomas. A practical approach in a multidisciplinary clinic. AJR AM J Roentgenol 2000;174:597–608 [DOI] [PubMed] [Google Scholar]
- 3.Ohgiya Y, Hashimoto T, Gokan T, Watanabe S, Kuroda M, Hirose M, et al. Dynamic MRI for distinguishing high-flow from low-flow peripheral vascular malformations. AJR AM J Roentgenol 2005;185:1131–7 [DOI] [PubMed] [Google Scholar]
- 4.Seline TH, Gross BH, Francis IR. CT and MR imaging of mediastinal hemangiomas. J Comput Assist Tomog 1990;14:766–8 [DOI] [PubMed] [Google Scholar]
- 5.McAdams PH, Rosado-de-Christenson ML, Moran CA. Mediastinal hemangioma: radiographic and CT features in 14 patients. Radiology 1994;193:399–402 [DOI] [PubMed] [Google Scholar]
- 6.Sakurai K, Hara M, Ozawa Y, Nakagawa M, Shibamoto Y. Thoracic hemangiomas: imaging via CT, MR, and PET along with pathologic correlation. J Thorac Imaging 2008;23:114–20 [DOI] [PubMed] [Google Scholar]
- 7.Hatayama K, Watanabe H, Ahmed AR, Yanagawa T, Shinozaki T, Oriuchi N, et al. Evaluation of hemangioma by positron emission tomography: role in a multimodality approach. J Comput Assist Tomog 2003;27:70–7 [DOI] [PubMed] [Google Scholar]
- 8.Sippel JM, Ravichandran PS, Antonovic R, Holden W. Extralobar pulmonary sequestration presenting as a mediastinal malignancy. Ann Thorac Surg 1997;63:1169–71 [DOI] [PubMed] [Google Scholar]
- 9.Tsitouridis I, Michaelides M, Pervana S. Posterior mediastinal arteriovenous malformation: report of a case. J Thorac Imaging 2008;23:127–30 [DOI] [PubMed] [Google Scholar]
- 10.Ching ASC, Chan PN, Cheung H, Chan YL, Metreweli C. CT and DSA appearances of a ruptured congenital arteriovenous malformation of the posterior mediastinal aorta. Br J Radiol 2000;73:1320–2 [DOI] [PubMed] [Google Scholar]
- 11.Busch T, Aleksic I, Schulze F, Sirbu H, Dalichau H. Retrocardiac arteriovenous malformation causing recurrent cerebral ischemia. Ann Thorac Surg 2000;70:663–5 [DOI] [PubMed] [Google Scholar]