Abstract
Intercellular spaces are often the first sites invaded by pathogens. In the spaces of tobacco mosaic virus (TMV)-infected and necrotic lesion-forming tobacco (Nicotiana tabacum L.) leaves, we found that an inducer for acidic pathogenesis-related (PR) proteins was accumulated. The induction activity was recovered in gel-filtrated fractions of low molecular mass with a basic nature, into which authentic spermine (Spm) was eluted. We quantified polyamines in the intercellular spaces of the necrotic lesion-forming leaves and found 20-fold higher levels of free Spm than in healthy leaves. Among several polyamines tested, exogenously supplied Spm induced acidic PR-1 gene expression. Immunoblot analysis showed that Spm treatment increased not only acidic PR-1 but also acidic PR-2, PR-3, and PR-5 protein accumulation. Treatment of healthy tobacco leaves with salicylic acid (SA) caused no significant increase in the level of endogenous Spm, and Spm did not increase the level of endogenous SA, suggesting that induction of acidic PR proteins by Spm is independent of SA. The size of TMV-induced local lesions was reduced by Spm treatment. These results indicate that Spm accumulates outside of cells after lesion formation and induces both acidic PR proteins and resistance against TMV via a SA-independent signaling pathway.
PAs are found in a wide range of organisms from bacteria to plants and animals, especially in proliferating cells. They are basic, small molecules believed to promote plant growth and development by activating synthesis of nucleic acids (Bertossi et al., 1965; Walden et al., 1997). Increases in endogenous PAs caused by environmental stresses such as high osmotic pressure, low temperature, and low pH have been reported (Young and Galston, 1983; Flores and Galston, 1984; McDonald and Kushad, 1986). Roles for PAs in plant-microbe interactions have also been proposed. Infection of the brown rust Puccinia hordei, a fungal pathogen, caused a remarkable increase in Spd levels in barley leaves (Greenland and Lewis, 1984). Infection of Phytophthora infestans raised both Spd and Spm levels in susceptible and resistant cultivars of potato (Stroinski and Szczotka, 1989). In resistant wheat cultivars, both fungal and bacterial pathogens elicit the production of amide conjugates of phenolic acid and PA, which may function as phytoalexins (Samborski and Rohringer, 1970). In barley seedlings synthesis of antifungal compounds, hordatines, which contain PAs, increases upon fungal infection (Smith and Best, 1978). These findings indicate a role for PAs in the resistance against bacterial or fungal attack in plants.
Among various host plant-pathogen combinations, an experimental system using TMV and tobacco (Nicotiana tabacum L.) cultivars resistant to TMV offers advantages to the study of certain defense mechanisms, because molecular markers of the defense response, such as SA, jasmonic acid, ethylene, and PR proteins, have been characterized and their analytical systems established. To our knowledge, no studies on the production and function of endogenous PAs in the TMV-tobacco system have been previously published. In this paper we describe the results of qualitative and quantitative analyses of PAs in TMV-infected and local lesion-forming tobacco leaves, and predict the roles of Spm in the induction of acidic PR proteins and resistance to TMV.
Systemic infection of tobacco plants by TMV causes severe mosaic symptoms on young leaves with a dwarf phenotype. However, in tobacco cultivars carrying the ”N” resistance gene against TMV, infected cells die, thus preventing further viral multiplication and translocation to the neighboring cells. Consequently, visible necrotic lesions are formed at the infection sites. This phenomenon is called the HR (Goodman and Novacky, 1994) and is thought to be a typical response to pathogen attack in plants. Lesion formation is accompanied by production of low-molecular-signaling compounds such as SA (Malamy et al., 1990) and ethylene (De Laat and Van Loon, 1983), with the associated induction of a number of PR proteins (Van Loon and Van Kammen, 1970). Tobacco PR proteins consist of at least five families, each of which contains both acidic and basic isoforms (Van Loon et al., 1994). PR-2 and PR-3 proteins have β-1,3-glucanase and chitinase activities, respectively (Kauffmann et al., 1987; Legrand et al., 1987). They could inhibit the growth of pathogens in vitro (Vigers et al., 1992; Niderman et al., 1995).
Transgenic plants overexpressing cDNAs such as those for PR-1, PR-3, and PR-5 were found to have enhanced resistance to fungal pathogen infection (Alexander et al., 1993; Vierheilig et al., 1993; Liu et al., 1994), suggesting their antifungal role in plants. Exogenously supplied SA induces accumulation of acidic PR proteins in healthy tobacco leaves (White, 1979; Ohshima et al., 1990). In addition, SA acts as a natural signal for PR proteins in local lesion-forming tobacco leaves (Klessig and Malamy, 1994). However, with the exception of SA and its derivatives, such as methyl SA (Shulaev et al., 1997), little is known about the natural inducers of acidic PR proteins. Plant hormones such as auxin, cytokinin, and GA3 (Ohashi and Matsuoka, 1987a; Ohashi and Ohshima, 1992), sugars (Herbers et al., 1996), and thiamine (Malamy et al., 1996) have all been reported as natural occurring inducers; however, there is little evidence for an increase in their concentration in response to the HR. Although synthetic compounds such as polyacrylic acid (Gianinazzi and Kassanis, 1974), eosin yellowish (Ohashi and Matsuoka, 1985), 2,6-dichloroisonicotinic acid and its methyl ester (Ward et al., 1991), and a benzothiadiazole derivative (Friedrich et al., 1996) have been shown to effectively induce acidic PR proteins, they are not natural compounds that are generally found in plants.
Acidic PR proteins that are induced by SA treatment or local lesion formation accumulate in the intercellular spaces to levels up to several percent of total soluble proteins (Parent and Asselin, 1984; Ohashi and Matsuoka, 1987b; Hosokawa and Ohashi, 1988). Because many phytopathogenic fungi invade intercellular spaces of host plants at early stages of infection, and since most phytopathogenic bacteria multiply in the intercellular spaces, PR proteins that accumulate in the spaces could directly inhibit pathogen growth. SA and its glucoside, known as inhibitors of the multiplication of certain pathogens, including TMV, were found in the intercellular spaces after local lesion formation (Seo et al., 1995), indicating that this area is an important battlefield in the fight against pathogens.
Here we describe the accumulation of Spm outside of cells in TMV-infected tobacco leaves, and discuss its possible role as an inducer of acidic PR gene expression in the defense of plants against pathogen infection.
MATERIALS AND METHODS
Plant Material
Tobacco (Nicotiana tabacum L. cv Samsun NN) plants, both wild-type and transformants harboring a PR1a-GUS chimeric gene, were grown in a greenhouse under natural light and controlled temperature at 20°C to 30°C. Well-expanded upper leaves of 6-week-old plants were detached and used for the following analyses: For TMV infection, a whole leaf was inoculated with purified TMV suspension (TMV-OM, an ordinary strain of TMV in Japan, 25 μg mL−1, unless otherwise described) using carborundum (mesh no. 600) and incubated at 22°C under continuous illumination at 100 μE m−2 s−1 for appropriate time intervals; for mock inoculation, only mechanical wounding was conducted by rubbing the leaf surface with water and carborundum. For chemical treatments, pieces or discs from healthy leaves were floated in a solution containing an appropriate concentration of sodium salicylate (pH 7.0) or PAs.
Recovery of PR-1 Inducers from the Intercellular Fluid of TMV-Infected Tobacco Leaves
Eight-hundred grams of necrotic lesion-forming tobacco leaves was submerged 4 d after TMV infection in 1 mm DTT solution in vacuo. The intercellular fluid of the leaves was recovered into the solution and then it was concentrated by freeze-drying. The resulting dried material from the intercellular spaces was solubilized in 1 mm DTT and subjected to gel filtration using a Sephadex G-15 column (10 × 300 mm, Pharmacia). The eluted solution (1 mL) was fractionated and evaluated for biological activity to induce GUS activity, as described below. Authentic Spm in free basic form was applied to the same column as a standard, and the eluted fractions were monitored by pH.
Analysis of PR-1 Gene Expression
Analysis of PR-1a gene expression was performed with transgenic tobacco plants harboring a PR1a-GUS gene. Previously, we used transgenic tobacco plants containing a GUS chimeric gene with 2.4 kb of the 5′ flanking region and +29 bp from the transcription start site of the PR-1a gene in the pTRA415 plasmid (Ohshima et al., 1990), in which SA treatment increased GUS activity 10- to 20-fold over a considerable level of basal constitutive GUS activity. In the current study we used new, improved plants containing the GUS coding region driven by 2.4 kb of the 5′ flanking region and +77 bp of the tobacco PR-1a gene. For generation of the PR1a-GUS plants, the 35S promoter region in the pBI121 vector (Jefferson et al., 1987) was replaced by the PR-1a promoter. In mature leaves of the adult transgenic plants, basal levels of GUS activity were almost null, and treatment with SA at 2 mm induced the basal activity by 1000-fold after 2 d of incubation (data not shown). We selected one representative plant for the analysis of PR-1a gene expression. In the transgenic plant the time-course analysis of GUS induction by SA showed similar kinetics to that of immunologically detected PR-1 proteins (Ohshima et al., 1990).
GUS activity was determined as described by Jefferson et al. (1987) with modifications by Kosugi et al. (1990). Leaf discs were cut out from fully expanded upper leaves of PR1a-GUS transgenic tobacco plants and used for GUS assay before and after chemical treatment. The fluorescence of the reactant was measured with a spectrofluorometer (model FP-777, Jasco, Easton, MD). GUS activity is given as nanomoles 4-methyl-umbelliferone per gram leaf fresh weight produced in 1 min at 37°C.
Immunoblot Analysis
Analysis of PR proteins was performed by immunoblotting. Leaf material was ground in 2 volumes of 84 mm citric acid-32 mm sodium phosphate buffer, pH 2.8. After centrifugation at 15,000g for 30 min, the resultant supernatant fluid was subjected to precipitation by 80% saturation with ammonium sulfate. The pellet was dialyzed against 50 mm sodium phosphate buffer, pH 7.0, containing 2 mm DTT. PR proteins were separated by two types of PAGE. For 15% SDS-PAGE, a protein solution from 3 mg leaf fresh weight (corresponding to 15 μg of protein) was used per lane, basically according to the standard procedure (Gallagher, 1996). For 15% native-PAGE separation, a protein solution from 3 mg leaf fresh weight was used per lane according to the method of Davis (1964). The separated proteins were blotted onto an Immobilon-P transfer membrane (PVDF, pore size 0.45 μm, no. IPVH 000 10, Millipore) using a semidry electroblotting system. Immunoblotting was performed basically according to the standard method of Gallagher et al. (1996). For immunodetection, rabbit polyclonal antibody against PR-1a protein (Ohashi and Matsuoka, 1985) and new antibodies prepared as described by Ohashi and Matsuoka (1985) using purified tobacco PR-N, PR-P, and PR-S proteins were used to detect PR-1, PR-2, PR-3, and PR-5 proteins, respectively. An alkaline phosphatase-conjugated anti-rabbit IgG was used as the secondary antibody. Each PR protein was identified in gel by mobility in comparison with known Mr markers for SDS-PAGE, or purified standard PR proteins for native-PAGE.
Quantification of PAs
Free PAs in both whole tobacco leaves and the intercellular spaces were quantified. For extraction of PAs in whole leaves, fresh leaf tissue (1.5 g) was homogenized and PAs were extracted with 5 mL of 0.5 m perchloric acid. For extraction of PAs in the intercellular fluid, 35 leaf discs (18 mm in diameter) were cut out from leaves, immediately weighed, washed with distilled water, and submerged in water in vacuo. Subsequently, the water-infiltrated leaf discs were subjected to centrifugation at 2000g for 20 min to recover the intercellular fluid from the discs that were placed in a 25-mL disposable Terumo syringe sitting inside a 50-mL disposable Falcon tube. PAs in these two extracts were derivatized with benzoyl chloride using diaminohexane as an internal standard basically according to the method described by Flores and Galston (1982). Separation and quantification of PA derivatives were carried out using a HLPC system (model LC-10A, Shimadzu, Tokyo, Japan) equipped with a UV detector under the following conditions: column, Shimadzu Shim-pack CLC-ODS (6 × 150 mm); column temperature, 45°C; mobile phase, 64% (v/v) methanol; flow rate, 0.8 mL min−1; and detection, 254 nm.
Quantification of SA
SA and its conjugate SAG were extracted from 2 g of leaf material and quantified essentially as described by Malamy et al. (1992). SAG was quantitated following enzymatic hydrolysis with β-glucosidase (EC 3.2.1.21; from almonds; Sigma). Separation and quantification were performed using a HPLC system equipped with a spectrofluorescence detector (model RF-550A, Shimadzu). Analysis conditions were as follows: column, μBondasphere 300 (Waters), 5-μm C-18 (3.9 × 150 mm); column temperature, 25°C; mobile phase, 23% (v/v) methanol in 20 mm sodium acetate, pH 5.0, isocratic; flow rate, 1 mL min−1; excitation wavelength, 313 nm; and emission wavelength, 405 nm. All data were corrected for losses.
Evaluation of Spm-Induced Resistance against TMV Infection
To elucidate acquired resistance to TMV induced by Spm treatment, the PA was fed through petioles of detached leaves at a final average concentration in the leaf tissue of 0, 150, 300, or 500 μm, and the leaves were incubated at 22°C under 100 μE m−2 s−1 of continuous light for 0 or 2 d. Then, the leaves were inoculated with TMV (10 μg mL−1). After an additional 4 d of incubation, the diameters of the necrotic lesions were measured separately in three areas: apical, middle, and basal, using enlarged photocopies of the leaves. At least 100 local lesions from four leaves were measured for each area. The mean values of the diameter and sd were calculated. The significance of differences in lesion size between Spm-treated sections and controls was assessed with a Student's t test.
RESULTS
Inducer Activity for PR Gene Expression in the Intercellular Fluid of TMV-Infected Leaves
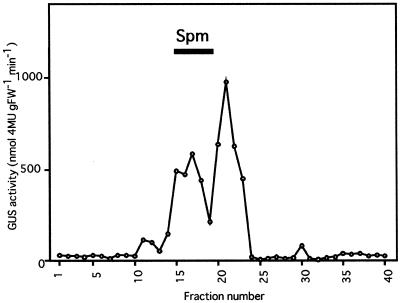
By vacuum-infiltration of 800 g of TMV-infected and local lesion-forming tobacco leaves in 1 mm DTT, we recovered the solution into which the intercellular fluid of the leaves was solubilized. The concentrate resulting from freeze-drying of the solution was subjected to gel filtration to identify compounds with inducer function for acidic PR-1 gene expression. When leaf discs from PR1a-GUS transgenic tobacco plants were treated with 500 μm SA, GUS activity was induced to 1038 nmol 4-methyl-umbelliferone g−1 fresh weight min−1 2 d after the treatment, which corresponds to 1000 times the activity in the water-treated control. Using this system, gel-filtrated fractions were subjected to induction of GUS activity. Two major peaks were found positive for induced expression of the acidic PR-1 gene, as shown in Figure 1. The fractions of numbers 15 to 18 in the first peak contained compounds with a molecular mass of less than 300 D, and without exception exhibited an alkaline pH. We thought that the fractions may contain PAs, which are representative organic compounds with similar characteristics, and tested whether authentic Spm molecules are present in the same fractions. As expected, the fractions for Spm completely overlapped the active fractions (Fig. 1). Therefore, we examined the occurrence and role of PAs in local lesion-forming tobacco plants.
Figure 1.
Inducer activity for PR gene expression in gel-filtrated fractions of the intercellular fluid from TMV-infected tobacco leaves. Intercellular fluid of TMV-infected and necrotic lesion-forming tobacco leaves was concentrated and applied to a Sephadex G-15 column. The gel-filtrated fractions were tested for their ability to induce acidic PR-1a gene expression in leaf discs of PR1a-GUS transgenic tobacco plants. GUS activity in the leaf discs was determined 3 d after treatment with each fraction. The underlining bar shows the fractions to which authentic Spm was eluted. The experiment was repeated twice with similar results. FW, Fresh weight.
The presence of the second major peak of GUS activity in the gel-filtrated fractions (Fig. 1) suggests the presence of other inducers of PR gene expression.
Increase in Free Spm in the Intercellular Fluid of TMV-Infected Leaves
We extracted total endogenous free PAs after homogenization of necrotic lesion-developing tobacco leaves 4 d after TMV inoculation, and determined quantitatively each PA by HPLC. Unexpectedly, free Put was increased by about 60% by mechanical wounding resulting from mock inoculation and TMV infection. However, although Cad was increased slightly by TMV infection, Spd and Spm were decreased after wounding and TMV infection (Table I). Next, we extracted free PAs in the intercellular fluid of tobacco leaves 3 and 5 d after TMV inoculation and found the Spm content to be 18- and 29-fold higher than in healthy leaves, respectively (Fig. 2). Mock inoculation did not induce an increase in Spm. Whether there was an increase in Put, Cad, and Spd in the spaces was not clear because their contents were relatively low and separation from other substances was unsuccessful under our analysis conditions.
Table I.
Content of free PAs in TMV-infected and local lesion-forming tobacco leaves
| Sample | PA Level
|
|||
|---|---|---|---|---|
| Put | Cad | Spd | Spm | |
| nmol g fresh wt−1 | ||||
| Healthy | 318.3 | 14.3 | 143.4 | 24.7 |
| Mock | 510.6 | 11.4 | 68.6 | 13.3 |
| TMV | 498.4 | 16.7 | 82.2 | 12.7 |
Free PAs in leaves of healthy plants (Healthy), 4-d mock-inoculated plants (Mock), and TMV-inoculated plants were quantified and expressed as nanomoles in 1 g of leaf fresh weight. The experiment was repeated twice with similar results.
Figure 2.
Induced accumulation of Spm in the intercellular spaces of local lesion-forming tobacco leaves. Free Spm in the intercellular fluid was quantified after TMV or mock inoculation. Data are the mean values of triplicate samples. Error bars indicate sd. The experiment was repeated twice with similar results. FW, Fresh weight.
Spm Induces Expression of the PR-1a Gene in Tobacco Leaves
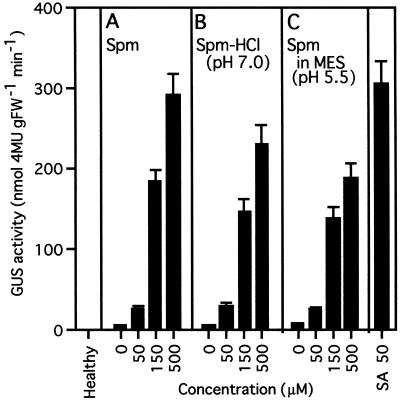
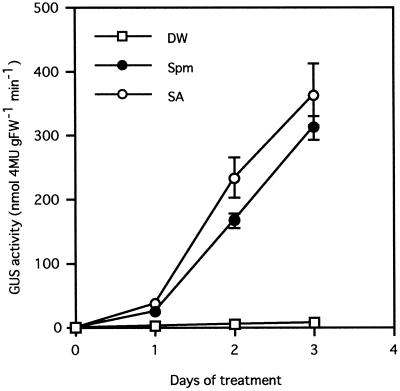
The effect of PAs on acidic PR-1 gene expression was studied in the PR1a-GUS transgenic tobacco system 3 d after treatment with various PAs. As shown in Figure 3, only very low levels of GUS activity were found in healthy and water-treated leaf discs. Although free Put or Cad treatment could not increase the activity, free Spd at a final concentration of 150 and 500 μm raised it 2.3- and 5.8-fold compared with the water-treated control, respectively. Free Spm at 50 μm (pH 8.5), 150 μm (pH 8.9), and 500 μm (pH 9.4) increased GUS activity 4-, 33-, and 48-fold, respectively. To confirm that the induction was specific for Spm rather than the alkaline pH, Spm was applied after neutralization with HCl to pH 7.0, or in Mes buffer at pH 5.5. The increase in GUS activity was lowered a minimal amount by alterations in pH. However, the extent of the increase was basically unchanged by Spm-HCl (Fig. 4B) and Spm in Mes buffer (Fig. 4C). Thus, the inducing effect of Spm was detected at all pH levels tested. Time-course analysis after treatment with 300 μm Spm showed that the kinetics of GUS induction by Spm was similar to that by 50 μm SA (Fig. 5). On the 1st d of treatment, only slight GUS activity was detected, but this activity increased linearly, reaching 910-fold and 1060-fold the control level 3 d after Spm and SA treatment, respectively.
Figure 3.
Induction of PR1a-GUS gene expression by PAs. GUS activity in the leaf discs of PR1a-GUS transgenic tobacco plants was determined 3 d after the treatment with various PAs. For a positive control, leaf discs were treated with 50 μm SA (pH 7.0). Data are the mean values of triplicate samples. Error bars indicate sd. The experiment was repeated twice with similar results. FW, Fresh weight.
Figure 4.
Induction of GUS activity by Spm at various pH levels. Leaf discs of PR1a-GUS plants were treated with free Spm in water (pH 8.5, 8.9, and 9.4 at 50, 150, and 500 μm, respectively) (A), with Spm neutralized with HCl (pH 7.0) (B), or with Spm in the presence of 10 mm Mes buffer (pH 5.5) (C). GUS activity in the leaf discs was determined 3 d after the treatment. For a positive control, leaf discs treated with 50 μm SA (pH 7.0) were used. Data are the mean values of triplicate samples. Error bars indicate sd. The experiment was repeated twice with similar results. FW, Fresh weight.
Figure 5.
Time-course analysis of PR1a-GUS gene expression induced by Spm and SA. Leaf discs of PR1a-GUS plants were treated with distilled water (DW, –□–), 300 μm Spm (pH 9.2) (–•–), or 50 μm SA (–○–), and GUS activity in the leaf discs was determined after 0 to 3 d. Data are the mean values of triplicate samples. Error bars indicate sd. The experiment was repeated twice with similar results. FW, Fresh weight.
In this experimental system, exogenously supplied Spd or Spm at 500 μm often induced small necrotic lesions in the edges of leaf discs. Treatment with 300 μm of these PAs sometimes caused faint necrotic lesions on young leaves. However, this necrosis barely affected the Spm-induced GUS activity in the leaf discs of PR1a-GUS transgenic tobacco plants. Also, 150 μm Spm, which did not cause visible necrosis during the experiment, clearly induced both GUS activity and expression of diverse PR proteins, as described below, suggesting that possible chemical injury by high levels of Spm is not the cause of PR gene activation.
Spm Induces Accumulation of a Set of Acidic PR Proteins
Induction of PR proteins by Spm was also confirmed at the protein level. Some acidic PR proteins were immunologically determined using specific antibodies. The results in Figure 6A show that acidic PR-1 proteins with similar Mrs, PR-1a, PR-1b, and PR-1c, migrated as one band in 15% SDS-polyacrylamide gels and that the signal was clearly increased by Spm treatment in a concentration-dependent manner. One of the acidic PR-5 proteins, PR-S, showed a more sensitive response to Spm treatment than acidic PR-1 proteins. Acidic PR-2 proteins carrying β-1,3-glucanase activity contain three isoforms with slightly different Mrs, PR-2, PR-N, and PR-O. These three isoforms were also increased by Spm treatment, although small amounts of PR-N and PR-O proteins were found in healthy leaves. Two acidic PR-3 proteins, PR-P and PR-Q, and basic chitinases were found in healthy leaves, resulting in faint bands upon immunodetection, and they were further increased by exogenously supplied Spm. To separate the acidic PR-2 and PR-3 proteins from the basic ones, native-PAGE was performed in a basic gel. In this gel system only the acidic proteins that have high mobilities can be separated from the basic proteins with low mobilities. As shown in Figure 6B, all acidic PR-2 and PR-3 proteins were shown to be induced by Spm treatment, as confirmed by mobility equal to that of standard acidic PR proteins, PR-2, PR-N, PR-O, PR-P, and PR-Q, respectively.
Figure 6.
Immunoblot analysis of acidic PR-1, PR-2, PR-3, and PR-5 proteins. Leaf pieces were treated with 150, 300, or 500 μm Spm for 3 d. After tissue homogenization, soluble protein was extracted. To evaluate acidic PR protein induction, 50 μm SA-treated leaves and leaves 3 d after TMV infection were also extracted as positive controls. Fifteen micrograms of protein (equivalent to 3 mg of fresh leaf material) was subjected to SDS-PAGE (A) or native-PAGE (B). Immunodetection was performed with specific antibodies against individual purified PR proteins (see Methods). Each PR protein was identified by comparison of its mobility with that of Mr markers or standard samples of purified PR proteins. BCs, Basic chitinases. The experiment was repeated twice with similar results.
Exogenously Supplied SA Does Not Induce Accumulation of Spm and Spm Fails to Induce SA
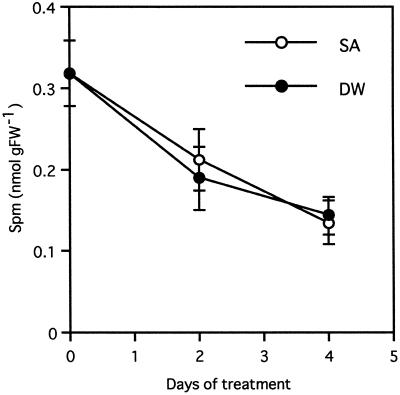
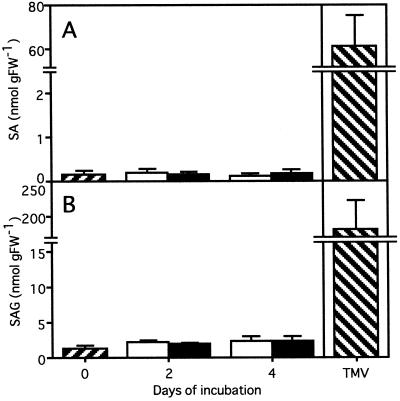
Time-course analysis showed that the kinetics of PR-1 gene induction by Spm was similar to that by SA (Fig. 5). To assess whether PR gene activation by Spm occurs through SA, the relationship between Spm and SA as signaling compounds for PR gene expression was analyzed. First, the Spm in the intercellular fluid was quantified in leaves floated in a 500 μm SA solution. The basal amount of Spm in the intercellular fluid decreased gradually with or without SA treatment, suggesting that SA had no significant effect on synthesis, secretion, or degradation of Spm at least within 4 d after treatment (Fig. 7). Second, SA and SAG were quantified in the leaves treated with 300 μm Spm. As shown in Figure 8, Spm failed to raise the levels of endogenous SA and SAG within 4 d after the treatment, whereas the levels increased to 61.4 and 181 nmol g fresh weight−1 in TMV-infected leaves, corresponding to 370 and 140 times the level in healthy leaves, respectively.
Figure 7.
Effect of SA treatment on the amount of Spm recovered from the intercellular fluid. Free Spm was quantified in the intercellular fluid of tobacco leaves that were incubated for 2 or 4 d in 500 μm SA solution or in distilled water (DW). Data are the mean values of triplicate samples. Error bars indicate sd. The experiment was repeated twice with similar results. FW, Fresh weight.
Figure 8.
Effect of exogenously supplied Spm on the accumulation of SA and SAG. SA (A) and SAG (B) were quantified 2 or 4 d after incubation with 300 μm Spm (▪) or distilled water (□). As a positive control, leaves were used 4 d after TMV inoculation (▧). Data are the mean values of triplicate samples. Error bars indicate sd. The experiment was repeated twice with similar results. FW, Fresh weight.
Spm Induces TMV Resistance in Tobacco Leaves
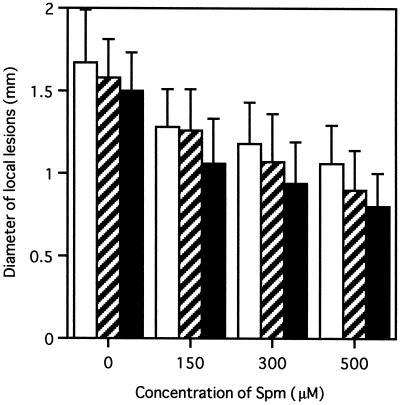
Spm was induced in the intercellular spaces of TMV-infected and local lesion-forming tobacco leaves. Because local acquired resistance against secondary infection by pathogens is established in the tissues around local lesions (Ross, 1961), we studied whether Spm induces resistance against TMV infection. Spm solution was fed through petioles of detached tobacco leaves at 300 μm. The leaves were inoculated with TMV immediately or 2 d after the treatment and incubated for an additional 4 d. The diameters of the lesions on the apical, middle, and basal parts of the leaves were measured separately. When TMV inoculation was performed immediately after Spm treatment, the size of the developed lesions showed only a slight reduction to 98%, 92%, and 85%, respectively, of that in water-treated control leaves. However, when leaves were inoculated 2 d after the treatment, Spm application reduced lesion size to 80%, 70%, and 56% of the respective controls (Fig. 9). Evaluation by a Student's t test resulted in a P value less than 0.001 for each part between Spm- and water-treated leaves. In another experiment, detached leaves were treated with 150, 300, and 500 μm Spm, and after 2 d of preincubation, TMV was inoculated. Four days later, the diameters of necrotic lesions formed on the leaves were measured. The result in Figure 10 shows that Spm treatment significantly reduced lesion size in all leaf parts (P < 0.001 in Student's t test). The reduction was dose dependent.
Figure 9.
Induced resistance against TMV infection by Spm. Detached tobacco leaves were inoculated with TMV 2 d after the treatment with 300 μm Spm solution or distilled water and incubated for a further 4 d at 22°C. Then, the lesion size was measured in three areas: apical (□), middle (▨), and basal (▪) sections were evaluated using enlarged photocopies. At least 100 lesions from four leaves were measured for each section. Data are mean values. Error bars indicate sd. Evaluation by a Student's t test resulted in P < 0.001 for each section between Spm- and water-treated leaves. Lesions that developed in basal parts of preincubated leaves are shown in B. The experiment was repeated twice with similar results.
Figure 10.
Induction of resistance against TMV by Spm. Detached tobacco leaves were treated with various concentrations of Spm for 2 d and inoculated with TMV. The diameter of developed local lesions was measured after 4 additional d of incubation. The measurement was performed separately in three areas: apical (□), middle (▨), and basal (▪). At least 100 lesions from 4 leaves were measured for each section. Data are mean values. Error bars indicate sd.
DISCUSSION
There is limited evidence that PAs play a role in plant self-defense. Here we report the quantitative analysis of PAs in both whole-leaf tissues and the intercellular spaces, and propose a new Spm signaling pathway for PR protein gene expression that differs from that of SA. The Spm accumulated in the intercellular spaces of TMV-infected leaves may function as a natural signal molecule to induce PR proteins and confer resistance against further TMV infection.
Low-molecular-mass substances exhibiting basic pH were found in the intercellular extract of TMV-infected tobacco leaves to have inducing activity for acidic PR-1 genes. The results of quantitative analysis of free PAs showed a specific increase in free Spm in the intercellular spaces after local lesion formation. However, we could not detect such an increase when whole-leaf tissues were used, suggesting the following possibilities. First, synthesis of Spm is highly activated in necrotic tissue, yet the total amount of Spm decreases because consumption is enhanced. This possibility is supported by the finding that Put and Spd, precursors of Spm, are synthesized and highly accumulated around necrotic lesions on TMV-infected tobacco (Torrigiani et al., 1997). We in the present study and Scalet et al. (1991) found that the amount of Put was significantly increased by mechanical wounding in both TMV-infected and mock-inoculated tobacco leaves and in wounded chickpea leaves, respectively. The second possibility is that the increase in Spm in the intercellular fluid is the result of leakage from damaged cells in necrotic lesions. In healthy tobacco leaves, only 1% of total Spm (0.3 nmol g−1 fresh weight) is found in the intercellular spaces. However, 60% of Spm (7.5 nmol g−1 fresh weight) is detected in these spaces in local lesion-forming leaves 4 d after TMV inoculation (Fig. 2). The area occupied by necrotic lesions in the TMV-infected leaves was less than 30% of total leaf area, and the other 70% was free from cell disruption. Even if all of the Spm in the disrupted cells in local lesions leaked out to the intercellular fluid, 7.5 nmol g−1 fresh weight, which is the value for Spm found in the intercellular spaces 4 d after TMV infection, could not be calculated from 12.7 nmol g−1 fresh weight, the value for total Spm of TMV-infected leaves (Table I), except by contribution of positive Spm transportation to the intercellular fluid in adjacent healthy cells. These results suggest enhanced production and secretion of Spm to the spaces in lesion-formed leaves but not leakage from disrupted cells. For the last possibility, it is also likely that increased Spm could be converted to conjugated forms such as hydroxycinnamic acid amides (for review, see Tiburcio et al., 1990).
In the analysis of PR1a-GUS plants, Spm, among various PAs, could increase GUS activity. Although alkaline pH enhanced the GUS induction by Spm, the induction was always observed at different pHs of the solution, suggesting that expression of the PR-1a gene is specifically induced by Spm rather than by alkaline pH. It is interesting that the kinetics of induction of GUS activity by Spm were almost the same as that by SA. Immunoblot analysis showed that Spm induces a diverse range, not only of acidic PR proteins such as PR-1a, PR-1b, PR-1c, PR-2, PR-N, PR-O, PR-P, PR-Q, and PR-S, but also of basic PR-3 proteins. These findings strongly suggest that Spm is an endogenous signal for accumulation of acidic and probably also basic PR proteins.
Because exogenously applied Spm induced acidic PR proteins, which are widely considered to be molecular markers of HR, we also expected Spm to induce acquired resistance against TMV. In fact, exogenously supplied Spm reduced the lesion size in a concentration-dependent manner, suggesting that Spm enhances the resistance of tobacco against TMV infection. This phenomenon is well correlated with dose-dependent induction of PR proteins by Spm (Fig. 6).
We showed that TMV spread was considerably inhibited by Spm, which also induced acidic PR proteins in a concentration-dependent manner in tobacco leaves. It is known that overexpression of acidic PR proteins results in acquisition of certain antifungal activities in tobacco plants (Alexander et al., 1993). However, as there is no direct evidence that PR proteins inhibit TMV multiplication, we speculate that Spm would induce PR protein accumulation and TMV resistance separately. PR protein induction appears to be one of the self-defense mechanisms induced by Spm, and other molecules contributing to virus resistance could be induced by Spm.
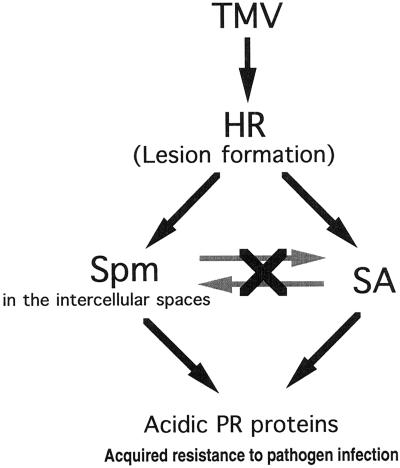
In the signaling pathway leading to induction of acidic PR proteins, the relationship between Spm and SA as the signals was evaluated. Exogenously supplied SA caused no significant change in the amount of Spm for at least 2 to 4 d after the treatment. Conversely, exogenously supplied Spm did not raise levels of endogenous SA and SAG. These results suggest two independent pathways for acidic PR protein induction: SA mediated and Spm mediated (Fig. 11).
Figure 11.
Proposed model for the pathway signaling acidic PR protein induction and acquisition of resistance. Spm accumulated in the intercellular spaces of TMV-infected tobacco leaves induced a high level of acidic PR protein expression and conferred resistance to further infection by TMV. This induction was not affected by SA. See text for details.
Based on the evidence described in this paper, we propose a critical role for the intercellular spaces in terms of Spm accumulation. The leaf tissues infected with TMV and the neighboring tissues gradually dehydrate with completion of local lesion formation, so the fluid in the intercellular spaces becomes concentrated. Therefore, the level of Spm accumulated in the intercellular spaces after lesion formation would be high enough to induce expression of PR proteins and to confer acquired resistance. It should be noted that induction of GUS activity by Spm in the PR1a-GUS transgenic plants is relatively weak when compared with that by SA. Spm reduces lesion size to a lesser extent than SA, which reduces it by about 80% (Conrath et al., 1995); however, this response may serve as a back-up system of SA signaling to ensure HR at leaves infected by TMV. To our knowledge, this is the first evidence directly linking PA to the plant-defense response against viral infection.
ACKNOWLEDGMENTS
We are grateful to Shinsuke Fujihara (Shikoku National Agricultural Experiment Station, Kagawa, Japan) for valuable advice regarding PA quantification. We also thank Hiroki Matsufuru, Shigemi Seo, Norihiro Ohtsubo, Ichiro Mitsuhara, Kamal A. Malik, Shunichi Kosugi, Tomoya Niki, Susumu Hiraga, and Taka Murakami for helpful discussions, and Y. Gotoh, H. Ochiai, Y. Naitoh, and Y. Matsuda for maintenance of the plants.
Abbreviations:
- Cad
cadaverine
- HR
hypersensitive reaction
- PA
polyamine
- PR
pathogenesis-related
- Put
putrescine
- SA
salicylic acid
- SAG
salicylic acid β-glucoside
- Spd
spermidine
- Spm
spermine
- TMV
tobacco mosaic virus
Footnotes
This work was supported by grants from the Center of Excellence and Core Research for Evolutional Science and Technology.
LITERATURE CITED
- Alexander D, Goodman RM, Gut-Rella M, Glascock C, Weymann K, Friedrich L, Maddox D, Ahl-Goy P, Luntz T, Ward E and others. Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proc Natl Acad Sci USA. 1993;90:7327–7331. doi: 10.1073/pnas.90.15.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertossi F, Bagni N, Moruzzi G, Caldarera CM. Spermine as a new growth-promoting substance for Helianthus tuberosus (Jerusalem artichoke) in vitro. Experientia. 1965;21:80–81. [Google Scholar]
- Conrath U, Chen Z, Ricigliano JR, Klessig DF. Two inducers of plant defense responses, 2,6-dichloroisonicotinic acid and salicylic acid, inhibit catalase activity in tobacco. Proc Natl Acad Sci USA. 1995;92:7143–7147. doi: 10.1073/pnas.92.16.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ. Method and application to human serum proteins. Ann NY Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Laat AMM, Van Loon LC. The relationship between stimulated ethylene production and symptom expression in virus-infected tobacco leaves. Physiol Plant Pathol. 1983;22:261–273. [Google Scholar]
- Flores HE, Galston AW. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982;69:701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores HE, Galston AW. Osmotic stress-induced polyamine accumulation in cereal leaves. Plant Physiol. 1984;75:102–109. doi: 10.1104/pp.75.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich L, Lawton K, Ruess W, Masner P, Specker N, Rella MG, Meier B, Dincher S, Staub T, Uknes S and others. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J. 1996;10:61–70. [Google Scholar]
- Gallagher S, Winston SE, Fuller SA, Hurrell JGR. Immunoblotting and immunodetection. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: Greene Publishing and Wiley-Interscience; 1996. pp. 10.8.1–10.8.17. [Google Scholar]
- Gallagher SR. One-dimensional SDS gel electrophoresis of proteins. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: Greene Publishing and Wiley-Interscience; 1996. pp. 10.2.1–10.2.35. [Google Scholar]
- Gianinazzi S, Kassanis B. Virus resistance induced in plants by polyacrylic acid. J Gen Virol. 1974;23:1–9. [Google Scholar]
- Goodman RN, Novacky AJ (1994) The Hypersensitive Reaction in Plants to Pathogens, A Resistance Phenomenon. APS Press, St. Paul, MN
- Greenland AJ, Lewis DH. Amines in barley leaves infected by brown rust and their possible relevance to formation of “green islands.”. New Phytol. 1984;96:283–291. [Google Scholar]
- Herbers K, Meuwly P, Métraux J-P, Sonnewald U. Salicylic acid-independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett. 1996;397:239–244. doi: 10.1016/s0014-5793(96)01183-0. [DOI] [PubMed] [Google Scholar]
- Hosokawa D, Ohashi Y. Immunochemical localization of pathogenesis-related proteins secreted into the intercellular spaces of salicylate-treated tobacco leaves. Plant Cell Physiol. 1988;29:1035–1040. [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann S, Legrand M, Geoffroy P, Fritig B. Biological function of ‘pathogenesis-related’ proteins: four PR proteins of tobacco have 1,3-β-glucanase activity. EMBO J. 1987;6:3209–3212. doi: 10.1002/j.1460-2075.1987.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig DF, Malamy J. The salicylic acid signal in plants. Plant Mol Biol. 1994;26:1439–1458. doi: 10.1007/BF00016484. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y, Nakajima K, Arai Y. An improved assay for β-glucuronidase in transformed cells: methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant Sci. 1990;70:133–140. [Google Scholar]
- Legrand M, Kauffmann S, Geoffroy P, Fritig B. Biological function of pathogenesis-related proteins: four tobacco pathogenesis-related proteins are chitinases. Proc Natl Acad Sci USA. 1987;84:6750–6754. doi: 10.1073/pnas.84.19.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Raghothama KG, Hasegawa PM, Bressan RA. Osmotin overexpression in potato delays development of disease symptoms. Proc Natl Acad Sci USA. 1994;91:1888–1892. doi: 10.1073/pnas.91.5.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Malamy J, Hennig J, Klessig DF. Temperature-dependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell. 1992;4:359–366. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J, Sánchez-Casas P, Hennig J, Guo A, Klessig DF. Dissection of the salicylic acid signaling pathway in tobacco. Mol Plant-Microbe Interact. 1996;9:474–482. [Google Scholar]
- McDonald RE, Kushad MM. Accumulation of putrescine during chilling injury of fruit. Plant Physiol. 1986;82:324–326. doi: 10.1104/pp.82.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niderman T, Genetet I, Bruyère T, Gees R, Stintzi A, Legrand M, Fritig B, Mösinger E. Pathogenesis-related PR-1 proteins are antifungal. Isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans. Plant Physiol. 1995;108:17–27. doi: 10.1104/pp.108.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y, Matsuoka M. Synthesis of stress proteins in tobacco leaves. Plant Cell Physiol. 1985;26:473–480. [Google Scholar]
- Ohashi Y, Matsuoka M. Induction and secretion of pathogenesis-related proteins by salicylate or plant hormones in tobacco suspension cultures. Plant Cell Physiol. 1987a;28:573–580. [Google Scholar]
- Ohashi Y, Matsuoka M. Localization of pathogenesis-related proteins in the epidermis and intercellular spaces of tobacco leaves after their induction by potassium salicylate or tobacco mosaic virus infection. Plant Cell Physiol. 1987b;28:1227–1235. [Google Scholar]
- Ohashi Y, Ohshima M. Stress-induced expression of genes for pathogenesis-related proteins in plants. Plant Cell Physiol. 1992;33:819–826. [Google Scholar]
- Ohshima M, Itoh H, Matsuoka M, Murakami T, Ohashi Y. Analysis of stress-induced or salicylic acid-induced expression of the pathogenesis-related 1a protein gene in transgenic tobacco. Plant Cell. 1990;2:95–106. doi: 10.1105/tpc.2.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent J-G, Asselin A. Detection of pathogenesis-related proteins (PR or b) and of other proteins in the intercellular fluid of hypersensitive plants infected with tobacco mosaic virus. Can J Bot. 1984;62:564–569. [Google Scholar]
- Ross AF. Localized acquired resistance to plant virus infection in hypersensitive hosts. Virology. 1961;14:329–339. doi: 10.1016/0042-6822(61)90318-x. [DOI] [PubMed] [Google Scholar]
- Samborski DJ, Rohringer R. Abnormal metabolites of wheat: occurrence, isolation and biogenesis of 2-hydroxyputrescine amides. Phytochemistry. 1970;9:1939–1945. [Google Scholar]
- Scalet M, Federico R, Angelini R. Time course of diamine oxidase and peroxidase activities, and polyamine changes after mechanical injury of chickpea seedlings. J Plant Physiol. 1991;137:571–575. [Google Scholar]
- Seo S, Ishizuka K, Ohashi Y. Induction of salicylic acid β-glucosidase in tobacco leaves by exogenous salicylic acid. Plant Cell Physiol. 1995;36:447–453. [Google Scholar]
- Shulaev V, Silverman P, Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. [Google Scholar]
- Smith TA, Best GR. Distribution of the hordatines in barley. Phytochemistry. 1978;17:1093–1098. [Google Scholar]
- Stroinski A, Szczotka Z. Effect of cadmium and Phytophthora infestans on polyamine levels in potato leaves. Physiol Plant. 1989;77:244–246. [Google Scholar]
- Tiburcio AF, Kaur-Sawhney R, Galston AW (1990) Polyamine metabolism. In BJ Miflin, PJ Lea, eds, The Biochemistry of Plants: Intermediary Nitrogen Metabolism. Academic Press, New York, pp 283–325
- Torrigiani P, Rabiti AL, Bortolotti C, Betti L, Marani F, Canova A, Bagni N. Polyamine synthesis and accumulation in the hypersensitive response to TMV in Nicotiana tabacum. New Phytol. 1997;135:467–473. [Google Scholar]
- Van Loon LC, Pierpoint WS, Boller Th, Conejero V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol Biol Rep. 1994;12:243–264. [Google Scholar]
- Van Loon LC, Van Kammen A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. “Samsun” and “Samsun NN.”. Virology. 1970;40:199–211. doi: 10.1016/0042-6822(70)90395-8. [DOI] [PubMed] [Google Scholar]
- Vierheilig H, Alt M, Neuhaus J-M, Boller T, Wiemken A. Colonization of transgenic Nicotiana sylvestris plants, expressing different forms of Nicotiana tabacum chitinase, by the root pathogen Rhizoctonia solani and by the mycorrhizal symbiont Glomus mosseae. Mol Plant-Microbe Interact. 1993;6:261–264. [Google Scholar]
- Vigers AJ, Wiedemann S, Roberts WK, Legrand M, Selitrennikoff CP, Fritig B. Thaumatin-like pathogenesis-related proteins are antifungal. Plant Sci. 1992;83:155–161. [Google Scholar]
- Walden R, Cordeiro A, Tiburcio AF. Polyamines: small molecules triggering pathways in plant growth and development. Plant Physiol. 1997;113:1009–1013. doi: 10.1104/pp.113.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward ER, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, Ahl-Goy P, Métraux J-P, Ryals JA. Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell. 1991;3:1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–412. doi: 10.1016/0042-6822(79)90019-9. [DOI] [PubMed] [Google Scholar]
- Young ND, Galston AW. Putrescine and acid stress. Plant Physiol. 1983;71:767–771. doi: 10.1104/pp.71.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]