Abstract
Volumetric modulated arc therapy (VMAT) is a novel radiation technique, which can achieve highly conformal dose distributions with improved target volume coverage and sparing of normal tissues compared with conventional radiotherapy techniques. VMAT also has the potential to offer additional advantages, such as reduced treatment delivery time compared with conventional static field intensity modulated radiotherapy (IMRT). The clinical worldwide use of VMAT is increasing significantly. Currently the majority of published data on VMAT are limited to planning and feasibility studies, although there is emerging clinical outcome data in several tumour sites. This article aims to discuss the current use of VMAT techniques in practice and review the available data from planning and clinical outcome studies in various tumour sites including prostate, pelvis (lower gastrointestinal, gynaecological), head and neck, thoracic, central nervous system, breast and other tumour sites.
There have been significant advances in the delivery of radiotherapy over the past few decades. These include increased sophistication of imaging techniques, which has resulted in improved accuracy of target volume definition and delineation [1], as well as developments in treatment planning systems and linear accelerator delivery capabilities leading to improved dose distributions and conformity [2]. These developments have been mainly driven by the need to reduce the dose to normal tissue structures and thereby minimise the risk of toxicity and morbidity, which then allows dose escalation to the tumour volume potentially leading to improved locoregional control. To that end, newer radiation techniques, e.g. intensity modulated radiotherapy (IMRT), have been developed. IMRT techniques employ variable intensity across multiple radiation beams leading to the construction of highly conformal dose distributions. This is achieved by subdividing each radiation beam into smaller radiation beamlets and varying the individual intensities of these beamlets [3-5]. The advantages of this technique are improved target volume conformity, particularly in volumes with complex concave shapes, and improved sparing of normal tissues and organs at risk (OARs) resulting in reduced acute and late toxicities [6-9]. IMRT also has the ability to produce inhomogeneous dose distributions, which allows the simultaneous delivery of different doses per fraction to separate areas within the target volume. This could facilitate localised dose escalation strategies without increasing total treatment time (for example, by using hypofractionated regimens), which may have the potential radiobiological benefit of reducing the impact of accelerated repopulation in tumour clonogens [10,11].
Despite the obvious benefits of IMRT, there are still some disadvantages. The planning and quality assurance (QA) processes required for IMRT are more complex and time-consuming compared with conventional conformal radiotherapy (CRT) techniques, which can have significant impact on departmental resources [12-15]. However, several commercial systems are now available that allow multiple plan measurement of IMRT plans and facilitate batching of patient QA measurements to improve efficiency. A standard IMRT plan often requires multiple fixed angle radiation beams, which can increase treatment delivery time. This can impact on patient comfort on the treatment couch, reproducibility of treatment position and intrafraction motion. There are also some concerns that the increased treatment time could have radiobiological implications owing to the possibility of increased tumour cell repair and repopulation during the extra time required to deliver the treatment [16-18].
IMRT plans use a larger number of monitor units (MU) compared with conventional CRT plans leading to an increase in the amount of low dose radiation to the rest of the body. The number of MU used in fixed field IMRT depends, to some degree, on the IMRT technique; usually more MU are required in the sliding window (SW) or dynamic IMRT technique [19,20]. In this technique, each radiation beam is modulated by continuously moving multileaf collimators (MLCs). This is in contrast to the step-and-shoot (SS) or static techniques in which each beam is subdivided into multiple segments with differing MLC shapes and the beam is switched off between segments. The increase in MU and subsequent increase in low dose radiation has led to concerns of increased risk of secondary radiation-induced malignancies, which is of particular relevance in paediatric patients or patients with long life expectancies [21-23]. There are estimates in the literature that the number of MU in an IMRT plan is two to three times higher than a conventional radiotherapy plan [21,24] with an increase in the incidence of radiation-induced secondary malignancies from 1−1.75% for patients who survive for 10 years or more [21].
More recently, there has been some interest in arc-based or rotational therapies in an attempt to overcome some of the limitations associated with fixed field IMRT. The basic concept of arc therapy is the delivery of radiation from a continuous rotation of the radiation source and allows the patient to be treated from a full 360° beam angle. Arc therapies have the ability to achieve highly conformal dose distributions and are essentially an alternative form of IMRT. However, a major advantage over fixed gantry IMRT is the improvement in treatment delivery efficiency as a result of the reduction in treatment delivery time and the reduction in MU usage with subsequent reduction of integral radiation dose to the rest of the body [25-27]. In addition to the subsequent advantages from the shorter treatment delivery time, a further potential benefit is the availability of extra time within a set treatment appointment time slot to employ image-guided radiotherapy (IGRT). IGRT involves the incorporation of imaging before and/or during treatment to enable more precise verification of treatment delivery and allow for adaptive strategies to improve the accuracy of treatment [28,29]. The main drawback of IGRT is the requirement for more time on the treatment couch and an increase in the total amount of radiation to the patient, especially with daily IGRT imaging schedules. These disadvantages are less of an issue with arc therapies, which have shorter treatment delivery times and fewer MU.
There are two main forms of arc-based therapies: tomotherapy and volumetric modulated arc therapy (VMAT). Tomotherapy (i.e. “slice therapy”) machines can be considered to be a combination of a CT scanner and a linear accelerator that can deliver the radiation in a fan-shaped distribution, similar to CT imaging with a continuously rotating radiation source, while the patient is moved through the machine. Tomotherapy techniques can be subdivided into axial or serial tomotherapy (where the radiation is delivered slice by slice) or helical tomotherapy (HT) (where the radiation is delivered in a continuous spiral) [30-33]. There is limited data on axial tomotherapy in comparison with fixed field IMRT. HT has been evaluated in a variety of tumour sites and it can generally achieve either similar or improved dose distributions compared with fixed field IMRT, with variable results on treatment time comparisons [34-40]. The details of these studies and a review of the use of HT have been discussed elsewhere [25] and will not be discussed in detail in this paper.
VMAT was first introduced in 2007 and described as a novel radiation technique that allowed the simultaneous variation of three parameters during treatment delivery, i.e. gantry rotation speed, treatment aperture shape via movement of MLC leaves and dose rate [41]. The earlier form of arc therapy, termed intensity modulated arc therapy (IMAT) was first described by Yu in 1995 [26] and required the use of multiple superimposed arcs to achieve a satisfactory dose distribution [42]. More recent VMAT techniques have allowed the whole target volume to be treated using one or two arcs, although complex cases may require more. In a recent review, VMAT is essentially described as a form of single arc IMAT technique that employs dose rate variation [43]. One benefit of VMAT compared with tomotherapy is the possibility of delivering this treatment on conventional linear accelerators, which are configured to have this capability. Currently there are several VMAT systems available under various names (RapidArc, Varian; SmartArc, Phillips; and Elekta VMAT, Elekta). The main aim of this review is to discuss the current use of VMAT techniques in practice, and review the available data from planning studies and the clinical outcomes in various tumour sites. A second aim is to identify future areas for research in this field. A systematic review was conducted using PubMed/MEDLINE with the keywords “volumetric” and “arc”. 165 articles were identified of which 65 were relevant for the purpose of this review.
Prostate cancer
Prostate cancer is one of the most common tumour sites treated with IMRT worldwide. The use of IMRT allows dose escalation, which has been shown to improve clinical outcomes while simultaneously reducing toxicity by improved OAR sparing [8,44-50]. As a result, IMRT is now the standard technique employed for primary prostate radiotherapy at several institutions. Therefore, this is a logical starting point for the evaluation of alternative IMRT techniques, such as VMAT. One of the earliest VMAT planning studies was performed by Palma et al [51] in which a planning comparison was performed in 10 patient datasets between standard three-dimensional (3D)-CRT, fixed field IMRT using 5 coplanar fields (SW), constant dose rate-VMAT (CDR-VMAT) and variable dose rate-VMAT (VDR-VMAT). The results report significantly improved OAR sparing with both IMRT and VMAT plans compared with 3D-CRT, with acceptable planning target volume (PTV) coverage. The lowest doses to the OARs were achieved in the VDR-VMAT plans, which required 42% fewer MU compared with the fixed field IMRT plans.
The improved OAR sparing with VMAT has been reported in other planning studies. A planning study of 11 prostate cancer patients at Memorial Sloan Kettering Cancer Centre compared 5-field fixed field IMRT (SS) with VMAT [52]. They report improved rectal wall sparing with a resultant improved Normal Tissue Complication Probability (NTCP) of rectal wall by 1.5%, and lower doses to the bladder wall (not statistically significant) and femoral heads. Similar findings were seen in a Danish study comparing single partial arc VMAT with fixed field IMRT (SW) which showed improved bladder and rectal sparing [53]. Hardcastle et al [54] also found lower doses to the rectum with resultant lower rectal NTCP in their study comparing VMAT to seven-field fixed field IMRT (SS). Ost et al [55] compared fixed field IMRT (SS) with VMAT for prostate radiotherapy with a simultaneous integrated dose-escalated boost to intraprostatic lesions defined with MRI with or without magnetic resonance spectroscopy. In this study, fixed field IMRT plans using 3 fields, 5 fields and 7 fields were generated for each of the 12 patient datasets. Compared with all three, the VMAT plans performed better in reducing the dose to the rectum which was statistically significant in the volumes receiving doses between 20 Gy and 50 Gy (e.g. V50 Gy was 45% in the 7-field IMRT vs 32% in VMAT, p = 0.001). Another study by Weber et al [56] compared 5-field IMRT (SW) with intensity modulated proton therapy (IMPT) and VMAT for recurrent prostate cancer previously treated with radiotherapy and found improved OAR sparing with IMPT and VMAT compared with IMRT. More recently a large study of 292 patient datasets comparing VMAT and 7-field fixed field IMRT (SW) showed that VMAT could achieve lower mean doses to the bladder and rectum, particularly in the high dose regions [57].
However, other planning studies have reported contradictory results in terms of OAR sparing. Yoo et al [58] performed a planning study comparing fixed field IMRT using 7 coplanar fields to single and double arc VMAT in 10 patients with high risk prostate cancer and reported lower mean doses to the OARs in the IMRT plans. For instance the mean dose to the rectum was 35.5 Gy in the IMRT plan compared with 40.2 Gy in the single arc and 37.5 Gy in the double arc VMAT plans. The differences were statistically significant for rectal and small bowel doses. Another study by Wolff et al [59] which compared VMAT with serial tomotherapy, fixed field IMRT (SS) and 3D-CRT found lower mean doses to the rectum with tomotherapy and IMRT compared with VMAT plans. Tsai et al [60] compared VMAT with fixed field IMRT (SS) and HT and found superior dose conformity and OAR sparing with HT. A similar planning study by Rao et al [61] comparing VMAT with HT and fixed field IMRT (SS) found largely equivalent sparing of OARs between the three techniques (although maximum dose to the femoral head was lower by an average of 1.3 Gy in the VMAT plans compared with HT).
Overall, most of these planning studies have reported comparable and acceptable PTV coverage with VMAT techniques compared with fixed field IMRT. The results on target volume homogeneity and conformity are more conflicting, with some studies reporting improved conformity and/or homogeneity with VMAT [58,61] while others reported better results with fixed field IMRT [59,64]. This variation could be due to a number of factors including the number of arcs used in the VMAT plans (in general, double arc plans can achieve higher conformity and homogeneity compared with single arc plans), the type of VMAT optimisation approach and the number of fields used in the fixed field IMRT plans. Generally, better quality IMRT plans can be obtained with larger number of fields and this could explain the better results with fixed field IMRT found in the studies by Yoo et al [58] and Wolff et al [59]. However, it is worth bearing in mind that many institutions have adopted a five-field technique over seven- or nine-fields as their class solution for prostate IMRT because of greater efficiency and ease of treatment delivery and verification. Another difficulty in analysing these planning studies is the differences in target volume definition and dose prescriptions. For example, some studies have defined their primary planning target volume (PTV) as the prostate or prostate and seminal vesicles only [52,59] while in the study by Yoo et al [58], the primary PTV was larger because the pelvic lymph nodes were included in the target volume. Dose prescriptions also varied with some studies also evaluating the feasibility of dose escalation [62,64]. Shaffer et al [62] evaluated the simultaneous integrated boost (SIB) technique with a boost to intraprostatic lesions (up to 88.8 Gy) and found improved coverage of the boost region with VMAT compared with fixed field IMRT (SW) with acceptable doses to the OARs.
One of the major concerns with any IMRT technique is the potential increased risk of radiation-induced secondary malignancy. In most studies, the volume of normal tissue receiving radiation dose is defined as the integral dose, which is proportional to the product of dose multiplied by the number of voxels for unit density. This is dependent on a number of factors including the number of MU, which is associated with scattered and leakage radiation from the linear accelerator. In these planning studies, VMAT plans generally use fewer MU (up to 65% fewer) compared with fixed field IMRT [52,53,58]. However, the results on integral dose are more varied. Some studies have reported no difference in integral doses between VMAT and IMRT [53,55], while others report a higher integral dose with VMAT compared with IMRT [58]. Yoo et al [58] have reported that a possible reason for this discrepancy is that integral dose is dependent not only on MU, but also on target volume and aperture size and shape. It is worth noting that the dose distribution obtained in VMAT plans generally show an increase in the volume of the area receiving low dose radiation compared with fixed field IMRT due to the spread of dose from the entire arc of 360°. In a study by Zhang et al [52] normal tissue doses in VMAT plans were reported as lower in the intermediate to high dose levels (28–48 Gy), but higher in the low dose levels (below 22 Gy) compared with fixed field IMRT. An argument for the use of IMRT techniques, including VMAT, is the increase in conformity with the resultant reduced higher dose to normal tissues outside the target volume, which may in fact compensate for the increased low dose radiation [63].
A common finding from these planning studies is the improved efficiency of VMAT delivery with a reduction in treatment delivery times [52,58-60]. A single arc VMAT treatment fraction can potentially be delivered in 1–1.5 min compared with 5–10 min with a 5- or 7-field IMRT fraction. The potential benefits of faster treatment times have already been discussed above. The issue of intrafraction motion may be of particular relevance in prostate radiotherapy as there may be significant changes in rectal and bladder volumes within the time period required to deliver an IMRT fraction. This could potentially compromise target volume coverage and reduce tumour local control. In addition, the use of hypofractionated treatments that use larger doses per fraction is becoming increasingly common, particularly in prostate cancer, which is estimated to have a lower α/β ratio than other tumours and therefore theoretically could benefit more from hypofractionated schedules [65-68]. The impact of this change in treatment schedules is increased MU and treatment time per fraction where faster VMAT delivery techniques may be an attractive solution. It is worth noting that the optimisation and dose calculation times for VMAT planning are longer compared with fixed field IMRT (up to ×4) [58]. However, VMAT technology is still developing and newer versions of the planning algorithm software as well as increasing experience and expertise may well speed up the optimisation and planning process.
Few studies have reported clinical outcome data because of the novelty of VMAT technology. Pesce et al [69] reported their results on 45 patients treated with VMAT (RapidArc, Varian) in their institution. In terms of acute toxicity (graded by the National Cancer Institute Common Terminology Criteria of Adverse Effects (NCI CTCAE) version 3), there was no acute Grade 2 or 3 rectal toxicity reported while 12% of patients experienced Grade 2 dysuria and 44% had preserved erectile function. Biochemical response recorded at 6 weeks showed median PSA levels reduced to 0.4. Further follow-up will be required to evaluate clinical outcome such as local control and survival as well as late toxicity parameters. The issue of secondary malignancy induction will be of particular interest given that it is still too early to quantify this risk accurately for IMRT and VMAT techniques.
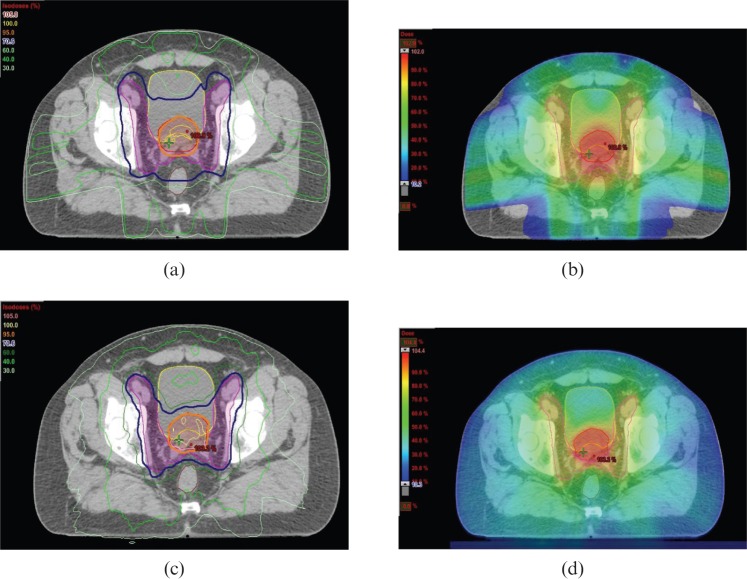
A summary of the comparative planning studies evaluating VMAT in prostate cancer is presented in Table 1. An example of the dose distributions achieved with VMAT and fixed field IMRT for prostate cancer is illustrated in Figure 1.
Table 1. Comparative planning studies in prostate cancer.
| Paper [ref] VMAT commercial system | Number of patients | Site and dose | Comparison | PTV | OAR | MU per fraction | Treatment time per fraction |
| Palma et al [51] Predecessor to RapidArc | 10 | Prostate alone 74 Gy in 37 fractions | 3D-CRT vs IMRT(5F,SW) vs CDR-VMAT (SA) vs VDR-VMAT (SA) | IMRT and VMAT – similar PTV coverage and homogeneity (homogeneity inferior to 3D-CRT). Conformity best with IMRT and VDR-VMAT | VDR-VMAT best (compared with IMRT for sparing of rectum and femoral heads; compared with CDR-VMAT for sparing of bladder and rectum) | CDR-VMAT, 491.6; VDR-VMAT, 454.2; IMRT, 788.8; 3D-CRT, 295.5 | |
| Zhang et al [52] | 11 | Prostate + proximal SV 86.4 Gy | IMRT (5F,SS) vs VMAT (SA) | IMRT – slightly higher dose to PTV (V95%, D95%, mean dose and TCP) and better homogeneity compared with VMAT | VMAT better then IMRT (sparing of rectum, bladder, femoral heads) | VMAT, 290; IMRT, 642 | VMAT, 1 min; IMRT, 5 min |
| Kjaer-Kristoffersen et al [53] RapidArc | 8 | Prostate + SV, 78 Gy (5 pts); 74 Gy (1 pt) Prostate bed, 66 Gy (2 pts) | IMRT (5F,SW) vs VMAT (partial SA) | IMRT – slightly better PTV coverage (V95%) but VMAT better in PTV minus rectum coverage. Hotspots higher in VMAT plans. | VMAT better than IMRT (sparing of bladder, rectum). Integral dose to body similar. Low dose bath (V5 Gy) to body larger for VMAT | VMAT, 529; IMRT, 647 | |
| Hardcastle et al [54] SmartArc | 10 | Prostate 78 Gy in 39 fractions | IMRT (7F,SS) vs VMAT (SA) | IMRT and VMAT − similar PTV coverage (except D95% where VMAT had lower values). | VMAT better than IMRT at rectal sparing at doses <50 Gy. VMAT – higher doses to femoral heads. No significant difference in bladder doses. | VMAT, 417; IMRT, 526 | VMAT, 1.3 min; IMRT, 4.5 min |
| Ost et al [55] | 12 | Prostate + SV (76 Gy) and IPL boost (82 Gy). Additional IPL dose level >85 Gy | IMRT (3F,5F,7F,SS) vs VMAT (SA) | IMRT (5F,7F) and VMAT – similar PTV coverage and all better than IMRT 3F. Dose escalation up to 95 Gy to IPL with VMAT | VMAT better at rectal sparing (significant at rectal volumes receiving 20−50 Gy). No difference in integral dose to body. | For 6 MV: VMAT, 447; IMRT (3F), 362; IMRT (5F), 407; IMRT (7F), 434 | VMAT, 1.95 min; IMRT (5F), 3.85 min; IMRT (7F), 4.82 min |
| Weber et al [56] RapidArc | 7 | Recurrent prostate carcinoma 56 Gy in 14 fractions | IMRT (5F,SW) vs IMPT vs VMAT (SA) | IMPT best for PTV coverage, VMAT better than IMRT for GTV and PTV coverage. VMAT (high definition MLC) – best for homogeneity. IMRT, VMAT better than IMPT for conformity | IMPT and RA better than IMRT (sparing of rectum, urethra, bladder). Integral doses to body lowest with IMPT. IMPT best at sparing penile bulb | ||
| Kopp et al [57] RapidArc | 292 | Prostate 77.4 Gy in 43 fraction | IMRT (7F,SW) vs VMAT (SA) | VMAT and IMRT similar PTV coverage (VMAT less homogeneous). VMAT – slightly higher D2% | VMAT better than IMRT (sparing of rectum at high doses, bladder, femoral heads, penile bulb) | ||
| Yoo et al [58] RapidArc | 10 | Prostate, SV and LN (primary) 46.8 Gy; prostate and SV (boost) 28.8 Gy (1.8 Gy per fraction) | IMRT (9F,7F) vs VMAT (SA) vs VMAT (DA) | Primary plans – IMRT better than VMAT (PTV coverage, conformity). Boost plans – similar PTV coverage, homogeneity; IMRT had worse conformity compared to VMAT | Primary plans-IMRT better than VMAT (sparing of bladder, rectum, small bowel). Boost plans – IMRT and DA VMAT better than SA VMAT. Higher integral doses to body with VMAT | Primary plans: VMAT (SA), 429; (DA), 444; IMRT, 1300. Boost plans: VMAT (SA), 443; VMAT (DA), 484; IMRT, 777 | Primary plans: VMAT (SA), 1.5 min; VMAT (DA), 3.1 min; IMRT, 8.1 min. Boost plans: VMAT (SA), 1.5 min; VMAT (DA), 3.1 min; IMRT, 4.9 min. |
| Wolff et al [59] ERGO++ | 9 | Prostate + SV 76 Gy | 3D-CRT vs IMRT (7F,SS) vs VMAT (SA,DA) vs ST | VMAT and ST− better PTV coverage compared with IMRT. Conformity better with IMRT. No difference in homogeneity | ST and IMRT better than VMAT for rectal sparing | VMAT (SA), 386; VMAT (DA), 371; IMRT, 544; ST, 2714 | VMAT (SA), 1.8 min; VMAT (DA), 3.7 min; IMRT, 6 min; ST, 12 min. |
| Tsai et al [60] ERGO++ | 12 | Prostate ± SV 78 Gy in 39 fraction | IMRT (5F,SS) vs VMAT (SA) vs HT | Similar PTV coverage between all three techniques. HT – better conformity | HT better than VMAT and IMRT at rectal sparing at 65 Gy and 40 Gy (VMAT slightly better than IMRT). No difference in bladder and femoral head sparing. | VMAT, 309.7; IMRT, 336.1; HT, 3368 | VMAT, 2.6 min; IMRT, 3.8 min; HT, 3.8 min |
| Rao et al [61] SmartArc | 6 (of 18) | Not specified | IMRT (7F,SS) vs VMAT (SA) vs HT | Similar PTV coverage between all three techniques. VMAT – slightly better homogeneity. | No significant difference between all three techniques VMAT – slightly lower maximum dose to femoral heads compared with HT and IMRT. | VMAT, 549; IMRT, 639 | VMAT, 2.2 min; IMRT, 8.1 min; HT, 4.0 min |
| Shaffer et al [62] Predecessor to RapidArc | 10 | Prostate 74 Gy in 37 fractions + SIB to prostate CTV up to 88.8 Gy | IMRT (9F,SW) vs VMAT (SA) | Volume of CTV boosted and mean dose within boost region higher with VMAT | VMAT, 949; IMRT, 1819 | VMAT, 3.7 min; IMRT, 9.6 min | |
| Crijns et al [64] RapidArc | 11 | Prostate + SV (SIB) 74 Gy + 55 Gy in 37 fractions | IMRT vs VMAT (SA) | No significant difference in PTV coverage (small differences depending on VMAT optimisation approach). IMRT – better homogeneity | No significant differences (except rectal maximum doses lower in some VMAT optimisation approaches). Mean rectal NTCP lower in VMAT | VMAT, 1.2−1.5 min | |
| Guckenberger et al [94] SmartArc | 5 (of 20) | Prostate + SV (SIB) 74 Gy + 60 Gy in 33 fractions | IMRT (7F,SS) vs VMAT (SA) vs VMAT (DA) | Similar PTV coverage (slightly better with DA VMAT). VMAT – better conformity | VMAT slightly better than IMRT at rectal, bladder sparing (except rectal V70 Gy which is higher with VMAT) | VMAT (SA), 465; VMAT (DA), 572; IMRT, 513 | VMAT (SA), 2.08 min; VMAT (DA), 3.87 min; IMRT, 5.82 min |
VMAT, volumetric modulated arc therapy; PTV, planning target volume; OAR, organs at risk; MU, monitor units; Gy, Gray; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity modulated radiotherapy; CDR, constant dose rate; VDR, variable dose rate; 5F, five field; 7F, seven field; 9F, nine field; SW, sliding window; SS, step-and-shoot; SA, single arc; DA, double arc; SV, seminal vesicles; V95%, volume receiving ≥95% prescribed dose; D95%, dose to 95% of volume; TCP, tumour control probability; V5 Gy, volume receiving ≥5 Gy; IPL, intraprostatic lesion; MV, megavoltage; IMPT, intensity modulated proton therapy; MLC, multileaf collimator; D2%, dose to 2% of volume; ST, serial tomotherapy; HT, helical tomotherapy; SIB, simultaneous integrated boost; CTV, clinical target volume; V70 Gy, volume receiving ≥70 Gy.
Figure 1.
Example of dose distributions in (a,b) IMRT and (c,d) VMAT plans for radiotherapy to prostate (primary planning target volume (PTV)) and pelvic lymph nodes (elective PTV). The dose prescribed to the primary PTV and elective PTV is 74 Gy and 55 Gy in 37 fractions, respectively. The primary PTV (red contour) is encompassed by the 95% isodose (orange line and colour wash) and the elective PTV (pink contour) is encompassed by the 70.6% isodose (dark blue line and light green colour wash). Some sparing of the rectum (brown contour) and bladder (yellow contour) is achieved. Figures courtesy of Department of Medical Physics, Royal Surrey County Hospital, UK.
Pelvic malignancies (lower gastrointestinal, gynaecological cancers)
Given the success of IMRT in prostate cancer, there has been interest in the use of these techniques in the treatment of other pelvic malignancies including lower gastrointestinal and gynaecological cancers. Conventional fixed field IMRT has been evaluated in anal and rectal cancer as well as cervix and endometrial cancers [70-74]. In general the results are positive with improved dose conformity and sparing of OARs seen with IMRT compared with conventional conformal radiotherapy.
Anal cancer
Several planning studies have evaluated VMAT in anal cancer. Clivio et al [75] conducted a planning study in 10 patients with Stage T2–4 N0/+ anal cancer comparing fixed field IMRT (7–9 fields, SW) with single and double arc VMAT. The results showed that PTV coverage was largely similar between the techniques, although double arc VMAT achieved slightly better coverage and dose homogeneity compared with IMRT while single arc was slightly inferior. For the primary tumour (PTV1), the dose received by 98% of the volume (D98%) was 95.9% in the double arc compared with 94.6% in the IMRT plan (this was statistically significant). IMRT was slightly superior in dose conformity (not statistically significant). Regarding OAR sparing, double arc VMAT plans reduced the volume of bladder treated to medium-low radiation dose levels (10–40 Gy) and significantly reduced mean doses to the femora. Doses to the small bowel and healthy tissue were not significantly different between the two techniques. Double arc VMAT allowed more sparing of the male external genitalia (testis and penile bulb) compared with IMRT and single arc VMAT.
Following this study, two other planning comparison studies for anal cancer were recently conducted. Vieillot et al [76] compared seven-field fixed field IMRT (SW) with single and double arc VMAT and found similar results to Clivio's study [75] with equivalent PTV coverage, dose homogeneity and conformity (double arc VMAT performed slightly better in conformity, but this was not statistically significant) and improved OAR sparing with double arc VMAT. Following the results of an initial evaluation comparing single and double arc VMAT, Stieler et al [77] then compared double arc VMAT with conventional CRT and nine-field fixed field IMRT (SS). Again the results showed largely similar PTV coverage with CRT showing the most homogeneous but least conformal dose distribution and IMRT achieving higher conformity compared with VMAT. They also reported that IMRT produced the best sparing of non-PTV healthy tissue with no significant difference in bladder and small bowel doses.
Direct comparisons between these planning studies are difficult due to the inherent differences in patient population, target volume delineation, dose prescriptions and planning techniques, which can all introduce bias. In particular, Clivio et al [75] discuss the paucity of data for conventional IMRT in anal cancer, which has made it difficult to set realistic dose constraints for OARs in the optimisation process. In fact, the OAR doses in their study were lower than those seen in previous IMRT studies, although a direct comparison without bias is not possible. A significant reduction in MU (of up to 70%) and treatment time was reported in these studies [75-78]. Although double arc VMAT used more MU than single arc, this was still considerably less than in the IMRT plans and would be the preferred option considering the better target coverage, homogeneity and OAR sparing.
Rectal cancer
Arc therapy was initially evaluated in rectal cancer by Duthoy et al [78] in a planning study comparing 3D-CRT and IMAT (3–6 arcs). They found similar PTV coverage, but significantly lower mean doses to small bowel and integral dose in the IMAT plans. Richetti et al [79] reported on their technical and clinical experience of 25 patients with locally advanced rectal cancer treated with VMAT and performed a planning comparison with a matched cohort of patients who underwent conventional conformal radiotherapy. Although PTV coverage was similar, single arc VMAT achieved significantly superior dose conformity with a trend to improvement in homogeneity and improved OAR sparing (small bowel, femora and healthy tissue). In terms of acute toxicity, up to 50% of patients had diarrhoea and 8% of patients who received VMAT experienced NCI CTCAE v3.0 Grade 3 bowel toxicity. Longer follow-up is required to assess the clinical outcome and late toxicity following VMAT.
Gynaecological cancer
For gynaecological cancers, IMAT was one of the first arc techniques evaluated for whole abdominopelvic radiotherapy (WAPRT) in the treatment of relapsed ovarian cancer [80]. This technique was also used in the study by Wong et al [81] to investigate patients with high risk endometrial cancer. Both studies have reported acceptable target volume coverage and OAR sparing that was similar to fixed field IMRT and superior to conventional techniques. Whereas multiple arcs were used in the Ghent study, Wong et al [81] reported that two anterior arcs were sufficient in treating the target volume adequately with acceptable sparing of OARs. VMAT has been evaluated as a next logical step given the possibility of treating the entire target volume in a single arc, which would reduce treatment delivery time.
Cozzi et al [82] conducted a planning study comparing VMAT with five-field conventional fixed field IMRT (SW) in eight patients with cervical cancer. The results show similar target volume coverage with improved homogeneity and conformity with VMAT. OAR sparing (bladder and rectum) was significantly improved with VMAT with lower mean doses and volume that received at least 40 Gy (V40 Gy). For the rectum, mean dose and V40 Gy in the VMAT plans were 36.3 Gy and 51.5%, respectively, compared with 42.5 Gy and 78.7% in the IMRT plans. A similar trend was found with small bowel sparing. This resulted in a potential relative reduction in NTCP estimates for rectal bleeding, bladder contracture/loss of volume and small bowel obstruction/perforation by 30–70%. Integral dose to healthy tissue was also reduced with VMAT by an average of 12% compared with IMRT. The superior results seen with VMAT in this study appear more pronounced compared with the previously mentioned studies for anal and rectal cancer, although direct comparisons are difficult given the numerous biases, for example, the difference in target volume size and definition (the PTV in anal cancer patients included the pelvic and inguinal nodes, while this study only included the pelvic nodes). Another possible explanation is that in Cozzi et al’s study [82] the IMRT plans were optimised using five coplanar fields while the other studies used between seven- and nine- fields. The increase in the number of fields could have improved the quality of the IMRT plans leading to less pronounced differences with the VMAT plans, but at the expense of higher MU and longer treatment times. While it is clear that IMRT techniques are superior to conventional CRT, it is less certain how IMRT compares with intracavitary brachytherapy. Brachytherapy has the advantage of organ immobilisation with very steep dose gradients and highly conformal dose distributions, which are not currently matched by IMRT techniques; therefore, the general consensus is that IMRT or VMAT will not replace the role of brachytherapy in gynaecological cancers [7].
The use of WAPRT for ovarian cancer is not considered standard practice in the UK. This is despite some studies reporting response rates and outcomes for WAPRT that are comparable with chemotherapy in the palliative and adjuvant setting [83-85]. One of the main concerns with WAPRT is the risk of increased toxicity due to irradiation of critical structures and normal tissues, which can limit the dose and coverage of the target volume. IMRT techniques may allow the facilitation of this approach by improved conformity, OAR sparing and the feasibility of dose escalation [86]. A recent planning study by Mahanshetty et al [87] compared double arc VMAT with fixed field IMRT (SW) in five patients undergoing WAPRT as consolidation therapy following treatment with surgery and chemotherapy. Both techniques were largely similar in terms of target coverage, homogeneity and OAR sparing, although IMRT was slightly better in sparing the volume of bladder and liver outside the PTV for doses over 20 Gy. Another study by Matsuzak et al [88] comparing VMAT with IMRT for WAPRT showed acceptable PTV coverage for both techniques, but slightly superior bone marrow sparing with VMAT (mean dose 19.8 Gy vs 21.9 Gy). Both studies found reduced MU use and treatment delivery times with VMAT compared with fixed field IMRT. The shortened treatment time may reduce the impact of intrafraction motion, which may be significant in intra-abdominal radiotherapy.
A summary of the comparative planning studies evaluating VMAT in pelvic malignancies (lower gastrointestinal and gynaecological cancer) is presented in Table 2.
Table 2. Comparative planning studies in pelvic malignancies (lower gastrointestinal, gynaecological).
| Paper [ref] VMAT commercial system | Number of patients | Site | Comparison | PTV | OAR | MU per fraction | Treatment time per fraction |
| Clivio et al [75] RapidArc | 10 | Anal | IMRT (7−9F, SW) vs VMAT (SA) vs VMAT (DA) | Similar PTV coverage and homogeneity (DA VMAT slightly better than SA VMAT and IMRT; SA VMAT slightly inferior to IMRT). No significant difference in conformity | VMAT better than IMRT at sparing bladder at medium-low dose levels (lower V30 Gy) and femora. No difference in small bowel sparing. DA VMAT better than SA VMAT and IMRT at sparing male external genitalia. No difference in healthy tissue doses. | IMRT, 1531; VMAT (SA), 468; VMAT (DA), 545 | IMRT, 9.4 min; VMAT (SA), 1.1 min; VMAT (DA), 2.6 min |
| Vieillot et al [76] RapidArc | 10 | Anal | IMRT (7F,SW) vs VMAT (SA) vs VMAT (DA) | Similar PTV coverage. DA VMAT and IMRT similar in conformity and homogeneity (both better than SA VMAT) | DA VMAT better than IMRT and SA VMAT at sparing bladder and external genitalia at medium-low dose levels and lower mean doses to small bowel and femoral heads. No difference in healthy tissue doses | IMRT, 1646; VMAT (SA), 330; VMAT (DA), 493 | IMRT, 14 min; VMAT (SA), 1.1 min; VMAT (DA), 2.3 min |
| Stieler et al [77] ERGO++ | 8 | Anal | 3D-CRT vs IMRT (9F,SS) vs VMAT (DA) | Similar PTV coverage. 3D-CRT – best homogeneity but worst conformity. IMRT slightly better than VMAT for conformity | IMRT better than VMAT at sparing non-PTV tissue. IMRT and VMAT better than 3D-CRT for bladder sparing. No significant differences between IMRT and VMAT for bladder and bowel sparing | IMRT, 477−1260; VMAT, 268; 3D-CRT, 225 | IMRT, 9.5−10.3 min; VMAT, 4.8 min; 3D-CRT, 3.7 min |
| Richetti et al [79] RapidArc | 25 | Rectal | 3D-CRT (3F) vs VMAT (SA) (matched cohort of 20 patients treated with 3D-CRT) | Similar PTV coverage. VMAT significantly better than 3D-CRT for conformity (with trend to improvement in homogeneity) | No significant difference in bladder sparing (although bladder volumes were different). VMAT better than 3D-CRT at sparing femora and bowels. VMAT – lower integral mean dose to body | VMAT, 276; 3D-CRT, 293 | VMAT, 2.05 min; 3D-CRT, 3.42 min |
| Cozzi et al [82] RapidArc | 8 | Cervix | IMRT (5F,SW) vs VMAT | VMAT better than IMRT for conformity and slightly better for PTV coverage and homogeneity | VMAT significantly better than IMRT at bladder and rectal sparing (similar trend with small bowel sparing). VMAT – 12% lower integral dose to body compared with IMRT | IMRT, 479; VMAT, 245 | IMRT, 15 min; VMAT, 1.0−2.3 min |
| Mahanshetty et al [87] RapidArc | 5 | WAPRT for ovarian cancer | IMRT (7F,SW) vs VMAT (TA, two full and one partial anterior arc) | Similar PTV coverage. VMAT and IMRT (15MV) better conformity compared with IMRT (6MV) | No significant differences. 15MV slightly better than 6MV (both IMRT and VMAT). IMRT (15MV) slightly better than VMAT (15MV) at sparing bladder minus PTV and liver for doses higher than 20 Gy | IMRT, 2841−3103; VMAT,538−635 | IMRT, 17.4−18 min; VMAT, 4.8 min |
VMAT, volumetric modulated arc therapy; PTV, planning target volume; OAR, organs at risk; MU, monitor units; IMRT, intensity modulated radiotherapy; 3F, three field; 5F, five field; 7F, seven field; 9F, nine field; SW, sliding window; SS, step-and-shoot; SA, single arc; DA, double arc; Gy, Gray; V30 Gy, volume receiving ≥30 Gy; 3DCRT, three-dimensional conformal radiotherapy; TA, triple arc; MV, megavoltage; WAPRT, whole abdominopelvic radiotherapy.
Head and neck cancer
Radiotherapy for head and neck cancer can be challenging due to the complex anatomy of the head and neck region with these tumours often located within close proximity to critical structures which can limit radiation dose. In addition, these tumours often display an aggressive phenotype and often grow rapidly due to the rich lymphatic supply in the head and neck region, and can therefore present at a locally advanced stage. Radiotherapy is an important treatment modality in these tumours as it offers an alternative treatment option to surgical resection which can cause unacceptable cosmetic disfigurement and functional impairment. Randomised evidence has shown that IMRT can reduce late toxicity parameters such as xerostomia by increasing sparing of the parotid glands [89]. Furthermore the ability of IMRT to produce inhomogeneous dose distributions can be exploited to simultaneously treat the primary and elective target volumes (areas at risk of microscopic spread of disease) to different dose per fractions without increasing overall treatment time. This SIB technique allows both volumes to be treated within one treatment plan without the need for matching fields therefore reducing the potential risk of reduced dose coverage in the areas of matching beams [90].
Several planning studies have compared dosimetric results achieved with VMAT plans with fixed field IMRT plans. Verbakel et al [91] compared single and double arc VMAT with 7-field fixed field IMRT (SW) in 12 patients with advanced tumours of the nasopharynx, oropharynx and hypopharynx. The PTV coverage was similar between IMRT and VMAT with improved homogeneity when using two arcs with VMAT. Similarly there were no significant differences in the doses to the OARs, although the authors report a slightly lower mean dose (average of 2 Gy) to the parotid glands with the double arc VMAT plans compared with the single arc and IMRT plans. The results in this study were relatively similar to a larger planning study by Vanetti et al [92] which compared single and double arc VMAT with 7–9 field fixed field IMRT (SW) in 29 patients with tumours of the oropharynx, hypopharynx and larynx. PTV coverage and conformity were similar in the two groups with better homogeneity in the double arc VMAT plans. In this study, the mean doses to the OARs were lower in the VMAT plans with double arc plans achieving significantly lower doses compared with single arc plans. For the spinal cord, the D2% was 39 Gy in the double arc VMAT plans and 42.8 Gy in the IMRT plans. For the brainstem D2% was 23.8 Gy for double arc VMAT and 38.2 Gy for IMRT. For the contralateral parotid glands, the mean dose was 28.2 Gy for double arc VMAT and 32.6 Gy for IMRT, while for the ipsilateral glands the mean dose was 34.4 Gy and 40.1 Gy for the double arc VMAT and IMRT plans, respectively. Additional OARs, including cochlea, vocal apparatus and oesophageal constrictors, were also defined and evaluated in this study; however, no specific dose constraints were set for these. Again there was greater sparing of these OARs with the VMAT plans achieving lower mean doses to these structures. Integral doses to the body were also lower in the VMAT plans by an average of 7% compared with the fixed field IMRT plans. A more recent planning study comparing VMAT with fixed field IMRT (SW) in nasopharyngeal and oropharyngeal cancer confirmed improved sparing of the contralateral parotid glands with comparable PTV coverage between the two techniques [93].
The number of arcs to use in VMAT plans has already been discussed. Owing to the complexity of the target volumes in head and neck radiotherapy, the general consensus is that more than one arc is required to achieve an acceptable dose distribution. Guckenberger et al [94] conducted a planning study that included patients receiving primary or post-operative radiotherapy for pharyngeal tumours (10 patients) and 5 patients with paranasal sinus tumours. Each patient had a nine-field fixed field IMRT plan (SS) and a single arc, double arc and triple arc VMAT plan. In the post-operative pharyngeal patients, PTV coverage was inferior in the single arc VMAT plan compared with the IMRT plan. The double arc plan was equivalent to IMRT and triple arc was superior in terms of PTV coverage and homogeneity. In primary pharyngeal patients, both single arc and double arc VMAT plans were inferior to the IMRT plan, while the triple arc plan was equivalent. In the paranasal sinus group, all VMAT plans were inferior to the IMRT plan for dose coverage, particularly in the region between the orbits. The mean dose to the lenses in this group was also higher in the VMAT plans compared with the IMRT plans. The superiority of double arc VMAT plans compared with single arc in terms of PTV coverage and OAR sparing was also confirmed in other planning studies [88,89,96]. However, another study by Bertelsen et al [95] that compared single arc VMAT plans with fixed field IMRT (SS) plans in 25 patients with oropharyngeal or hypopharyngeal cancer found similar PTV coverage with slightly better conformity in the elective nodal volume with VMAT. It is worth noting that in this study the IMRT plans used five or seven fields compared with the other studies which used seven or nine beams and as discussed previously, the quality of IMRT plan improves with increasing number of beams, but at the expense of a greater number of MU and longer treatment times.
The degree of OAR sparing in the majority of these planning studies are either not significantly different or slightly better in the VMAT plans compared with fixed field IMRT [91,92] (this excludes the paranasal sinus group in Guckenberger's study [94]). Some studies have also reported lower integral doses to the body with VMAT plans [92]. However, the volume of area receiving the lower radiation dose range is greater in VMAT plans. For instance in the Bertelsen et al study [95], the volume of the contralateral parotid glands receiving doses less than 23 Gy was greater in the VMAT plans, but this was the contrary for doses higher than 23 Gy. This was also the case for the ipsilateral parotid glands where the intersection was at approximately 11 Gy. There is a correlation between the incidence of xerostomia and mean doses to the parotid glands; it is generally accepted that mean dose thresholds are used as constraints in the optimisation process [97,98]. It remains unclear what the clinical significance is of the greater volume of glands receiving low dose radiation. There are preclinical data suggesting that radiation tolerances of some OARs (parotid glands and spinal cord) may be reduced when the OAR regions receiving higher doses are surrounded by areas receiving lower doses (“the bath and shower” effect) [99]. Longer term follow-up will be required to assess the impact of this on late toxicity.
There is limited data on the comparison between HT and VMAT in head and neck cancer. Clemente et al [100] performed a planning study in eight patients with oropharyngeal tumours comparing a nine-field fixed field IMRT (SS), double arc VMAT and HT plan. There was no significant difference in PTV coverage for the high and intermediate dose levels, but the HT plans were better than VMAT and IMRT in the coverage of the elective PTV (D98% was 97.1% in HT plans compared with 94.5% with IMRT and 92.6% with VMAT). HT was superior to VMAT and IMRT in terms of dose conformity and homogeneity while VMAT plans were superior to IMRT in dose conformity. Dose to brain, parotid, oral mucosa and oesophagus were all lowest in the HT plans while VMAT and IMRT plans achieved lower doses to the mandible. The authors also point out that although there was better sparing of the OARs outside the PTV, the doses to OARs embedded in the PTV were higher in the HT plans compared with VMAT. Another planning study by Rao et al [61] compared IMRT, VMAT and HT showed no significant difference in PTV coverage, but similarly showed lowest mean doses to OARs in the HT plans. Overall it is felt that HT and VMAT can produce comparable dose distributions although HT may be slightly better at treating more complex volumes, for example if there are multiple targets within a larger irradiated volume [61].
As in the prostate VMAT planning studies, a universal finding in these studies was the reduction in MU (up to 46%) with VMAT plans compared with fixed field IMRT [91-93]. Many of the above studies used seven to nine fields in their fixed field IMRT plans which used a larger number of MUs compared with the five-field plans [91,92,95]. It is worth bearing in mind again, the number of MU in fixed field IMRT depends on the IMRT technique; usually more MU are required in the SW or dynamic IMRT technique [19,20]. The differences in MU between IMRT and VMAT found in studies using step and shoot IMRT are smaller than in the studies using the SW technique. Another reported benefit of VMAT is the shorter delivery time [92,96]. However, this is dependent on the number of fields used in the IMRT plans with seven- or nine-field plans taking slightly longer to deliver compared with five-field plans [94,100]. Clemente et al [100] also commented that in more complex cases, VMAT delivery time was prolonged and resulted in longer treatment times compared with HT. Preliminary data suggests that acute toxicity with VMAT in head and neck cancer is acceptable. Scorsetti et al [101] reported 45 patients who were treated with VMAT (RapidArc, Varian), of which 78% also received concomitant chemotherapy. In their patient series, the incidence of NCI CTCAE v.3.0 Grade 3 mucositis was 28%, Grade 3 dermatitis was 14% and Grade 2 dysphagia was 44%. Late toxicity and clinical outcome data are awaited.
Dose escalation strategies in head and neck cancer have been evaluated using fixed field IMRT. The rationale behind this is the high locoregional relapse rate despite improvements in therapy and the observation that the majority of these treatment failures are occurring within the high dose radiotherapy volume [102-104]. These regions are thought to represent areas of hypoxia and radioresistance within the tumour volume and may require higher doses to improve local control. The feasibility of dose escalation using fixed field IMRT has been tested in Phase I studies with acceptable toxicity rates [105,106]. Larger randomised trials evaluating this strategy are now in progress or in set-up. The concept of dose painting using biological imaging, such as positron emission tomography (PET) to guide the delineation of these boost regions has also been of significant interest in recent years. Fluorine-18-fluorodeoxyglucose (18F-FDG) has been proposed as a potential tracer to be used in this way due to its wide availability and correlation with many biological processes that are associated with radioresistance including hypoxia, increased cell proliferation and accelerated repopulation. A Phase I trial of 41 head and neck cancer patients has demonstrated the feasibility and safety of this approach using fixed field IMRT to boost 18F-FDG avid regions within the gross target volume (GTV) [107]. At present, one study has reported on the feasibility of dose painting using VMAT. Korreman et al [108] tested voxel-based dose painting in a head and neck cancer patient using 61Cu-diacetyl-bis (N4-methylthiosemicarbazone) (61Cu-ATSM) PET to guide delineation of boost volumes and reported this to be feasible. Further studies are required to evaluate the potential benefits of this strategy.
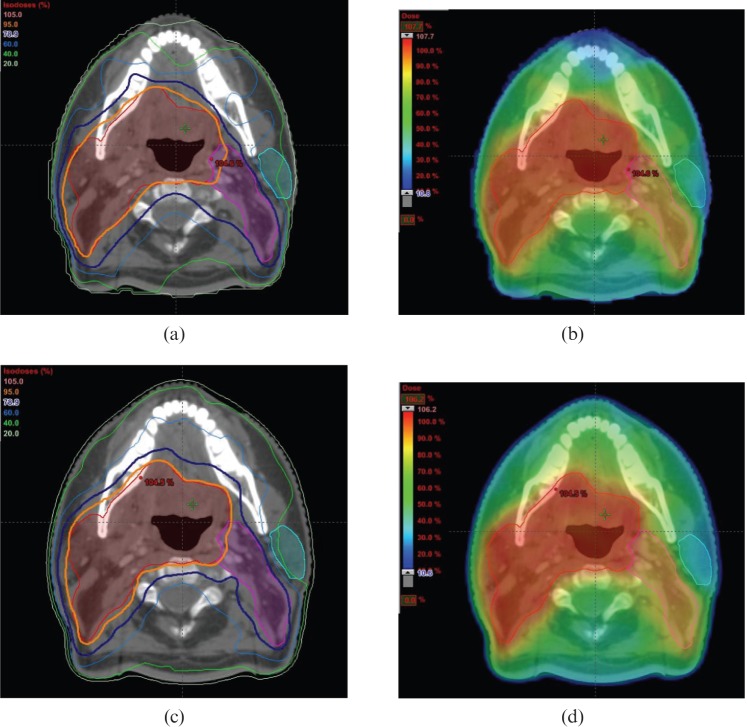
A summary of the comparative planning studies evaluating VMAT in head and neck cancer is presented in Table 3. An example of the dose distributions achieved with VMAT and fixed field IMRT for head and neck cancer is illustrated in Figure 2.
Table 3. Comparative planning studies in head and neck cancer.
| Paper [ref] VMAT commercial system | Number of patients | Primary tumour site | Comparison | PTV | OAR | MU per fraction | Treatment time per fraction |
| Verbakel et al [91] RapidArc | 12 | Nasopharynx, oropharynx and hypopharynx | IMRT (7F,SW) vs VMAT (SA) vs VMAT (DA) | Similar PTV coverage. DA VMAT better than SA VMAT and IMRT for homogeneity | No significant difference. Parotid dose lower with DA VMAT (by average 2Gy) compared with SA VMAT and IMRT | VMAT (SA), 439; VMAT (DA), 459; IMRT, 1108 | |
| Vanetti et al [92] RapidArc | 29 | Oropharynx, hypopharynx and larynx | IMRT (7−9F,SW) vs VMAT (SA) vs VMAT (DA) | Similar PTV coverage and conformity. DA VMAT better than SA VMAT and IMRT for homogeneity (SA VMAT slightly inferior to IMRT) | VMAT better than IMRT at sparing spinal cord (D2%, mean dose), brainstem (D2%, mean dose) and parotid glands (mean dose). DA VMAT better than SA VMAT. VMAT – lower integral doses to body | VMAT (SA), 463; VMAT (DA), 584; IMRT, 1126 | VMAT (SA), 1.2−1.5 min; VMAT (DA), 3 min; IMRT, 15 min |
| Johnston et al [93] RapidArc | 10 | Nasopharynx and oropharynx | IMRT (9F,SW) vs VMAT (DA) | Similar PTV coverage IMRT slightly better than VMAT for conformity and homogeneity | No significant differences for spinal cord, brainstem doses. VMAT better than IMRT for contralateral parotid gland sparing | VMAT, 529; IMRT, 1628 | |
| Guckenberger et al [94] SmartArc | 15 (of 20) | Post-operative pharynx/ larynx, primary pharynx, paranasal sinus | IMRT (9F,SS) vs VMAT (1−3 arcs) | For PTV coverage and homogeneity: (post-operative pharynx/larynx) SA VMAT inferior to IMRT, DA VMAT = IMRT TA VMAT better than IMRT; (primary pharynx) SA and DA VMAT inferior to IMRT TA VMAT = IMRT; (paranasal sinus) All VMAT plans inferior to IMRT; (decreased coverage between orbits) | (Post-operative pharynx/larynx, primary pharynx) No significant difference (SA VMAT inferior to DA VMAT; TA VMAT and IMRT) (paranasal sinus) All VMAT plans inferior to IMRT for lens sparing | IMRT, 430−688; VMAT (SA), 358−440; VMAT (DA), 460–519; VMAT (TA), 506−560 | IMRT, 9.55−12.25 min; VMAT (SA), 1.85−2 min; VMAT (DA), 3.83−3.98 min; VMAT (TA), 4.42−4.58 min |
| Bertelsen et al [95] Smartarc | 25 | Oropharynx and hypopharynx | IMRT (5−7F,SS) vs VMAT (SA) | Similar PTV coverage and homogeneity. VMAT better than IMRT for elective PTV coverage and conformity | VMAT better than IMRT at sparing spinal cord, parotid glands, submandibular glands at high dose levels. VMAT − lower volumes of normal tissue (outside PTV) irradiated to higher doses | VMAT, 460; IMRT, 503 | VMAT, 4.02 min; IMRT, 6.2 min |
| Alvarez-Moret [96] Oncentra Masterplan | 4 | Oral cavity, hypopharynx, nasal cavity | IMRT (7−9F,SS) vs VMAT (SA) vs VMAT (DA) | IMRT and DA VMAT similar PTV coverage, homogeneity (SA VMAT inferior to IMRT and DA VMAT) | IMRT and DA VMAT largely similar OAR sparing (SA VMAT inferior to IMRT and DA VMAT) | VMAT (SA), 491.3; VMAT (DA), 596.4; IMRT, 575.4 | VMAT (SA), 1.86 min; VMAT (DA), 3.64 min; IMRT, 11.7 min |
| Clemente et al [100] SmartArc | 8 | Oropharynx | IMRT (9F,SS) vs VMAT (DA) vs HT | HT better than VMAT and IMRT for coverage of elective PTV, homogeneity and conformity. VMAT better than IMRT in conformity (no difference in homogeneity) | HT – lower doses to brain, parotid, oral mucosa, oesophagus. VMAT and IMRT− lower doses to mandible. VMAT – slightly lower mean dose to ipsilateral parotid gland compared with IMRT | VMAT, 672.4; IMRT, 931 | VMAT, 8.13 min; IMRT, 13.94 min; HT, 7.26 min |
| Rao et al [61] SmartArc | 6 (of 18) | IMRT (9F,SS) vs VMAT (DA) vs HT | Similar PTV coverage | HT better than VMAT and IMRT at sparing of spinal cord, brainstem, parotid glands. No difference in mean dose to body | IMRT, 777; VMAT, 620 | IMRT, 11.1 min; VMAT, 4.6 min; HT, 7.0 min |
VMAT, volumetric modulated arc therapy; PTV, planning target volume; OAR, organs at risk; MU, monitor units; IMRT, intensity modulated radiotherapy; 5F, five field; 7F, seven field; 9F, nine field; SW, sliding window; SS, step-and-shoot; SA, single arc; DA, double arc; TA, triple arc; Gy, gray; D2%, dose to 2% of volume; TA, triple arc; HT, helical tomotherapy.
Figure 2.
Example of dose distributions in (a,b) IMRT and (c,d) VMAT plans for oropharyngeal cancer. The dose prescribed to the primary planning target volume (PTV) (encompasses the tumour and involved lymph nodes) and elective PTV (regional lymph nodes at risk of microscopic spread) is 65 Gy and 54 Gy in 30 fractions, respectively. The primary PTV (red contour) is encompassed by the 95% isodose (orange line and colour wash) and the elective PTV (pink contour) is encompassed by the 78.9% isodose (dark blue line and light green colour wash). Some sparing of the parotid gland (light blue contour) is achieved. Figures courtesy of Department of Medical Physics, Royal Surrey County Hospital, UK.
Thoracic tumours
The use of IMRT has been evaluated in the treatment of lung cancer and has been shown to improve dose conformity and OAR sparing [109,110]. The impact of intrafraction motion in these cases can be significant and can potentially reduce target volume coverage or cause a geographical miss and increase doses to OARs. Various approaches to manage intrafraction motion include using four-dimensional (4D)-CT scanning that allow visualisation of all the possible tumour positions within the respiratory cycle, which can then be incorporated with a safety margin in the PTV, breath-hold techniques (e.g. active breathing control), delivery gating and marker tracking [111-113]. The issue of treatment delivery time per fraction is also important as the degree of intrafraction motion has been found to increase with time [114]. VMAT would be an attractive solution because IMRT quality dose distributions can still be achieved, but in a shorter treatment time that could minimise the effect of intrafraction motion. Although motion-adaptive radiotherapy is currently widely used with conventional fixed field IMRT, it has not yet been routinely implemented with VMAT. The feasibility of tracking target motion for arc therapy using a dynamic MLC algorithm has been evaluated in a recent study and has shown encouraging results, but further research in this area is warranted [115].
Lung cancer
For early stage lung cancers e.g. Stage I non-small cell lung cancer (NSCLC), stereotactic body radiotherapy (SBRT) has emerged as an alternative treatment option to surgical resection for patients who are medically inoperable, giving excellent local control rates (up to 95%) [116,117]. SBRT is usually delivered with hypofractionated radiotherapy schedules and using multiple non-coplanar fixed beams occasionally combined with dynamic arcs. IMRT and HT have also been evaluated using this approach. These techniques can improve dose conformity compared with conventional radiotherapy, but at the expense of prolonged delivery time [118,119]. Several planning studies have evaluated the performance of VMAT in delivering SBRT for lung cancer. Mcgrath et al [120] compared VMAT to conventional 3D-CRT (using 7–10 non-coplanar beams) in 21 patient datasets with Stage Ia NSCLC. VMAT was planned using a single 180° partial arc with the arc range selected to avoid as much of the contralateral lung as possible. The dose prescription to the PTV/internal target volume was 48 Gy in 12 fractions. The results report improved conformity with VMAT at the 80% and 50% isodose levels, although there was no significant difference at the 95% isodose level. VMAT achieved improved sparing of the lung parenchyma with significantly lower doses to the volume receiving 20 Gy, 12.5 Gy, 10 Gy and 5 Gy.
The improved dose conformity at the 80% and 60% isodose levels was also seen in the study by Ong et al [121], which evaluated 18 patients with Stage I NSCLC comparing double arc VMAT with conventional 3D-CRT, dynamic conformal arcs and IMRT (SW). However, they report higher doses to the ipsilateral and contralateral lungs in the VMAT plans compared with the conventional CRT plans. A possible reason for this is that partial arcs avoiding the contralateral lung were not used in this study. In addition, the authors also specify that the plans were optimised taking into consideration dose constraints to the chest wall because there is evidence that lower V30 Gy for the chest wall is associated with lower risk of late toxicity e.g. rib fractures. As a result, the doses to the chest wall in this study were significantly lower in the VMAT plans, but at the cost of increased dose to the lungs. A recent study conducted by a Dutch group compared coplanar VMAT with non-coplanar and coplanar IMRT in 27 patient datasets [122]. The VMAT plans used double partial arcs avoiding the contralateral lung. While PTV coverage was similar between all three techniques, both non-coplanar IMRT and coplanar VMAT performed better then coplanar IMRT in reducing dose to healthy lung tissue. Non-coplanar IMRT had slightly better conformity and lower V20 Gy for normal lung compared with coplanar VMAT. Another recent study by Brock et al [123] reported improved target volume coverage with VMAT and non-coplanar CRT techniques compared with coplanar techniques. VMAT resulted in slightly higher lung V11 Gy but lower V20 Gy compared with the non-coplanar plans (not statistically significant).
All these studies report improved treatment efficiency with reduction in treatment delivery time. Mcgrath et al [120] reported a reduction in delivery time with VMAT of 37–63%. In the Ong et al study [121], three fractionation schedules (54 Gy in 3 fractions, 55 Gy in 5 fractions or 60 Gy in 8 fractions) were evaluated based on a risk-adapted fractionation scheme that is dependent on factors including tumour size and position. Unsurprisingly the delivery times for the schedules with larger dose per fractions were longer (10.3 min for 54 Gy in 3 fractions and 3.9 min for 60 Gy in 8 fractions). This was compared with an average delivery time of 12 min with IMRT and 11.6 min with conventional 3D-CRT, which did not vary significantly between the different fractionation schedules. In Ong et al’s study [121], MU with VMAT were higher than conventional CRT but significantly lower compared with IMRT (average MU/Gy was 240 with VMAT with 445 with IMRT). However, this was not seen in the study by Holt et al [122] where the number of MUs used in the VMAT and IMRT plans were not significantly different.
There is limited data on the comparison of HT and VMAT in lung cancer. In the study by Rao et al [61] comparing VMAT with HT and fixed field IMRT (SS), 6 cases of lung cancer (out of a total of 18 patient datasets) were evaluated. All three plans resulted in largely similar PTV coverage and OAR sparing. Lung mean doses and V20 Gy were slightly lower in the IMRT plans compared with HT and VMAT, but this was not statistically significant. The main benefit seen with VMAT is the reduction in treatment delivery time (average of 2.1 min per fraction compared with 5.4 and 7.9 min for HT and IMRT, respectively).
Limited data is available for the use of VMAT in more advanced lung tumours. One early paper presented a case study of small cell lung cancer that was treated with VMAT and reported slightly improved PTV coverage and a slightly reduced lung V20 Gy compared with conventional CRT techniques [124]. Scorsetti et al [125] evaluated planning and clinical outcomes in 24 patients with locally advanced NSCLC (Stage IIIA−IIIB) treated with VMAT (RapidArc, Varian) at their unit. They report satisfactory target coverage and homogeneity with doses to OARs within acceptable tolerances. Acute toxicity was also acceptable with no Grade 3 toxicities reported. Target volumes in these advanced cases are often large, which results in a large volume of normal lung receiving low dose radiation (V5 Gy) with any IMRT technique including fixed field IMRT and VMAT.
The increase in normal tissue volume receiving low dose radiation seen in VMAT has raised some concern. In addition to the potential increased risk of secondary malignancy induction, there have been anecdotal observations that the rate of radiological pneumonitis may be higher in patients treated with VMAT compared with conventional 3D-CRT (this is in SBRT follow-up). Palma et al [126] conducted a matched analysis of patients who had received SBRT with VMAT or conventional 3D-CRT to evaluate the patterns of radiological changes and severity of pneumonitis. They report no statistically significant differences in either endpoint, although 12% of patients receiving VMAT had what was classified as severe radiological changes compared with 2% of conventional 3D-CRT patients. However, despite over 50% of patients showing acute radiological changes, only 5% of patients had the clinical symptoms of pneumonitis. It is worth noting that in their practice, patients are treated with at least two full arcs with or without the addition of partial arcs, as opposed to exclusive partial arcs with avoidance of the contralateral lung. In addition, these patients were assessed and imaged at 3 months post-treatment in this study, which may be too early to evaluate the rates of late onset pneumonitis and fibrosis.
Mesothelioma
In terms of other thoracic histological tumour types, IMRT techniques have been evaluated in the treatment of malignant pleural mesothelioma (MPM). Post-operative radiotherapy following chemotherapy and surgery (extrapleural pneumonectomy) has been used to try to improve locoregional control and survival in this disease, which is associated with a poor prognosis [127]. Conventional techniques provide suboptimal dose coverage owing to the complexity of the target volume, which is often large, irregular in shape and in close proximity to numerous critical structures (lung, liver, spinal cord, heart, oesophagus and kidneys). Double arc VMAT was evaluated in a recent planning study of six patients with MPM and compared with nine-field fixed field IMRT (SW) [128]. PTV coverage, dose homogeneity and conformity were largely equivalent between the two techniques. In terms of OAR sparing, VMAT plans achieved significantly lower mean doses to the contralateral kidney, heart, liver and oesophagus, and lower V20 Gy for the contralateral lung. The reduction in MU (average MU per 2 Gy was 734 vs 2195) and treatment delivery time (3.7 min vs 13.4 min) was a further benefit of VMAT found in this study.
A summary of the comparative planning studies evaluating VMAT in thoracic tumours is presented in Table 4.
Table 4. Comparative planning studies in thoracic tumours.
| Paper [ref] VMAT commercial system | Number of patients | Stage and dose | Comparison | PTV | OAR | MU per fraction | Treatment time per fraction |
| McGrath et al [120] | 21 | Stage Ia NSCLC SBRT 48 Gy in 12 fractions | 3D-CRT (7−10 non-coplanar) vs VMAT (single partial arc) | VMAT better than 3D-CRT for conformity at 80% and 50% isodose levels. No difference in homogeneity | VMAT better than 3D-CRT at sparing lung (V20 Gy, V12.5 Gy, V10 Gy, V5 Gy). No significant difference in mean dose to other OARs | VMAT, 2360; 3D-CRT, 2235 | VMAT reduced treatment time by 37−63% compared with 3D-CRT |
| Ong et al [121] Rapidarc | 18 | Stage I NSCLC SBRT 54Gy in 3 fractions 55Gy in 5 fractions 60Gy in 8 fractions | 3D-CRT non-coplanar (10F) vs DCA vs IMRT (9−10F coplanar), SW vs VMAT (DA) | VMAT better than 3D-CRT, DCA and IMRT for conformity at 80% and 60% isodose levels | VMAT – higher lung doses (V20Gy,V5Gy) compared with 3D-CRT (no significant difference with IMRT). VMAT − better sparing of chest wall (V45Gy, V30Gy, V20Gy) compared to 3D-CRT, DCA and IMRT | VMAT,1800−4320; 3D-CRT, 1343−3222; DCA, 1402−3364; IMRT, 3338−8010 | VMAT, 3.9−10.5 min; 3D-CRT, 11.6 min; IMRT, 12 min |
| Holt et al [122] SmartArc | 27 | Stage I/IIa NSCLC SBRT 54Gy in 3 fractions | Coplanar IMRT (9F) vs non-coplanar IMRT (12−16F) vs VMAT (DA) | Similar PTV coverage VMAT better than coplanar IMRT but inferior to non-coplanar IMRT for conformity at 50% isodose level. Non-coplanar IMRT better than coplanar IMRT for conformity at 75% and 50% isodose levels | VMAT inferior to non-coplanar IMRT for lung V20Gy, spinal cord (Dmax), oesophagus (Dmax) and chest wall V30Gy. VMAT and non-coplanar IMRT better than coplanar IMRT for lung V20Gy, spinal cord and oesophageal Dmax | VMAT, 3428; coplanar IMRT, 3335; non-coplanar IMRT, 3313 | VMAT, 6.5 min; Coplanar IMRT, 17 min; non-coplanar IMRT, 23.7 min |
| Brock et al [123] | 5 | Stage I NSCLC SBRT 60Gy in 8 fractions | Coplanar and non-coplanar CRT (3F, 5F, 7F, 9F) vs VMAT | Non-coplanar and VMAT better than co-planar for PTV coverage | Non-coplanar CRT better than coplanar CRT for lung V11Gy (no significant difference for V20Gy). VMAT slightly higher V11Gy and lower V20Gy compared with non-coplanar CRT (not statistically significant) | VMAT, 2.13 min; non-coplanar (5F), 12.67 min; (7F), 7.75 min | |
| Rao et al [61] SmartArc | 6 (of 18) | Not specified | IMRT (7F,SS) vs VMAT (SA) vs HT | Similar PTV coverage, homogeneity | IMRT – slightly lower lung mean dose and V20Gy (not statistically significant) | VMAT, 476; IMRT, 569 | VMAT, 2.1 min; IMRT, 7.9 min; HT, 5.4 min |
| Scorsetti [128] RapidArc | 6 | Mesothelioma | IMRT (9F,SW) vs VMAT (DA) | Similar PTV coverage | VMAT better than IMRT at sparing some OAR (contralateral lung V20Gy, kidney D1%, heart mean dose, liver mean dose) | VMAT, 734; IMRT, 2195 | VMAT, 3.7 min; IMRT, 13.4 min |
VMAT, volumetric modulated arc therapy; PTV, planning target volume; OAR, organs at risk; MU, monitor units; NSCLC, non small cell lung cancer; SBRT, stereotactic body radiotherapy; Gy, Gray; 3D-CRT, three-dimensional conformal radiotherapy; V20Gy, volume receiving ≥20Gy; V12.5Gy, volume receiving ≥12.5Gy; V10Gy, volume receiving ≥10Gy, V5Gy, volume receiving ≥5Gy; DCA, dynamic conformal arcs; IMRT, intensity modulated radiotherapy; V45Gy, volume receiving ≥45Gy; V30Gy, volume receiving ≥30Gy;3F, three field; 5F, five field; 7F, seven field; 9F, nine field; 12F, twelve field, 16F, sixteen field; SW, sliding window; SS, step-and-shoot; SA, single arc; DA, double arc; Dmax, maximum dose; V11Gy, volume receiving ≥11Gy; HT, helical tomotherapy; D1%, dose to 1% of volume.
Central nervous system tumours
Benign lesions
Radiotherapy for intracranial tumours can be challenging owing to the proximity of these tumours to numerous critical structures. In particular, the use of radiation in the management of benign intracranial tumours where long life expectancies are predicted raises the need for highly conformal techniques to reduce radiation dose to the surrounding normal tissue. IMRT, Cyberknife, HT and stereotactic techniques have all been evaluated in this clinical setting. Fogliata et al [129] evaluated VMAT in comparison with 5–7 field fixed field IMRT (SW) and HT in a planning study of 12 patients with benign intracranial tumours. These included five acoustic neuromas, five meningiomas and two pituitary adenomas. The results showed equivalent PTV coverage with arc therapies performing slightly better than IMRT. VMAT and IMRT plans were superior in OAR sparing and reducing integral dose compared with HT. IMRT performed slightly better than VMAT plans in reducing doses to OARs, healthy brain and healthy tissue especially at low dose levels (below 10 Gy). This may be significant in this patient cohort where the risk of radiation-induced secondary malignancy should be minimised as much as possible.
Another study by Lagerwaard et al [130] compared single arc VMAT with their standard technique used for frameless radiosurgery of five non-coplanar dynamic conformal arcs (5DCA) and a third plan of single dynamic conformal arc (1DCA). In the three patient datasets with varying sizes of acoustic neuroma (small, 0.5 cm3; intermediate, 2.8 cm3 and large, 14.8 cm3), PTV coverage was similar between the three plans with VMAT plans showing improved conformity compared with the 5DCA and 1DCA plans. Maximum doses to OARs were lower in the VMAT and 5DCA plans compared with the 1DCA plans and there was a reduction in the volume of normal brain receiving low dose radiation (below 1 Gy) with VMAT and 1DCA compared with 5DCA plans. No significant difference was found in the number of MU between VMAT and 5DCA plans, but treatment time was reduced. This is important as the risk of inaccuracies due to intrafraction motion can be reduced. The authors also postulate that the quality of VMAT plans may possibly be further improved if the high definition MLCs (2.5 mm width compared with standard 5 mm width) were used. Both these studies used coplanar fields for the VMAT plans and the authors discuss the possibility of improving the quality of these plans by using non-coplanar and/or multiple arcs.
Malignant glioma
For malignant gliomas, IMRT has been evaluated with several planning studies showing a dosimetric superiority for non-coplanar IMRT techniques compared with conventional 3D-CRT [131]. Malignant gliomas are widely infiltrative in their extension with indistinct tumour margins that are difficult to accurately define. There is therefore a concern of increased risk of insufficient dosage of the target volume, especially with the steep dose gradients in IMRT plans. However, with more sophisticated imaging modalities to guide definition of tumour margins, the ability to co-register diagnostic MRI with planning CT images for improved accuracy of target volume delineation as well as the potential benefit of improved OAR sparing and facilitation of dose escalation, IMRT should be considered as a potentially useful technique for the treatment of these often aggressive tumours. Wagner et al [132] conducted a planning study of 14 patients with malignant glioma (World Health Organization (WHO) Grade 3 or 4) comparing single arc VMAT with 5–9 field fixed field IMRT (SW) and 3D-CRT. Conformity was higher for VMAT and IMRT compared with 3D-CRT; VMAT performed slightly better than IMRT in this respect. However, PTV coverage (which in this study was calculated as the ratio of target volume covered by the 95% isodose line divided by the PTV volume) was superior in IMRT compared with VMAT (94.7% vs 90.5%). For PTVs that were distant to OARs, 3D-CRT performed as well as IMRT in terms of PTV coverage, but was significantly inferior to both IMRT and VMAT for PTVs that were situated close to OARs. Regarding OAR sparing, VMAT achieved slightly better sparing compared with the other two techniques. The volume of healthy tissue receiving low dose radiation (V5 Gy) and mean dose of healthy brain was the highest in VMAT plans and lowest in 3D-CRT plans (mean dose 27.9 Gy vs 25.8 Gy).
Another study by Shaffer et al [133] evaluated VMAT in 10 patients with WHO Grade 3 or 4 glioma and compared the VMAT plans with 7-field fixed field IMRT (SW). The authors attempted to reduce bias in their study by cross-planning between two experienced planners, each generating five new IMRT and VMAT plans to avoid systematic planner bias. The patient datasets were also selected to include only cases where the PTV overlapped with at least one OAR therefore increasing the difficulty and complexity of planning. The results essentially showed equivalence in PTV coverage, conformity and homogeneity between the two techniques. For OAR sparing, VMAT and IMRT achieved similar sparing of midline OARs (brainstem and optic chiasm), but VMAT was better at sparing peripheral OARs with a lower mean dose to the retina, optic nerve and lens. Similar to Wagner et al’s study [132], the mean dose to normal brain was significantly higher in the VMAT plans (by 12%) compared with IMRT.
While it is difficult to make definite conclusions from these planning studies, owing to their limitations, some recommendations have been discussed. Wagner et al [132] suggest, for PTVs situated distant to OARs, 3D-CRT can achieve comparable and acceptable PTV coverage with better sparing of healthy brain and normal tissue and therefore would be the technique of choice. However, for PTVs close to OARs, either IMRT or VMAT would be preferable depending on the adequacy of PTV coverage, while bearing in mind the added benefit of reduced MU and treatment time with VMAT. Therefore, the preferred radiation technique for these tumours should be selected on an individual case basis and in certain situations IMRT or VMAT may not always offer the optimal solution. Regarding the issue of increased radiation to healthy brain tissue, Shaffer et al [133] postulate that this could possibly be reduced by setting constraints for normal brain in the optimisation process or using multiple, partial and/or non-coplanar arcs to avoid entry and exit beams through critical normal tissue structures.
Metastatic lesions
In the palliative setting, randomised data has shown improvements in survival with the combination of radiosurgery and whole brain radiotherapy (WBRT) compared with WBRT alone for brain metastases [134]. Stereotactic radiosurgery can either be delivered using linear accelerator-based treatment with multiple static or conformal arcs, or using gamma knife radiosurgery with multiple highly collimated cobalt sources. Historically, this strategy has been reserved for patients with solitary metastasis or oligometastatic diseases (≤3 lesions). However, several studies have recently evaluated the feasibility of using VMAT to deliver either a single fraction radiosurgical boost or fractionated “stereotactic” boost to multiple brain metastases. Lagerwaard et al [135] compared WBRT with SIB to the metastatic lesions using double arc VMAT, with their conventional strategy of WBRT followed sequentially by a single stereotactic boost (21 Gy to 80% isodose) using multiple non-coplanar conformal arcs. For the integrated VMAT plan, the total dose to the metastatic lesions was 40 Gy in 5 fractions (WBRT dose prescription was 20 Gy in 5 fractions). They found satisfactory coverage for the boost and WBRT PTV in the integrated plans and much steeper dose gradients outside the boost PTV, which resulted in improved conformity compared with the conventional strategy. The volume of normal brain receiving between 25 Gy and 35 Gy was lower in the integrated VMAT plans but the maximum dose to the lenses was higher compared with the conventional technique (9.5 Gy vs 5 Gy). Ma et al [136] conducted a planning study evaluating hypofractionated stereotactic radiotherapy (dose prescription of 50 Gy in 10 fractions) in 10 patients with between 2 and 4 brain metastases. Single and double arc VMAT plans were compared with seven-field fixed field IMRT (SS). PTV coverage was similar between VMAT and IMRT with slightly better conformity and homogeneity in the double arc VMAT plans. Double arc VMAT plans also resulted in slightly lower maximum doses to the brainstem and optic structures compared with fixed field IMRT. However, the percentage of healthy tissue volume receiving 5 Gy was larger with VMAT (56.7% single arc, 57.1% double arc) compared with fixed field IMRT (52.9%).
In another study by Clark et al [137], three VMAT plans were generated for four simulated cases that varied with regard to spacing between the lesions (either 3 cm or 6 cm apart) and axial planes (either same or different planes). The VMAT plans included a single arc/single isocentre (SASI), triple arc/single isocentre (TASI) and triple arc/triple isocentre (TATI) plan. Conformity was better in the multi-arc plans; the TASI plan performed better than the TATI plan. This was also the case with the size of the 12 Gy isodose volumes, which is considered an important predictor of normal tissue complications including radiation necrosis. These differences were more pronounced in the cases where lesions were situated in close proximity, leading the authors to conclude that for less demanding cases, where lesions are spaced widely apart, a single arc technique can produce adequate plans. For more complex cases, multi-arc techniques are superior to single arc with no significant differences between single or triple isocentre techniques. Single isocentre techniques would be preferable as treatment time is shorter and there is less potential for set-up error. Another feasibility study by Hsu et al [138] found that VMAT was able to deliver WBRT with SIB for 1–3 brain metastases with the addition of hippocampal avoidance, which could potentially reduce the risk of neurocognitive dysfunction.
Spinal metastatic disease is another palliative setting where IMRT techniques could play a role. This common complication of many malignant tumours can lead to significant morbidity in terms of severe pain and neurological dysfunction including paralysis in the case of spinal cord compression. The radiation dose that can be delivered using conventional radiotherapy techniques are limited by dose constraints set to minimise spinal cord toxicity. This limitation may be overcome by the use of SBRT using highly conformal intensity modulated radiation techniques. Two separate planning studies by Kuijper et al [140] and Wu et al [139] compared VMAT with fixed field IMRT to deliver SBRT to vertebral metastases. In Wu et al’s study [139], IMRT plans (SW) using 8–12 static beams were compared with single and double arc VMAT. PTV coverage was comparable, but conformity was best with double arc VMAT. IMRT was best at spinal cord sparing while double arc VMAT was better than single arc in this respect. In Kuijper et al's study [140], fewer beams were used in the IMRT plans (7–9 fields, SW) and they evaluated two or three arcs in the VMAT plans. Their results showed equivalence in terms of PTV coverage and OAR sparing, although conformity was better with VMAT. This study did not show any reduction in MU and treatment time, which is contrary to Wu et al's [139] findings. This could be explained by the greater number of beams in the IMRT and fewer arcs in the VMAT plans in Wu et al's study [139]. A further finding from Kuijper et al's study [140] is that although IMRT and VMAT can achieve adequate PTV coverage in cases where only the vertebral body was treated, the PTV coverage is compromised with both techniques where the target volume was more complex and included the entire vertebra (vertebral body, pedicles and posterior elements). Furthermore, while these results are encouraging, these advanced radiotherapy techniques require lengthy planning and QA procedures and may be unrealistic for case scenarios such as spinal cord compression where urgent initiation of treatment is of paramount importance. As a result, most of these patients will still receive standard conventional radiotherapy. A proportion of these patients (25–40%) may develop troublesome in-field local recurrences following their initial treatment. The feasibility of reirradiation with conventional, and more recently IMRT techniques, has been studied. Mancosu et al [141] evaluated VMAT in this setting and found that this technique could achieve satisfactory PTV coverage and spinal cord sparing. Further trials to evaluate clinical outcomes (local control and symptom palliation) with this strategy are in progress.
A summary of the comparative planning studies evaluating VMAT in central nervous system tumours is presented in Table 5. An example of dose distributions achieved with VMAT and fixed field IMRT for malignant glioma is illustrated in Figure 3.
Table 5. Comparative planning studies in central nervous system tumours.
| Paper [ref] VMAT commercial system | Number of patients | Type + Dose | Comparison | PTV | OAR | MU per fraction | Treatment time per fraction |
| Fogliata et al [129] RapidArc | 12 | Acoustic neuroma, meningioma, pituitary adenoma | Non-coplanar IMRT (5−7F,SW) vs VMAT vs HT | VMAT and HT slightly better than IMRT for PTV coverage (D99%, D98%). VMAT and IMRT slightly better than HT for conformity | VMAT and IMRT better than HT at sparing OARs (brainstem, optic structures). VMAT and IMRT – lower integral dose to body compared with HT | ||
| Lagerwaard et al [130] RapidArc | 3 | Acoustic neuroma (radiosurgery single 12.5Gy) | VMAT (SA) vs 1DCA vs 5DCA | Similar PTV coverage. VMAT better than DCA for conformity | VMAT and 5DCA – similar maximum doses for cochlea, brainstem, trigeminal nerve (1DCA gave higher doses compared with VMAT and 5DCA). VMAT and 1DCA – smaller volume of normal brain receiving low dose radiation compared with 5DCA | VMAT, 2763−2869; 5DCA, 2483−2769 | VMAT, 4−5 min; 5DCA, 20 min |
| Wagner et al [132] RapidArc | 14 | High grade glioma | 3D-CRT (2−8F) vs IMRT (5−9F,SW) vs VMAT (SA) | IMRT better than VMAT and 3D-CRT for PTV coverage. 3D-CRT as good as IMRT and VMAT for coverage of PTVs distant from OAR but significantly inferior for PTVs close to OARs. VMAT slightly better than IMRT for conformity (both VMAT and IMRT better than CRT) | VMAT slightly better than IMRT and 3D-CRT for OAR sparing (chiasm, brainstem). VMAT – highest mean dose to normal brain and V5Gy of healthy tissue | VMAT, 321.1; IMRT, 587.8; CRT, 224 | VMAT 5× faster than IMRT; 1.2× faster than CRT |
| Shaffer et al [133] RapidArc | 10 | High grade glioma | IMRT (7F,SW) vs VMAT (SA) | Similar PTV coverage, conformity and homogeneity | VMAT better than IMRT at sparing lateralised OARs (retina, lens, optic nerves). No significant differences in sparing of centralised OARs (brainstem, chiasm). VMAT – higher mean dose to normal brain (by 12%) | VMAT, 363; IMRT, 789 | VMAT, 1.8 min; IMRT; 5.1 min |
| Lagerwaard et al [135] RapidArc | 8 | Brain metastases (1−5) | WBRT + SIB with VMAT 40Gy in 5 fractions (integrated plans) vs conventional WBRT + stereotactic boost 21Gy (summated plans) | Integrated plans (VMAT) significantly better than summated plans for conformity | Integrated plans (VMAT) – smaller volume of normal brain receiving 25−35Gy. Integrated plans (VMAT) – higher maximum dose to lenses | Integrated plans (VMAT), 1600 | Integrated plans (VMAT), 3 min |
| Ma et al [136] RapidArc | 10 | 2–4 brain metastases | IMRT (7F,SS) vs VMAT (SA) vs VMAT (DA) | Similar PTV coverage. DA VMAT slightly better than SA VMAT and IMRT for conformity and homogeneity | DA VMAT better than IMRT at sparing brainstem, optic nerves, lenses (lower maximum doses). VMAT – higher V5Gy healthy tissue, but lower V15Gy and V20Gy compared with IMRT | IMRT, 1944; VMAT (SA), 1199; VMAT (DA), 1387 | (Beam on time) IMRT, 6.5 min; VMAT (SA), 1.25 min; VMAT (DA), 2.5 min |
| Wu et al [139] RapidArc | 10 | Spinal metastases SBRT 16Gy single fraction | IMRT, (8−12F,SW) vs VMAT (SA) vs VMAT (DA) | Similar PTV coverage. DA VMAT better than SA VMAT and IMRT for conformity | IMRT better than VMAT for spinal cord sparing (significant difference for SA VMAT vs IMRT but not significant for DA VMAT vs IMRT) | VMAT (SA), 7730; VMAT(DA), 6317; IMRT, 8711 | VMAT, 7.9−8.6 min; IMRT, 15.9 min |
| Kuijper et al [140] RapidArc | 5 | Spinal metastases SBRT 16Gy Group 1: PTV = vertebral body only Group 2: PTV = entire vertebra | IMRT (7−9F,SW) vs VMAT (2−3 arcs) | Group 1: Similar and acceptable PTV coverage. VMAT better than IMRT for conformity; Group 2: Similar but inadequate PTV coverage. VMAT better than IMRT for conformity | Group 1: Similar OAR sparing; Group 2: VMAT slightly better than IMRT for spinal cord sparing | Group 1: VMAT, 7816; IMRT, 5660; Group 2: VMAT, 9019; IMRT, 9399 | Group 1: VMAT, 13.5 min; IMRT, 12.5 min; Group 2: VMAT, 16 min; IMRT, 19−20 min |
VMAT, volumetric modulated arc therapy; PTV, planning target volume; OAR, organs at risk; MU, monitor units; IMRT, intensity modulated radiotherapy; 5F, five field; 7F, seven field; 9F, nine field; 2F, two field; 8F, eight field; 12F, twelve field; SW, sliding window; SS, step-and-shoot; HT; helical tomotherapy; D99%, dose to 99% of volume; D98%, dose to 98% of volume; SA, single arc; DA, double arc; DCA, dynamic conformal arc; 3D-CRT, three-dimensional conformal radiotherapy; V5Gy, volume receiving ≥5Gy; WBRT, whole brain radiotherapy; SIB, simultaneous integrated boost; V15Gy, volume receiving ≥15Gy; V20Gy, volume receiving ≥20Gy; SBRT, stereotactic body radiotherapy.
Figure 3.
Example of dose distributions in (a) IMRT and (b) VMAT plans for malignant glioma. The dose prescribed to the planning target volume (PTV) (red contour) is 60 Gy in 30 fractions. The 95% isodose (green line) is encompassing most of the PTV. There is compromise of PTV coverage to allow sparing of the optic nerves (dark blue contour) and brain stem (pink contour). Figures courtesy of Dr R Shaffer. Reprinted from Int J Radiat Oncology Biol Phys, Vol. 76, No.4, Shaffer R, Nichol AM, Vollans E, Fong M, Nakano S, Moiseenko V et al. A comparison of volumetric modulated arc therapy and conventional intensity-modulated radiotherapy for frontal and temporal high-grade gliomas, pp. 1177-1184, Copyright 2010, with permission from Elsevier [133].
Breast cancer
VMAT has been investigated in a number of different scenarios. Qiu et al [142] conducted a planning study for partial breast radiotherapy in eight patients comparing conventional CRT using four to five non-coplanar fields with VMAT using a modified partial arc. The results showed similar target volume coverage; VMAT had slightly better conformity, although this was not statistically significant. The doses to the ipsilateral lung and ipsilateral normal breast tissue were significantly reduced with VMAT. V20 Gy for the ipsilateral lung was 0.5% in VMAT plans compared with 1.6% in CRT plans while V5 Gy for the ipsilateral breast was 59.6% and 70% for the VMAT and CRT plans, respectively. VMAT also used fewer MU and reduced treatment time compared with conventional radiotherapy. Although partial breast radiotherapy is increasingly considered acceptable for selected patients, this strategy is still not widely used outside of clinical trials and longer follow-up of these patients is needed to assess the long term clinical outcomes. Radiotherapy to the whole breast and/or regional lymph nodes remains the gold standard.
The irradiation of internal mammary lymph nodes is not considered standard practice in the UK, but it is performed for selected patients with high risk breast cancer at several institutions worldwide. Conventionally this is done using the modified wide tangent (MWT) technique, which increases the volume of normal tissue (particularly lung and heart) receiving radiation. Popescu et al [143] evaluated VMAT against the conventional MWT technique and nine-field fixed field IMRT (SW) in a group of patients with left-sided breast cancer who also received radiotherapy to the internal mammary nodes. They report similar PTV coverage, dose homogeneity and conformity, but improved sparing of OARs with VMAT, particularly for the heart, ipsilateral lung and contralateral breast. Another similar study by Johansen et al [144] found similar PTV coverage, but improved conformity with IMRT (SW) and VMAT compared with conventional techniques and better homogeneity with VMAT. In their study, there was improved sparing of the ipsilateral lung and contralateral breast with VMAT, but no significant differences in cardiac doses. However, only 3 out of the 8 were left-sided tumours, therefore, definite conclusions on cardiac-sparing cannot be made from this study.
Another potential area where IMRT techniques could play a useful role is in the treatment of large target volumes where there is risk of increased radiation dose to OARs (e.g. in bilateral breast cancer) or complex-shaped target volumes (e.g. in patients with an unusually shaped chest wall, such as in cases of pectus excavatum). Nicolini et al [145] conducted a planning study in 10 patients treated for bilateral breast cancer with an SIB technique comparing VMAT and fixed field IMRT (SW). Similar target coverage was found with better dose homogeneity with VMAT. The doses to the heart were lower with VMAT while for the lungs VMAT achieved better sparing at the mid- to high-dose levels (e.g. V20 Gy right lung 10.3% (VMAT) vs 14.5% (IMRT)) compared with IMRT, which gave better sparing at low dose levels (e.g.V5 Gy right lung 58.3% (VMAT) vs 44.4% (IMRT)). The mean and integral dose to healthy tissue was higher with VMAT in this study. In Popescu et al's study [143], the mean dose to healthy tissue was lower with VMAT, but V5 Gy for healthy tissue was higher, which is consistent with inferior sparing at low dose levels. Another important factor in this patient cohort is the effect of intrafraction motion, which could lead to increased doses to OARs. Although the shorter treatment time with VMAT could reduce the impact of motion, other methods to account for this (e.g. breath-hold or target tracking techniques) should also be considered.
The increase in low dose radiation to healthy tissues with IMRT techniques is a concern in this patient cohort. Breast cancer mortality is decreasing owing to a combination of factors including earlier diagnosis via screening and improvements in therapy. Many patients now survive for many years after diagnosis and treatment for breast cancer. It is therefore important to minimise late side effects that could arise from their treatment. Apart from cardiovascular disease, secondary malignancy is a significant cause of non-breast cancer mortality in long-term survivors [146]. The increased risk of secondary malignancy secondary to low dose radiation is currently not accurately quantifiable but should be borne in mind when deciding on the treatment strategy or radiation technique for these patients. IMRT will still play an important role in breast radiotherapy, particularly within the setting of partial breast dose escalation for high-risk disease, which is currently being investigated in the IMPORT-HIGH trial [147]. IMRT techniques can be refined to minimise the amount of low dose radiation to healthy tissues e.g. by setting dose constraints on additional normal tissue structures in the optimisation process or, in the case of VMAT, using partial arcs. While inverse planned IMRT is necessary for complex target volumes, simpler forward planned techniques using multiple segmented tangential fields may be able to produce acceptable dose distributions for less complex cases while also minimising low dose radiation to surrounding normal tissue. As with other tumour sites, it may be that there is no universal optimal solution and the selection of the most appropriate radiation technique, be it conventional CRT, IMRT or VMAT, must be made on an individual case basis.
A summary of the comparative planning studies evaluating VMAT in breast cancer is presented in Table 6.
Table 6. Comparative planning studies in breast cancer.
| Paper [ref] VMAT commercial system | Number of patients | Site | Comparison | PTV | OAR | MU per fraction | Treatment time per fraction |
| Qiu et al [142] Rapidarc | 8 | Breast (partial breast radiotherapy) | 3D-CRT (non coplanar, 4–5F) vs VMAT (modified partial arc) | Similar PTV coverage. VMAT slightly better than 3D-CRT at conformity (not statistically significant) | VMAT better than 3D-CRT at sparing ipsilateral normal breast tissue, ipsilateral lung | VMAT, 488.6; 3D-CRT, 634.1 | VMAT, 1.21 min; 3D-CRT, 6.3 min |
| Popescu et al [143] Predecessor to RapidArc | 5 | Breast (+ regional nodes including internal mammary nodes) | 3D-CRT vs IMRT (9F,SW) vs VMAT (2 partial arcs) | Similar PTV coverage, homogeneity, conformity | VMAT better than IMRT and 3D-CRT at sparing heart and ipsilateral lung (low and intermediate doses), contralateral breast (mean dose). VMAT – lower mean dose to healthy tissue but higher V5Gy compared with 3D-CRT and IMRT | VMAT, 862; IMRT, 1254; 3D-CRT,489 | VMAT, 3.9 min; IMRT, 8.8 min; 3D-CRT, 5 min |
| Johansen et al [144] RapidArc | 8 | Breast (chest wall and nodes including internal mammary nodes) | CRT (4F) vs IMRT (7F,SW) vs VMAT | Similar PTV coverage. VMAT and IMRT better than CRT for conformity. VMAT better than IMRT and CRT for homogeneity | VMAT and IMRT better than CRT at sparing ipsilateral lung. CRT – lowest doses to contralateral lung. VMAT – lowest doses to contralateral breast | ||
| Nicolini et al [145] RapidArc | 10 | Breast (Bilateral, SIB to tumour bed) | IMRT (12F,SW) vs VMAT (DA) | Similar PTV coverage. VMAT better than IMRT at homogeneity | VMAT better than IMRT at sparing heart and lungs (medium-high dose level) (for lungs, IMRT better at sparing at low dose levels). VMAT – higher mean and integral dose to healthy tissue | VMAT, 796; IMRT, 1398 | VMAT, 3 min; IMRT, 11.5 min |
VMAT, volumetric modulated arc therapy; PTV, planning target volume; OAR, organs at risk; MU, monitor units; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity modulated radiotherapy; 4F, four field; 5F, five field; 7F, seven field; 9F, nine field; 12F, twelve field; SW, sliding window; SS, step-and-shoot; V5Gy, volume receiving ≥5Gy; SIB, simultaneous integrated boost.
Other tumour sites and types
Lymphoma
VMAT for early Hodgkin's lymphoma was evaluated in a recent planning study [148]. In this patient cohort the remission rates following treatment are high, which can lead to long life expectancies and the need to reduce the rates of late toxicity, such as secondary cancers and cardiac morbidity. Weber et al [148] compared single arc VMAT with nine-field fixed field IMRT (SW) and found largely equivalent target coverage, homogeneity and conformity, but improved sparing of lung and breast tissue in the intermediate dose levels with VMAT (improvement in V10 Gy and D33%). No significant difference was seen in cardiac sparing between the two techniques. This study did not include conventional CRT techniques in their comparison, which would have resulted in lower doses to healthy tissue and OAR distant to the target volume (e.g. breast). This needs to be balanced against the potential of IMRT and VMAT to improve sparing of OARs within or close to the target volumes (e.g. lung). The authors discuss that there may be a significant role for smaller radiation fields in the treatment of this disease, using involved nodal radiotherapy (INRT) as opposed to involved field radiotherapy (IFRT). These two strategies were also compared using both techniques in this study and there was a universally significant reduction in OAR doses with INRT compared with IFRT.
Intra-abdominal tumours
VMAT has also been evaluated in intra-abdominal malignancy. Eppinga et al [149] evaluated VMAT in a planning study for advanced pancreatic cancer. VMAT plans achieved better conformity and OAR sparing (left kidney, liver, stomach, small bowel and duodenum) compared with fixed field IMRT (SW). Llacer-Moscardo et al [150] performed a dosimetric feasibility study of VMAT in retroperitoneal sarcoma and found acceptable target volume coverage and OAR sparing. Benthuysen et al [151] evaluated VMAT in distal oesophageal cancers and found comparable PTV coverage and OAR sparing to fixed field IMRT with a 42–67% reduction in MU, but greater low dose radiation to the body (V5 Gy 18% greater for VMAT plans). Hawkins et al [152] compared VMAT with 4-field CRT in 10 patient datasets with locally advanced or inoperable distal oesophageal cancers. They found improved OAR sparing (V30 Gy to heart 31% vs 55%) and improved PTV conformity with VMAT. Bignardi et al [153] conducted a planning study to compare VMAT with conventional CRT and nine-field fixed field IMRT (SW) in SBRT for abdominal metastatic lymph nodes (solitary metastasis or oligometastatic disease). The conventional technique used 4−8 static beams or 3−4 conformal short arcs. The results showed VMAT achieved the best PTV coverage (V95%, 90.2% (VMAT) vs 82.5% (CRT) vs 84.5% (IMRT)) and slightly superior dose conformity. OAR sparing was better with IMRT and VMAT compared with CRT. Scorsetti et al [154] reported on early clinical results with VMAT (RapidArc, Varian) in the treatment of primary or metastatic tumours at abdominal sites, which showed promising results in terms of local control and acute toxicity rates. An important factor to consider in intra-abdominal radiotherapy is the effect of intrafraction motion, which can reduce the accuracy of treatment, and methods to account for this should be considered.
Paediatric cases
There has been some debate on the use of IMRT techniques for paediatric cases owing to the concerns of a potential increase in radiation-induced secondary malignancy. IMRT plans typically use a larger number of MU and require longer treatment delivery times compared with conventional radiotherapy techniques, which can result in higher low dose radiation to normal tissues. VMAT techniques could theoretically lower the risk because VMAT plans generally require fewer MU and shorter treatment times compared with fixed field IMRT. The shorter treatment time could also be beneficial in cases where there may be issues with the treatment position causing patient discomfort or difficulties with airway access where general anaesthesia is required. One such example is craniospinal irradiation (CSI), which is an important part of the treatment of paediatric tumours, such as medulloblastoma. In general, patients are treated in the prone position because this allows better visualisation of the matching field junctions between the cranial and spinal fields, although several institutions have now adopted the supine technique as this is more comfortable for patients. Two recent studies evaluating VMAT in this setting have been recently published. Fogliata et al [155] reported on their experience using VMAT to deliver CSI in five patients from five different institutions (age range, 7–45 years). They found that VMAT could achieve highly conformal plans with acceptably low doses to OARs. A further benefit of VMAT (or IMRT technique) is the ability to treat the entire target volume without the need for matching field junctions, which is required in conventional techniques. This could reduce the risk of over dosage at the junction that could lead to increased risk of radiation myelitis or insufficient dosage that could potentially increase the risk of treatment failure. Another study by Lee et al [156] compared VMAT with conventional radiotherapy techniques for CSI in 5 patient datasets (3 patients were under 16 years old) and report improved target volume conformity and heterogeneity with VMAT, as well as significantly lower doses to OARs (heart, oesophagus, optic nerve and lens). V15 Gy and V10 Gy to the body were reduced in the VMAT plans but V2 Gy and V5 Gy were higher with VMAT, with a higher integral dose to non-PTV body volume in 4 of the 5 patients.
A planning study by Shaffer et al [157] evaluated single arc VMAT in 8 paediatric cases with retroperitoneal tumours in comparison with 7-field fixed field IMRT (SW), 3D-CRT and a parallel-opposed pair (POP) plan. VMAT and IMRT were able to achieve improved conformity and lower dose to the liver compared with 3D-CRT and POP, but at the expense of greater MU usage. VMAT has also been evaluated in the setting of total body irradiation (TBI), which is part of the pre-conditioning regimen used in haematological malignancies prior to bone marrow transplantation. A significant number of these patients will include children and young adults; therefore, the priority should be placed on reducing the rates of late toxicity and morbidity as a result of radiotherapy. Two recent planning studies evaluating VMAT in this setting have been published. Fogliata et al [158] reported on the feasibility of using VMAT to deliver TBI, which achieved satisfactory target coverage and acceptably low doses to the OARs (median dose for OARs ranged from 2.3 Gy for the oral cavity to 7.3 Gy for the bowels). Aydogan et al [159] compared VMAT with fixed field IMRT and tomotherapy and found comparable dose distributions between the techniques. With respect to OAR sparing, VMAT achieved lower median doses to the heart, liver and bowel compared with fixed field IMRT and tomotherapy, and lower median doses to lungs, kidneys, eyes and oral cavity compared with tomotherapy. Treatment delivery time with VMAT was reduced to approximately 18 min compared with 45 min with fixed field IMRT.
A summary of comparative planning studies in the tumour sites/types as specified above is presented in Table 7. An example of dose distributions achieved with VMAT and fixed field IMRT in paediatric retroperitoneal tumours is illustrated in Figure 4.
Table 7. Comparative planning studies in other tumour sites (lymphoma, intra-abdominal malignancies and paediatric tumours).
| Paper [ref] VMAT commercial system | Number of patients | Site and type | Comparison | PTV | OAR | MU per fraction | Treatment time per fraction |
| Weber et al [148] RapidArc | 10 | Stage I, II Hodgkins lymphoma (IFRT vs INRT) | IMRT (9F,SW) vs VMAT (SA) | IFRT: similar PTV coverage, homogeneity; VMAT slightly better than IMRT for conformity. INRT: VMAT slightly better than for PTV coverage (not statistically significant). No difference in homogeneity | VMAT better than IMRT at sparing lung (mid-dose level), breast (high to mid-dose level). No difference in cardiac sparing. VMAT – lower integral dose to body compared with IMRT | IMRT, 1020−1255; VMAT, 367−376 | |
| Eppinga et al [149] RapidArc | 10 | Pancreas | IMRT (5F,SW) vs VMAT (DA) | Similar PTV coverage. VMAT better than IMRT for conformity | VMAT better than IMRT for OAR sparing (kidneys, liver, stomach, bowel and duodenum) | VMAT, 561; IMRT, 800 | (Beam-on time) VMAT, less than 3 min; IMRT, 8 min |
| Benthuysen et al [151] | 14 | Oesophagus | IMRT (7F) vs VMAT (SA or DA) | Similar PTV coverage. IMRT slightly better than VMAT for homogeneity | Similar OAR sparing. VMAT – higher body V5Gy compared to IMRT | VMAT (SA), 401; VMAT (DA), 449.5; IMRT, 782 | |
| Hawkins et al [152] | 10 | Oesophagus | 3D-CRT (4F) vs VMAT | Similar PTV coverage. VMAT better than 3D-CRT for conformity | VMAT better than IMRT at sparing heart. VMAT – higher volumes of lung receiving low dose (V5Gy, V10Gy). No difference in V20Gy and mean lung dose | VMAT, 260; IMRT, 320 | VMAT, 1.1−1.47 min; IMRT, 3.25 min |
| Bignardi et al [153] RapidArc | 14 | Abdominal metastases SBRT 45Gy in 6 fractions | CRT (4−8 static beams, or 3−4 conformal short arcs) vs IMRT (9F,SW) vs VMAT (SA) | VMAT better than CRT and IMRT for PTV coverage (V95%) and slightly better for conformity | VMAT and IMRT better than CRT for OAR sparing | VMAT, 2186; IMRT, 2583; CRT, 1554 | VMAT, 3.7 min; IMRT, 10.6 min; CRT, 6.3 min |
| Lee et al [156] SmartArc | 5 | Craniospinal radiotherapy | Conventional vs VMAT | VMAT better than conventional for conformity and homogeneity | VMAT better than conventional at sparing heart, thyroid, oesophagus, lenses, optic nerves, eyes. VMAT – higher mean doses to kidneys, liver. VMAT – higher mean lung dose but lower V20Gy. VMAT – lower body V15Gy and V10Gy, but higher V5Gy and V2Gy | VMAT, 622.4; conventional, 600.4 | VMAT, 9.42 min; conventional, 15 min |
| Shaffer et al [157] RapidArc | 8 | Retroperitoneal paediatric tumours (5 neuroblastoma) | POP vs 3D-CRT vs IMRT (7F,SW) vs VMAT (SA) | Similar and adequate PTV coverage. IMRT and VMAT better than POP and 3D-CRT for conformity | No difference in ability to meet dose constraints to kidney. VMAT and IMRT better than POP and 3D-CRT at sparing liver | VMAT, 325; IMRT, 665; 3D-CRT, 235; POP, 203 | VMAT, 1.25 min; IMRT, 4.02 min; 3D-CRT, 2.6 min; POP, 1.38 min; |
| Aydogan et al [159] RapidArc | 6 | Total body irradiation | VMAT vs IMRT vs tomotherapy | VMAT – lower mean dose to heart, liver and bowel compared to fixed field IMRT and tomotherapy; lower median doses to lungs, kidneys, eyes and oral cavity compared with tomotherapy | VMAT, 18 min; IMRT, 45 min |
VMAT, volumetric modulated arc therapy; PTV, planning target volume; OAR, organs at risk; MU, monitor units; IFRT, involved field radiotherapy; INRT, involved nodal radiotherapy; IMRT, intensity modulated radiotherapy; 4F, four field; 5F, five field; 7F, seven field; 9F, nine field; SW, sliding window; SS, step-and-shoot; SA, single arc; DA, double arc; V5Gy, volume receiving ≥5Gy; 3D-CRT, three dimensional conformal radiotherapy; V10Gy, volume receiving ≥10Gy; V20Gy, volume receiving ≥20Gy; SBRT, stereotactic body radiotherapy; V95%, volume receiving ≥95% of prescribed dose; V15Gy, volume receiving ≥15Gy; V2Gy, volume receiving ≥2Gy; POP, parallel opposed pair.
Figure 4.
Example of dose distributions in (a) IMRT and (b) VMAT plans for radiotherapy to a retroperitoneal tumour in a paediatric patient. The planning target volume (PTV) (red contour) is encompassed by the 95% isodose (green line). The organs at risk include liver (blue contour), kidneys (dark orange and light blue), vertebral body (pink contour) and spinal cord (lilac contour). Figures courtesy of Dr R Shaffer. This material is reproduced with permission of John Wiley & Sons, Inc from Pediatr Blood Cancer, 2011, Vol 56, Shaffer R, Vollans E, Vellani R, Welsh M, Moiseenko V, Goddard K. A radiotherapy planning study of RapidArc, Intensity Modulated Radiotherapy, Three-Dimensional Conformal Radiotherapy, and Parallel Opposed Beams in the Treatment of Pediatric Retroperitoneal Tumors, pp.16-23, Copyright 2011Wiley-Liss, Inc [157].
Conclusion
VMAT is a new radiation technique that combines the ability to achieve highly conformal dose distributions with highly efficient treatment delivery. Most of the published data in the literature are dosimetric planning studies with limited clinical outcome data. However, VMAT is still a novel technology and as increasing numbers of patients are treated with this technique, more clinical data will emerge. Most of the planning studies in various tumour sites have compared VMAT with either fixed field IMRT or conventional CRT techniques. VMAT has clear superiority over conventional CRT with regard to improving dose conformity and OAR sparing. However, the distinction between VMAT and fixed field IMRT is less clear. The data suggests that for most tumour sites VMAT and fixed field IMRT will produce largely equivalent target volume coverage, dose conformity and homogeneity. The absolute difference in dosimetric parameters reported as statistically significant in some of the planning studies is relatively small and may not be clinically significant. Similarly with OAR sparing, some planning studies have reported equivalent results between VMAT and fixed field IMRT. However, for sites such as prostate or cervical cancer, some studies have reported significant improvement in OAR sparing with VMAT. The similarities between VMAT and fixed field IMRT are not surprising given that VMAT is essentially an alternative form of IMRT. The significant difference between VMAT and fixed field IMRT is the reduction in MU and treatment delivery time, which was an almost universal finding in all the planning studies.
There are inherent limitations with these planning studies. Even if the same strict planning objectives and calculation algorithms were used, it is extremely difficult to completely eliminate planner bias especially if multiple planners are involved in the process. Direct comparisons between different studies are not possible because of significant differences in target volume definitions, dose prescription and treatment schedules. Radiation techniques also vary between the studies, for example in the number of fields and arcs used in the fixed field IMRT and VMAT plans, and IMRT technique (SW or SS). As a result, it is not surprising that the results on PTV coverage and OAR sparing can appear conflicting between the studies.
A major source of concern with VMAT and IMRT is the increase in low dose radiation to surrounding normal tissue, which potentially increases the risk of secondary malignancy. It is estimated that the incidence of secondary malignancies could almost double with IMRT compared with conventional techniques (from 1% to 1.75% for patients surviving 10 years) [21]. Although the theoretical risk of secondary malignancy induction with VMAT should be lower as VMAT generally uses fewer MU compared with conventional fixed field IMRT, this could be counteracted by the increase of normal tissue volume receiving low dose radiation, which has been seen in a number of studies [9,129,132,143,156]. Longer follow-up of patients treated with these techniques will be required to accurately quantify this risk.
Finally, although there is evidence to show that VMAT has a definite place in the treatment of many tumours, it cannot be considered the universal solution for all clinical scenarios. Each case must be evaluated on an individual basis to select the most appropriate radiation technique that will give optimal results.
References
- 1.Newbold K, Partridge M, Cook G, Sohaib SA, Charles-Edwards E, Rhys-Evans P, et al. Advanced imaging applied to radiotherapy planning in head and neck cancer: a clinical review. Br J Radiol 2006;79:554–61 [DOI] [PubMed] [Google Scholar]
- 2.Thwaites DI, Tuohy JB. Back to the future: the history and development of the clinical linear accelerator. Phys Med Biol 2006;51:R343–62 [DOI] [PubMed] [Google Scholar]
- 3.Sternick ES, editor. The theory and practice of intensity modulated radiation therapy. Madison WI: Advanced Medical Publishing 1997. [Google Scholar]
- 4.Webb S. Intensity-modulated radiation therapy. Bristol: Institute of Physics Publishing 2000. [Google Scholar]
- 5.Webb S. Advances in treatment with intensity-modulated conformal radiotherapy. Tumori 1998;84:112–26 [DOI] [PubMed] [Google Scholar]
- 6.Veldeman L, Madani I, Hulstaert F, De Meerleer G, Mareel M, De Neve W. Evidence behind the use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol 2008;9:367–75 [DOI] [PubMed] [Google Scholar]
- 7.Staffurth J. Radiotherapy Development Board. A review of the clinical evidence for intensity-modulated radiotherapy. Clin Oncol 2010;22:643–57 [DOI] [PubMed] [Google Scholar]
- 8.Guerrero Urbano MT, Nutting CM. Clinical use of intensity-modulated radiotherapy: part I. Br J Radiol 2004;77:88–96 [DOI] [PubMed] [Google Scholar]
- 9.Guerrero Urbano MT, Nutting CM. Clinical use of intensity-modulated radiotherapy: part II. Br J Radiol 2004;77:177–82 [DOI] [PubMed] [Google Scholar]
- 10.Wu Q, Mohan R, Morris M, Lauve A, Schmidt-Ullrich R. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas: I – dosimetric results. Int J Radiat Oncol Biol Phys 2003;56:573–85 [DOI] [PubMed] [Google Scholar]
- 11.Mohan R, Wu Q, Manning M, Schmidt-Ullrich R. Radiobiological considerations in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys 2000;46:619–30 [DOI] [PubMed] [Google Scholar]
- 12.Saw CB, Ayyangar KM, Zhen W, Yoe-Sein M, Pillai S, Enke CA. Clinical implementation of intensity-modulated radiation therapy. Med Dosim 2002;27:161–9 [DOI] [PubMed] [Google Scholar]
- 13.McNair HA, Adams EJ, Clark CH, Miles EA, Nutting CM. Implementation of IMRT in the radiotherapy department. Br J Radiol 2003;76:859–6 [DOI] [PubMed] [Google Scholar]
- 14.Miles EA, Clark CH, Guerrero Urbano MT, Bidmead M, Dearnaley DP, Harrington KJ, et al. The impact of introducing intensity modulated radiotherapy into routine clinical practice. Radiother Oncol 2005;77:241–6 [DOI] [PubMed] [Google Scholar]
- 15.Intensity Modulated Radiation Therapy Collaborative Working Group Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys 2001;51:880–914 [DOI] [PubMed] [Google Scholar]
- 16.Wang JZ, Li XA, D'Souza WD, Stewart RD. Impact of prolonged fraction delivery times on tumour control: a note of caution for intensity-modulated radiation therapy (IMRT). Int J Radiat Oncol Biol Phys 2003;57:543–52 [DOI] [PubMed] [Google Scholar]
- 17.Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumour clonogen repopulation during radiotherapy. Acta Oncol 1988;27:131–46 [DOI] [PubMed] [Google Scholar]
- 18.Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumour sites and practical methods for compensation. Int J Radiat Oncol Biol Phys 2007;68:654–61 [DOI] [PubMed] [Google Scholar]
- 19.Adams EJ, Convery DJ, Cosgrove VP, McNair HA, Staffurth JN, Vaarkamp J, et al. Clinical implementation of dynamic and step-and-shoot IMRT to treat prostate cancer with high risk of pelvic lymph node involvement. Radiother Oncol 2004;70:1–10 [DOI] [PubMed] [Google Scholar]
- 20.Longobardi B, De Martin E, Fiorino C, Dell'Oca I, Broggi S, Cattaneo GM, et al. Comparing 3DCRT and inversely optimized IMRT planning for head and neck cancer: equivalence between step-and-shoot and sliding window techniques. Radiother Oncol 2005;77:18–56 [DOI] [PubMed] [Google Scholar]
- 21.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 2003;56:83–8 [DOI] [PubMed] [Google Scholar]
- 22.Verellen D, Vanhavere F. Risk assessment of radiation-induced malignancies based on whole-body equivalent dose estimates for IMRT treatment in the head and neck region. Radiother Oncol 1999;53:199–203 [DOI] [PubMed] [Google Scholar]
- 23.Ruben JD, Davis S, Evans C, Jones P, Gagliardi F, Harnes M, Hunter A. The effect of intensity-modulated radiotherapy on radiation-induced second malignancies. Int J Radiat Oncol Biol Phys 2008;70:1530–6 [DOI] [PubMed] [Google Scholar]
- 24.Gershkevitsh E, Clark CH, Staffurth J, Dearnaley DP, Trott KR. Dose to bone marrow using IMRT techniques in prostate cancer patients. Strahlenther Onkol 2005;181:172–8 [DOI] [PubMed] [Google Scholar]
- 25.Palma DA, Verbakel WF, Otto K, Senan S. New developments in arc radiation therapy: a review. Cancer Treat Rev 2010;36:393–9 [DOI] [PubMed] [Google Scholar]
- 26.Yu CX, Li XA, Ma L, Chen D, Naqvi S, Shepard D, et al. Clinical implementation of intensity-modulated arc therapy. Int J Radiat Oncol Biol Phys 2002;53:453–63 [DOI] [PubMed] [Google Scholar]
- 27.Fenwick JD, Tome WA, Soisson ET, Mehta MP, Rock Mackie T. Tomotherapy and other innovative IMRT delivery systems. Semin Radiat Oncol 2006;16:199–208 [DOI] [PubMed] [Google Scholar]
- 28.Verellen D, De Ridder M, Storme G. A (short) history of image-guided radiotherapy. Radiother Oncol 2008;86:4–13 [DOI] [PubMed] [Google Scholar]
- 29.Verellen D, Ridder MD, Linthout N, Tournel K, Soete G, Storme G. Innovations in image-guided radiotherapy. Nat Rev Cancer 2007;7:949–60 [DOI] [PubMed] [Google Scholar]
- 30.Mackie TR, Holmes T, Swerdloff S, Reckwerdt P, Deasy JO, Yang J, et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys 1993;20:1709–19 [DOI] [PubMed] [Google Scholar]
- 31.Welsh JS, Patel RR, Ritter MA, Harari PM, Mackie TR, Mehta MP. Helical tomotherapy: an innovative technology and approach to radiation therapy. Technol Cancer Res Treat 2002;1(4):311–6 [DOI] [PubMed] [Google Scholar]
- 32.Mackie TR. History of tomotherapy. Phys Med Biol 2006;51:R427–53 [DOI] [PubMed] [Google Scholar]
- 33.Beavis AW. Is tomotherapy the future of IMRT? Br J Radiol 2004;77:285–95 [DOI] [PubMed] [Google Scholar]
- 34.Sheng K, Molloy JA, Larner JM, Read PW. A dosimetric comparison of non-coplanar IMRT versus helical tomotherapy for nasal cavity and paranasal sinus cancer. Radiother Oncol 2007;82:174–8 [DOI] [PubMed] [Google Scholar]
- 35.Joseph KJ, Syme A, Small C, Warkentin H, Quon H, Ghosh S, et al. A treatment planning study comparing helical tomotherapy with intensity-modulated radiotherapy for the treatment of anal cancer. Radiother Oncol 2010;94:60–6 [DOI] [PubMed] [Google Scholar]
- 36.Van Vulpen M, Field C, Raajmakers CP, Parliament MB, Terhaard CH, Mackenzie MA, et al. Comparing step-and-shoot IMRT with dynamic helical tomotherapy IMRT plans for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2005;62:1535–9 [DOI] [PubMed] [Google Scholar]
- 37.Mavroidis P, Shi C, Plataniotis GA, Delichas MG, Costa Ferreira B, Rodriguez S, et al. Comparison of the helical tomotherapy against the multileaf collimator-based intensity-modulated radiotherapy and 3D conformal radiation modalities in lung cancer radiotherapy. Br J Radiol 2011;84:161–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee TF, Fang FM, Chao PJ, Su TJ, Wang LK, Leung SW. Dosimetric comparisons of helical tomotherapy and step-and-shoot intensity-modulated radiotherapy in nasopharyngeal carcinoma. Radiother Oncol 2008;89:89–96 [DOI] [PubMed] [Google Scholar]
- 39.Sterzing F, Sroka-Perez G, Schubert K, Münter MW, Thieke C, Huber P, et al. Evaluating target coverage and normal tissue sparing in the adjuvant radiotherapy of malignant pleural mesothelioma: helical tomotherapy compared with step-and-shoot IMRT. Radiother Oncol 2008;86:251–7 [DOI] [PubMed] [Google Scholar]
- 40.Marnitz S, Lukarski D, Kohler C, Wlodarczyk W, Ebert A, Budach V, et al. Helical tomotherapy versus conventional intensity-modulated radiation therapy for primary chemoradiation in cervical cancer patients: an intraindividual comparison. Int J Radiat Oncol Biol Phys 2011;81:424–30 [DOI] [PubMed] [Google Scholar]
- 41.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys 2008;35:310–7 [DOI] [PubMed] [Google Scholar]
- 42.Yu CX. Intensity-modulated arc therapy with dynamic multileaf collimation: an alternative to tomotherapy. Phys Med Biol 1995;40:1435–49 [DOI] [PubMed] [Google Scholar]
- 43.Yu CX, Tang G. Intensity-modulated arc therapy: principles, technologies and clinical implementation. Phys Med Biol 2011;56:R31–R54 [DOI] [PubMed] [Google Scholar]
- 44.Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, et al. RT01 collaborators. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007;8:475–87 [DOI] [PubMed] [Google Scholar]
- 45.Zelefsky MJ, Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 2002;53:1111–6 [DOI] [PubMed] [Google Scholar]
- 46.Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124–9 [DOI] [PubMed] [Google Scholar]
- 47.De Meerleer GO, Fonteyne VH, Vakaet L, Villeirs GM, Denoyette L, Verbaeys A, et al. Intensity-modulated radiation therapy for prostate cancer: late morbidity and results on biochemical control. Radiother Oncol 2007;82:160–6 [DOI] [PubMed] [Google Scholar]
- 48.Zelefsky MJ, Yamada Y, Fuks Z, Zhang Z, Hunt M, Cahlon O, et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys 2008;71:1028–33 [DOI] [PubMed] [Google Scholar]
- 49.Kupelian PA, Reddy CA, Carlson TP, Altsman KA, Willoughby TR. Preliminary observations on biochemical relapse-free survival rates after short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy/fraction) for localized prostate cancer. Int J Radiat Oncol Biol Phys 2002;53:904–12 [DOI] [PubMed] [Google Scholar]
- 50.De Meerleer GO, Vakaet LA, De Gersem WR, De Wagter C, De Naeyer B, De Neve W. Radiotherapy of prostate cancer with or without intensity modulated beams: a planning comparison. Int J Radiat Oncol Biol Phys 2000;47:639–48 [DOI] [PubMed] [Google Scholar]
- 51.Palma D, Vollans E, James K, Nakano S, Moiseenko V, Shaffer R, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:996–1001 [DOI] [PubMed] [Google Scholar]
- 52.Zhang P, Happersett L, Hunt M, Jackson A, Zelefsky M, Mageras G. Volumetric modulated arc therapy: planning and evaluation for prostate cancer cases. Int J Radiat Oncol Biol Phys 2010;76:1456–62 [DOI] [PubMed] [Google Scholar]
- 53.Kjaer-Kristoffersen F, Ohlhues L, Medin J, Korreman S. RapidArc volumetric modulated therapy planning for prostate cancer patients. Acta Oncol 2009;48:227–32 [DOI] [PubMed] [Google Scholar]
- 54.Hardcastle N, Tome WA, Foo K, Miller A, Carolan M, Metcalfe P. Comparison of prostate IMRT and VMAT biologically optimised treatment plans. Med Dosim 2011;36:292–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ost P, Speleers B, De Meerleer G, De Neve W, Fonteyne V, Villeirs G, et al. Volumetric arc therapy and intensity-modulated radiotherapy for primary prostate radiotherapy with simultaneous integrated boost to intraprostatic lesion with 6 and 18 MV: a planning comparison study. Int J Radiat Oncol Biol Phys 2011;79:920–6 [DOI] [PubMed] [Google Scholar]
- 56.Weber DC, Wang H, Cozzi L, Dipasquale G, Khan HG, Ratib O, et al. RapidArc, intensity modulated photon and proton techniques for recurrent prostate cancer in previously irradiated patients: a treatment planning comparison study. Radiat Oncol 2009;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kopp RW, Duff M, Catalfamo F, Shah D, Rajecki M, Ahmad K. doi: 10.1016/j.meddos.2010.09.004. VMAT vs 7-field IMRT: Assessing the dosimetric parameters of prostate cancer treatment with a 292-patient sample. Med Dosim 2011;4:[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Yoo S, Wu QJ, Lee WR, Yin FF. Radiotherapy treatment plans with RapidArc for prostate cancer involving seminal vesicles and lymph nodes. Int J Radiat Oncol Biol Phys 2010;76:935–42 [DOI] [PubMed] [Google Scholar]
- 59.Wolff D, Stieler F, Welzel G, Lorenz F, Abo-Madyan Y, Mai S, et al. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol 2009;93:226–33 [DOI] [PubMed] [Google Scholar]
- 60.Tsai CL, Wu JK, Chao HL, Tsai YC, Cheng JC. Treatment and dosimetric advantages between VMAT, IMRT, and helical tomotherapy in prostate cancer. Med Dosim 2011;36:264–71 [DOI] [PubMed] [Google Scholar]
- 61.Rao M, Yang W, Chen F, Sheng K, Ye J, Mehta V, et al. Comparison of Elekta VMAT with helical tomotherapy and fixed field IMRT: plan quality, delivery efficiency and accuracy. Med Phys 2010;37:1350–9 [DOI] [PubMed] [Google Scholar]
- 62.Shaffer R, Morris WJ, Moiseenko V, Welsh M, Crumley C, Nakano S, et al. Volumetric modulated arc therapy and conventional intensity-modulated radiotherapy for simultaneous maximal intraprostatic boost: a planning comparison study. Clin Oncol 2009;21:401–7 [DOI] [PubMed] [Google Scholar]
- 63.Alvarez Moret J, Koelbl O, Bogner L. Quasi-IMAT technique and secondary cancer risk in prostate cancer. Strahlenther Onkol 2009;185:248–53 [DOI] [PubMed] [Google Scholar]
- 64.Crijns W, Budiharto T, Defraene G, Verstraete J, Depuydt T, Haustermans K, et al. IMRT-based optimization approaches for volumetric modulated single arc radiotherapy planning. Radiother Oncol 2010;95:149–52 [DOI] [PubMed] [Google Scholar]
- 65.Miles EF, Lee WR. Hypofractionation for prostate cancer: a critical review. Semin Radiat Oncol 2008;18:41–7 [DOI] [PubMed] [Google Scholar]
- 66.Ritter M. Rationale, conduct, and outcome using hypofractionated radiotherapy in prostate cancer. Semin Radiat Oncol 2008;18:249–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeoh EE, Botten RJ, Butters J, Di Matteo AC, Holloway RH, Fowler J. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase iii randomized Trial. Int J Radiat Oncol Biol Phys 2010:7:[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Rene N, Faria S, Cury F, David M, Duclos M, Shenouda G. et al. Hypofractionated radiotherapy for favorable risk prostate cancer. Int J Radiat Oncol Biol Phys 2010;77:805–10 [DOI] [PubMed] [Google Scholar]
- 69.Pesce GA, Clivio A, Cozzi L, Nicolini G, Richetti A, Salati E, et al. Early clinical experience of radiotherapy of prostate cancer with volumetric modulated arc therapy. Radiat Oncol 2010;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mundt AJ, Lujan AE, Rotmensch J, Waggoner SE, Yamada SD, Fleming G, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2002;52:1330–7 [DOI] [PubMed] [Google Scholar]
- 71.Portelance L, Chao KS, Grigsby PW, Bennet H, Low D. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys 2001;51:261–6 [DOI] [PubMed] [Google Scholar]
- 72.Meyer JJ, Willett CG, Czito BG. Emerging role of intensity-modulated radiation therapy in anorectal cancer. Expert Rev Anticancer Ther 2008;8:585–93 [DOI] [PubMed] [Google Scholar]
- 73.Pepek JM, Willett CG, Wu QJ, Yoo S, Clough RW, Czito BG. Intensity-modulated radiation therapy for anal malignancies: a preliminary toxicity and disease outcomes analysis. Int J Radiat Oncol Biol Phys 2010;78:1413–9 [DOI] [PubMed] [Google Scholar]
- 74.Guerrero Urbano MT, Henrys AJ, Adams EJ, Norman AR, Bedford JL, Harrington KJ, et al. Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys 2006;65:907–16 [DOI] [PubMed] [Google Scholar]
- 75.Clivio A, Fogliata A, Franzetti-Pellanda A, Nicolini G, Vanetti E, Wyttenbach R, et al. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: A treatment planning comparison with fixed field IMRT. Radiother Oncol 2009;92:118–24 [DOI] [PubMed] [Google Scholar]
- 76.Vieillot S, Azria D, Lemanski C, Moscardo CL, Gourgou S, Dubois JB, et al. Plan comparison of volumetric-modulated arc therapy (RapidArc) and conventional intensity-modulated radiation therapy (IMRT) in anal canal cancer. Radiat Oncol 2010;5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stieler F, Wolff D, Lohr F, Steil V, Abo-Madyan Y, Lorenz F, et al. A fast radiotherapy paradigm for anal cancer with volumetric modulated arc therapy (VMAT). Radiat Oncol 2009;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duthoy W, De Gersem W, Vergote K, Boterberg T, Derie C, Smeets P, et al. Clinical implementation of intensity-modulated arc therapy (IMAT) for rectal cancer. Int J Radiat Oncol Biol Phys 2004;60:794–806 [DOI] [PubMed] [Google Scholar]
- 79.Richetti A, Fogliata A, Clivio A, Nicolini G, Pesce G, Salati E, et al. Neo-adjuvant chemo-radiation of rectal cancer with volumetric modulated arc therapy: summary of technical and dosimetric features and early clinical experience. Radiat Oncol 2010;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duthoy W, De Gersem W, Vergote K, Coghe M, Boterberg T, De Deene Y, et al. Whole abdominopelvic radiotherapy (WAPRT) using intensity-modulated arc therapy (IMAT): first clinical experience. Int J Radiat Oncol Biol Phys 2003;57:1019–32 [DOI] [PubMed] [Google Scholar]
- 81.Wong E, D'Souza DP, Chen JZ, Lock M, Rodrigues G, Coad T, et al. Intensity-modulated arc therapy for treatment of high-risk endometrial malignancies. Int J Radiat Oncol Biol Phys 2005;61:830–41 [DOI] [PubMed] [Google Scholar]
- 82.Cozzi L, Dinshaw KA, Shrivastava SK, Mahantshetty U, Engineer R, Deshpande DD, et al. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol 2008;89:180–91 [DOI] [PubMed] [Google Scholar]
- 83.Tinger A, Waldron T, Peluso N, Katin MJ, Dosoretz DE, Blitzer PH, et al. Effective palliative radiation therapy in advanced and recurrent ovarian carcinoma. Int J Radiat Oncol Biol Phys 2001;51:1256–63 [DOI] [PubMed] [Google Scholar]
- 84.Hepp R, Baeza MR, Olfos P, Suarez E. Adjuvant whole abdominal radiotherapy in epithelial cancer of the ovary. Int J Radiat Oncol Biol Phys 2002;53:360–5 [DOI] [PubMed] [Google Scholar]
- 85.Hamilton CA, Cheung MK, Osann K, Balzer B, Berman ML, Husain A, et al. The effect of adjuvant chemotherapy versus whole abdominopelvic radiation on the survival of patients with advanced stage uterine papillary serous carcinoma. Gynecol Oncol 2006;103:679–83 [DOI] [PubMed] [Google Scholar]
- 86.Hong L, Alektiar K, Chui C, LoSasso T, Hunt M, Spirou S, et al. IMRT of large fields: whole-abdomen irradiation. Int J Radiat Oncol Biol Phys 2002;54:278–89 [DOI] [PubMed] [Google Scholar]
- 87.Mahantshetty U, Jamema S, Engineer R, Deshpande D, Sarin R, Fogliata A, et al. Whole abdomen radiation therapy in ovarian cancers: a comparison between fixed beam and volumetric arc based intensity modulation. Radiat Oncol 2010;5:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsuzak MM, Yan D, Grills I, Martinez A. Clinical applications of volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 2010;77:608–16 [DOI] [PubMed] [Google Scholar]
- 89.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et alonbehalfofthe PARSPORT., trial management group Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu Q, Mohan R, Morris M, Lauve A, Schmidt-Ullrich R. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas: I – dosimetric results. Int J Radiat Oncol Biol Phys 2003;56:573–85 [DOI] [PubMed] [Google Scholar]
- 91.Verbakel WF, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity-modulated arc therapy vs. conventional IMRT in head-and-neck cancer: a comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys 2009;74:252–9 [DOI] [PubMed] [Google Scholar]
- 92.Vanetti E, Clivio A, Nicolini G, Fogliata A, Ghosh-Laskar S, Agarwal JP, et al. Volumetric modulated arc radiotherapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: a treatment planning comparison with fixed field IMRT. Radiother Oncol 2009;92:111–7 [DOI] [PubMed] [Google Scholar]
- 93.Johnston M, Clifford S, Bromley R, Back M, Oliver L, Eade T. Volumetric-modulated arc therapy in head and neck radiotherapy: A planning comparison using simultaneous integrated boost for nasopharynx and oropharynx carcinoma. Clin Oncol (R Coll Radiol) 2011;23:503–11 [DOI] [PubMed] [Google Scholar]
- 94.Guckenberger M, Richter A, Krieger T, Wilbert J, Baier K, Flentje M. Is a single arc sufficient in volumetric-modulated arc therapy (VMAT) for complex-shaped target volumes? Radiother Oncol 2009;93:259–65 [DOI] [PubMed] [Google Scholar]
- 95.Bertelsen A, Hansen CR, Johansen J, Brink C. Single arc volumetric modulated arc therapy of head and neck cancer. Radiother Oncol 2010;95:142–8 [DOI] [PubMed] [Google Scholar]
- 96.Alvarez-Moret J, Pohl F, Koelbl O, Dobler B. Evaluation of volumetric modulated arc therapy (VMAT) with Oncentra MasterPlan for the treatment of head and neck cancer. Radiat Oncol 2010;5:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999;45:577–87 [DOI] [PubMed] [Google Scholar]
- 98.Blanco AI, Chao KS, El Naqa I, Franklin GE, Zakarian K, Vicic M, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys 2005;62:1055–69 [DOI] [PubMed] [Google Scholar]
- 99.van Luijk P, Faber H, Schippers JM, Brandenburg S, Langendijk JA, Meertens H, et al. Bath and shower effects in the rat parotid gland explain increased relative risk of parotid gland dysfunction after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2009;74:1002–5 [DOI] [PubMed] [Google Scholar]
- 100.Clemente S, Wu B, Sanguineti G, Fusco V, Ricchetti F, Wong J, et al. SmartArc-based volumetric modulated arc therapy for oropharyngeal cancer: a dosimetric comparison with both intensity-modulated radiation therapy and helical tomotherapy. Int J Radiat Oncol Biol Phys 2011;80:1248–55 [DOI] [PubMed] [Google Scholar]
- 101.Scorsetti M, Fogliata A, Castiglioni S, Bressi C, Bignardi M, Navarria P, et al. Early clinical experience with volumetric modulated arc therapy in head and neck cancer patients. Radiat Oncol 2010;5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pigott K, Dische S, Saunders MI. Where exactly does failure occur after radiation in head and neck cancer? Radiother Oncol 1995;37:17–9 [DOI] [PubMed] [Google Scholar]
- 103.Chao KS, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2003;55:312–21 [DOI] [PubMed] [Google Scholar]
- 104.Bussels B, Maes A, Hermans R, Nuyts S, Weltens C, Van denBogaert W. Recurrences after conformal parotid-sparing radiotherapy for head and neck cancer. Radiother Oncol 2004;72:119–27 [DOI] [PubMed] [Google Scholar]
- 105.Guerrero Urbano MT, Clark CH, Hansen VN, Adams EJ, A'Hern R, Miles EA, et al. A phase I study of dose-escalated chemoradiation with accelerated intensity modulated radiotherapy in locally advanced head and neck cancer. Radiother Oncol 2007;85:36–41 [DOI] [PubMed] [Google Scholar]
- 106.Lauve A, Morris M, Schmidt-Ullrich R, Wu Q, Mohan R, Abayomi O, et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas: II–clinical results. Int J Radiat Oncol Biol Phys 2004;60:374–87 [DOI] [PubMed] [Google Scholar]
- 107.Madani I, Duthoy W, Derie C, De Gersem W, Botergerg T, Saerens M, et al. Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2007;68:126–35 [DOI] [PubMed] [Google Scholar]
- 108.Korreman SS, Ulrich S, Bowen S, Deveau M, Bentzen SM, Jeraj R. Feasibility of dose painting using volumetric modulated arc optimization and delivery. Acta Oncol 2010;49:964–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murshed H, Liu HH, Liao Z, Barker JL, Wang X, Tucker SL, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;58:1258–67 [DOI] [PubMed] [Google Scholar]
- 110.Chapet O, Khodri M, Jalade P, N'Guyen D, Flandin I, D'Hombres A, et al. Potential benefits of using non coplanar field and intensity modulated radiation therapy to preserve the heart in irradiation of lung tumors in the middle and lower lobes. Radiother Oncol 2006;80:333–40 [DOI] [PubMed] [Google Scholar]
- 111.Keall P. 4-dimensional computed tomography imaging and treatment planning. Semin Radiat Oncol 2004;14:81–90 [DOI] [PubMed] [Google Scholar]
- 112.McNair HA, Brock J, Symonds-Tayler JR, Ashley S, Eagle S, Evans PM, et al. Feasibility of the use of the Active Breathing Co ordinator (ABC) in patients receiving radical radiotherapy for non-small cell lung cancer (NSCLC). Radiother Oncol 2009;93:424–9 [DOI] [PubMed] [Google Scholar]
- 113.Suh Y, Weiss E, Zhong H, Fatyga M, Siebers JV, Keall PJ. A deliverable four-dimensional intensity-modulated radiation therapy-planning method for dynamic multileaf collimator tumor tracking delivery. Int J Radiat Oncol Biol Phys 2008;71:1526–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoogeman MS, Nuyttens JJ, Levendag PC, Heijmen BJ. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys 2008;70:609–18 [DOI] [PubMed] [Google Scholar]
- 115.Zimmerman J, Korreman S, Persson G, Cattell H, Svatos M, Sawant A, et al. DMLC motion tracking of moving targets for intensity modulated arc therapy treatment: a feasibility study. Acta Oncol 2009;48:245–50 [DOI] [PubMed] [Google Scholar]
- 116.Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;70:685–92 [DOI] [PubMed] [Google Scholar]
- 117.Xia T, Li H, Sun Q, Wang Y, Fan N, Yu Y, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;66:117–25 [DOI] [PubMed] [Google Scholar]
- 118.Videtic GM, Stephans K, Reddy C, Gajdos S, Kolar M, Clouser E, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: excellent local control. Int J Radiat Oncol Biol Phys 2010;77:344–9 [DOI] [PubMed] [Google Scholar]
- 119.Baisden JM, Romney DA, Reish AG, Cai J, Sheng K, Jones DR, et al. Dose as a function of lung volume and planned treatment volume in helical tomotherapy intensity-modulated radiation therapy-based stereotactic body radiation therapy for small lung tumors. Int J Radiat Oncol Biol Phys 2007;68:1229–37 [DOI] [PubMed] [Google Scholar]
- 120.McGrath SD, Matuszak MM, Yan D, Kestin LL, Martinez AA, Grills IS. Volumetric modulated arc therapy for delivery of hypofractionated stereotactic lung radiotherapy: A dosimetric and treatment efficiency analysis. Radiother Oncol 2010;95:153–7 [DOI] [PubMed] [Google Scholar]
- 121.Ong CL, Verbakel WF, Cuijpers JP, Slotman BJ, Lagerwaard FJ, Senan S. Stereotactic radiotherapy for peripheral lung tumors: a comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol 2010;97:437–42 [DOI] [PubMed] [Google Scholar]
- 122.Holt A, van Vliet-Vroegindeweij C, Mans A, Belderbos JS, Damen EM. Volumetric-modulated arc therapy for stereotactic body radiotherapy of lung tumors: a comparison with intensity-modulated radiotherapy techniques. Int J Radiat Oncol Biol Phys 2011;5:[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 123.Brock J, Bedford J, Nioutsikou E, Partridge M, Ashley S, McNair H, et al. Optimising stereotactic body radiotherapy for non-small cell lung cancer with volumetric intensity-modulated arc therapy – a planning study. Clin Oncol 2011;9:[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 124.Bedford JL, Nordmark Hansen V, McNair HA, Aitken AH, Brock JE, Warrington AP, et al. Treatment of lung cancer using volumetric modulated arc therapy and image guidance: a case study. Acta Oncol 2008;47:1438–43 [DOI] [PubMed] [Google Scholar]
- 125.Scorsetti M, Navarria P, Mancosu P, Alongi F, Castiglioni S, Cavina R, et al. Large volume unresectable locally advanced non-small cell lung cancer: acute toxicity and initial outcome results with rapid arc. Radiat Oncol 2010;5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Palma DA, Senan S, Haasbeek CJ, Verbakel WF, Vincent A, Lagerwaard F. Radiological and clinical pneumonitis after stereotactic lung radiotherapy: a matched analysis of three-dimensional conformal and volumetric-modulated arc therapy techniques. Int J Radiat Oncol Biol Phys 2011;80:506–13 [DOI] [PubMed] [Google Scholar]
- 127.de Perrot M, Feld R, Cho BC, Bezjak A, Anraku M, Burkes R, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413–8 [DOI] [PubMed] [Google Scholar]
- 128.Scorsetti M, Bignardi M, Clivio A, Cozzi L, Fogliata A, Lattuada P, et al. Volumetric modulation arc radiotherapy compared with static gantry intensity-modulated radiotherapy for malignant pleural mesothelioma tumor: a feasibility study. Int J Radiat Oncol Biol Phys 2010;77:942–9 [DOI] [PubMed] [Google Scholar]
- 129.Fogliata A, Clivio A, Nicolini G, Vanetti E, Cozzi L. Intensity modulation with photons for benign intracranial tumours: a planning comparison of volumetric single arc, helical arc and fixed gantry techniques. Radiother Oncol 2008;89:254–62 [DOI] [PubMed] [Google Scholar]
- 130.Lagerwaard FJ, Meijer OW, van derHoorn EA, Verbakel WF, Slotman BJ, Senan S. Volumetric modulated arc radiotherapy for vestibular schwannomas. Int J Radiat Oncol Biol Phys 2009;74:610–5 [DOI] [PubMed] [Google Scholar]
- 131.MacDonald SM, Ahmad S, Kachris S, Vogds BJ, DeRouen M, Gittleman AE, et al. Intensity modulated radiation therapy versus three-dimensional conformal radiation therapy for the treatment of high grade glioma: a dosimetric comparison. J Appl Clin Med Phys 2007;8:47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wagner D, Christiansen H, Wolff H, Vorwerk H. Radiotherapy of malignant gliomas: comparison of volumetric single arc technique (RapidArc), dynamic intensity-modulated technique and 3D conformal technique. Radiother Oncol 2009;93:593–6 [DOI] [PubMed] [Google Scholar]
- 133.Shaffer R, Nichol AM, Vollans E, Fong M, Nakano S, Moiseenko V, et al. A comparison of volumetric modulated arc therapy and conventional intensity-modulated radiotherapy for frontal and temporal high-grade gliomas. Int J Radiat Oncol Biol Phys 2010;76:1177–84 [DOI] [PubMed] [Google Scholar]
- 134.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665–72 [DOI] [PubMed] [Google Scholar]
- 135.Lagerwaard FJ, van derHoorn EA, Verbakel WF, Haasbeek CJ, Slotman BJ, Senan S. Whole-brain radiotherapy with simultaneous integrated boost to multiple brain metastases using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 2009;75:253–9 [DOI] [PubMed] [Google Scholar]
- 136.Ma Y, Li M, Yin Y, Kong L, Sun X, Lin X, et al. Hypofractionated stereotactic radiotherapy for brain metastases: a dosimetric and treatment efficiency comparison between volumetric modulated arc therapy and intensity modulated radiotherapy. Technol Cancer Res Treat 2010;9:499–507 [DOI] [PubMed] [Google Scholar]
- 137.Clark GM, Popple RA, Young PE, Fiveash JB. Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. Int J Radiat Oncol Biol Phys 2010;76:296–302 [DOI] [PubMed] [Google Scholar]
- 138.Hsu F, Carolan H, Nichol A, Cao F, Nuraney N, Lee R, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1-3 brain metastases: a feasibility study using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 2010;76:1480–5 [DOI] [PubMed] [Google Scholar]
- 139.Wu QJ, Yoo S, Kirkpatrick JP, Thongphiew D, Yin FF. Volumetric arc intensity-modulated therapy for spine body radiotherapy: comparison with static intensity-modulated treatment. Int J Radiat Oncol Biol Phys 2009;75:1596–604 [DOI] [PubMed] [Google Scholar]
- 140.Kuijper IT, Dahele M, Senan S, Verbakel WF. Volumetric modulated arc therapy versus conventional intensity modulated radiation therapy for stereotactic spine radiotherapy: a planning study and early clinical data. Radiother Oncol 2010;94:224–8 [DOI] [PubMed] [Google Scholar]
- 141.Mancosu P, Navarria P, Bignardi M, Cozzi L, Fogliata A, Lattuada P, et al. Re-irradiation of metastatic spinal cord compression: a feasibility study by volumetric-modulated arc radiotherapy for in-field recurrence creating a dosimetric hole on the central canal. Radiother Oncol 2010;94:67–70 [DOI] [PubMed] [Google Scholar]
- 142.Qiu JJ, Chang Z, Wu QJ, Yoo S, Horton J, Yin FF. Impact of volumetric modulated arc therapy technique on treatment with partial breast irradiation. Int J Radiat Oncol Biol Phys 2010;78:288–96 [DOI] [PubMed] [Google Scholar]
- 143.Popescu CC, Olivotto IA, Beckham WA, Ansbacher W, Zavgorodni S, Shaffer R, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys 2010;76:287–95 [DOI] [PubMed] [Google Scholar]
- 144.Johansen S, Cozzi L, Olsen DR. A planning comparison of dose patterns in organs at risk and predicted risk for radiation induced malignancy in the contralateral breast following radiation therapy of primary breast using conventional, IMRT and volumetric modulated arc treatment techniques. Acta Oncol 2009;48:495–503 [DOI] [PubMed] [Google Scholar]
- 145.Nicolini G, Clivio A, Fogliata A, Vanetti E, Cozzi L. Simultaneous integrated boost radiotherapy for bilateral breast: a treatment planning and dosimetric comparison for volumetric modulated arc and fixed field intensity modulated therapy. Radiat Oncol 2009;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–106 [DOI] [PubMed] [Google Scholar]
- 147.Donovan EM, Ciurlionis L, Fairfoul J, James H, Mayles H, Manktelow S, et al. planning with intensity-modulated radiotherapy and tomotherapy to modulate dose across breast to reflect recurrence risk (IMPORT high trial). Int J Radiat Oncol Biol Phys 2011;75:1064–72 [DOI] [PubMed] [Google Scholar]
- 148.Weber DC, Peguret N, Dipasquale G, Cozzi L. Involved-node and involved-field volumetric modulated arc vs. fixed beam intensity-modulated radiotherapy for female patients with early-stage supra-diaphragmatic Hodgkin lymphoma: a comparative planning study. Int J Radiat Oncol Biol Phys 2009;75:1578–86 [DOI] [PubMed] [Google Scholar]
- 149.W, Lagerwaard F, Verbakel W, Slotman B, Senan S. Volumetric modulated arc therapy for advanced pancreatic cancer. Strahlenther Onkol 2010;186:382–7 [DOI] [PubMed] [Google Scholar]
- 150.Llacer-Moscardo C, Quenet F, Azria D, Fenoglietto P. Feasibility study of volumetric modulated arc therapy for the treatment of retroperitoneal sarcomas. Radiat Oncol 2010;5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Benthuysen LV, Hales L, Podgorsak MB. doi: 10.1016/j.meddos.2010.09.009. Volumetric modulated arc therapy vs IMRT for the treatment of distal oesophageal cancer. Med Dosim 2011;4:[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 152.Hawkins MA, Bedford JL, Warrington AP, Tait DM. doi: 10.1259/bjr/25428720. Volumetric modulated arc therapy planning for distal oesophageal malignancies. Br J Radiol 2011;22:[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bignardi M, Navarria P, Mancosu P, Cozzi L, Fogliata A, Tozzi A, et al. Critical appraisal of volumetric modulated arc therapy in stereotactic body radiation therapy for metastases to abdominal lymph nodes. Int J Radiat Oncol Biol Phys 2009;75:1570–7 [DOI] [PubMed] [Google Scholar]
- 154.Scorsetti M, Bignardi M, Alongi F, Fogliata A, Mancosu P, Navarria P, et al. Stereotactic body radiation therapy for abdominal targets using volumetric intensity modulated arc therapy with RapidArc: Feasibility and clinical preliminary results. Acta Oncol 2011;50:528–38 [DOI] [PubMed] [Google Scholar]
- 155.Fogliata A, Bergstrom S, Cafaro I, Clivio A, Cozzi L, Dipasquale G, et al. Cranio-spinal irradiation with volumetric modulated arc therapy: A multi-institutional treatment experience. Radiother Oncol 2011;99:79–85 [DOI] [PubMed] [Google Scholar]
- 156.Lee YK, Brooks CJ, Bedford JL, Warrington AP, Saran FH. Development and evaluation of multiple isocentric volumetric modulated arc therapy technique for craniospinal axis radiotherapy planning. Int J Radiat Oncol Biol Phys 2011:22:[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 157.Shaffer R, Vollans E, Vellani R, Welsh M, Moiseenko V, Goddard K. A radiotherapy planning study of RapidArc, intensity modulated radiotherapy, three-dimensional conformal radiotherapy, and parallel opposed beams in the treatment of pediatric retroperitoneal tumors. Pediatr Blood Cancer 2011;56:16–23 [DOI] [PubMed] [Google Scholar]
- 158.Fogliata A, Cozzi L, Clivio A, Ibatici A, Mancosu P, Navarria P, et al. Preclinical assessment of volumetric modulated arc therapy for total marrow irradiation. Int J Radiat Oncol Biol Phys 2011;80:628–36 [DOI] [PubMed] [Google Scholar]
- 159.Aydogan B, Yeginer M, Kavak GO, Fan J, Radosevich JA, Gwe-Ya K. Total marrow irradiation with RapidArc volumetric arc therapy. Int J Radiat Oncol Biol Phys 2011;81:592–9 [DOI] [PubMed] [Google Scholar]