Abstract
Objectives
Therapeutic partial breast irradiation can be delivered intra-operatively using the Intrabeam 50 kVp compact X-ray device. Spherical applicators are added to the source to give an isotropic radiation dose. The low energy of this unit leads to rapid attenuation with distance, but dose rates are much greater than for diagnostic procedures.
Methods
To investigate the shielding requirements for this unit, attenuation measurements were carried out with manufacturer-provided tungsten–rubber sheets, lead, plasterboard and bricks. A prospective environmental dose rate survey was also conducted in the designated theatre.
Results
As a result of isotropic geometry, the scattered dose around shielding can be 1% of primary and thus often dominates measured dose rates compared with transmission. The absorbed dose rate of the unshielded source at 1 m was 11.6 mGy h−1 but this was reduced by 95% with the shielding sheets. Measured values for the common shielding materials were similar to reference data for the attenuation of a 50 kVp diagnostic X-ray beam. Two lead screens were constructed to shield operators remaining in the theatre and an air vent into a service corridor. A lead apron would also provide suitable attenuation, although a screen allows greater flexibility for treatment operators. With these measures, staff doses were reduced to negligible quantities. Survey measurements taken during patient treatments confirmed no additional measures were required, but the theatre should be a controlled area and access restricted.
Conclusion
Results from this study and reference data can be used for planning other facilities.
Radiotherapy is commonly delivered in multiple fractions using a linear accelerator. These operate at megavoltage energies and are located within dedicated shielded bunkers [1]. Treatment can also be delivered intra-operatively as a single fraction soon after surgical excision of the tumour. Electron-only linear accelerators have been used for this approach, and recent mobile devices can be used in unmodified theatres if the workload is limited or thick mobile shielding panels are used [2-4]. The Intrabeam system (Carl Zeiss Surgical, Oberkochen, Germany) is a compact X-ray unit used for intra-operative partial breast irradiation [5-8], currently in an international randomised controlled trial (Targit) [9,10]. It generates photons using an accelerating voltage of 50 kVp. This unit has the advantage of rapid attenuation of dose compared with megavoltage photon accelerators or brachytherapy using iridium-192 sources. The radiation can be readily switched on and off, and there is no risk of contamination compared with nuclear medicine procedures. It has been reported that the technique can be performed in standard operating theatres using thin shields over the treated area [5] and lead screens [11-13] or aprons [14] to protect the small number of staff who remain in the theatre during the irradiation. Although theatre staff may be familiar with diagnostic X-ray procedures in this energy range, the absorbed dose rate close to the source can be of the order of 1 Gy min−1, which is much greater. UK legislation [15] limits the effective dose to an individual radiation worker to 20 mSv per year, and 1 mSv per year for members of the public and other workers. It also requires prior risk assessment, designation of areas, local rules, arrangements for pregnant staff and appropriate training.
Implementation of partial breast irradiation using this unit has been described by several authors, including brief descriptions of radiation protection measures [5,12-14]. However, the aim of this study was to investigate the theoretical shielding requirements of the unit, including an environmental dose rate survey of a simulated treatment as part of the prior risk assessment. Second, these results were compared with measurements taken regularly during patient treatments, and with reference data [16], which could be used to plan shielding requirements at other centres.
Methods
Intra-operative treatment
The Intrabeam PRS500 is used in our centre for partial breast irradiation according to the following procedure. The unit generates X-rays in an approximately isotropic distribution from the tip of a narrow probe. Isotropy and output are checked before each treatment using quality control equipment provided with the system. Spherical applicators with diameters ranging from 1.5 cm to 5.0 cm are fitted to the probe to treat a range of tumour cavity sizes. The whole apparatus is mounted on a balanced mobile gantry arm, covered in a sterile sheath. The surgeon positions the applicator within the tumour cavity and applies a purse-string stitch [5]. This gives good conformance of the tissue with the applicator surface and reduces dose to the skin. Two packets of thermoluminescent dosimeters (TLDs) are sealed within sterile Tegaderm (3M, St. Paul, MN) and placed at known distances at least 10 mm from the applicator shank on the skin surface. These are used to determine the patient skin dose and monitor the consistency of the technique set-up. Sheets of tungsten-impregnated rubber (nominally 0.05 mm lead equivalent (50 keV), Carl Zeiss Surgical) are cut to fit over the treatment area to reduce external dose rates. An absorbed dose of 6 Gy at 10 mm from the applicator surface is prescribed. This takes typically between 25 and 40 min to deliver, depending on the size of applicator used. The X-ray unit is connected to a control terminal positioned behind a large lead screen, described further below. Two radiotherapy operators and an anaesthetist stand behind this screen during treatment to monitor the equipment and patient. All other staff leave the theatre. Figure 1 shows a typical set-up with the shielding in place.
Figure 1.
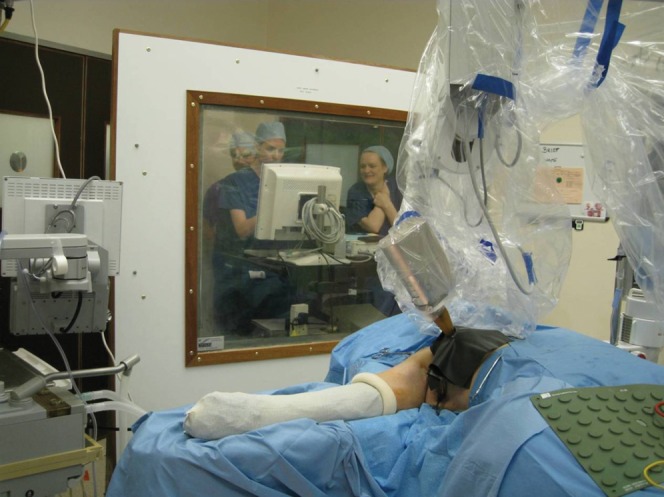
Typical intra-operative breast treatment set-up, with shielding in place.
Attenuation measurements
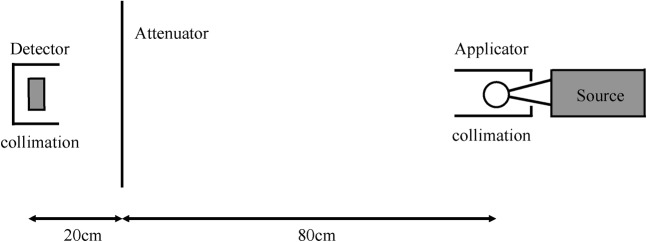
Attenuation measurements were performed in a radiotherapy department bunker using an Unfors Xi survey detector (Unfors, Billdal, Sweden). The smallest diameter applicator was attached to the source probe and the combination placed on polystyrene blocks to reduce scatter. The detector was positioned in a retort stand at a distance of 1 m from the effective source position at the centre of the applicator sphere. The following materials were placed between the source and detector, at a distance of 20 cm from the detector: single and double thickness of the tungsten–rubber sheets used for treatment; 0.5 mm and 1.0 mm thick lead sheets; 1 cm thick plasterboard (gypsum wallboard); and dry bricks (10 cm thick, gross density 1825 kg m−3, Blockleys Brick, Telford, UK). A 1 mm thick lead cylinder was placed around the applicator to collimate the normally isotropic beam. This technique has been used previously for measurement of the half value layer (HVL) with this unit as an index of beam quality [17]. It is referred to below as the “collimated beam” set-up. Measurements were repeated with additional 1 mm thick lead sheets placed around the detector. This set-up is illustrated in Figure 2. Further readings were acquired with each of the following modifications from this figure: with the attenuating material located at the end of the source collimation aperture (the “covered beam” set-up); with no collimation around source or detector (the “uncollimated beam” set-up).
Figure 2.
Diagram of one set-up for attenuation measurements, with both source and detector shielded by lead collimation.
The large lead screen to be used in theatre was constructed in-house. It contained a 3.6 mm equivalent leaded glass window (Wardray Premise, Thames Ditton, UK) recycled from a decommissioned simulator control room. The surrounding area was wood with 5 mm thick lead sheet, to give a total screen size of 1.5 m wide and 2.0 m high. Attenuation measurements were taken using this screen without source or detector collimation and with the window positioned between the source and detector. The attenuator-to-detector distance was increased to 60 cm for these measurements.
Reference transmission values were derived using the data for 50 kVp beams in Sutton and Williams [16]. To assess the applicability of the inverse square model for dose fall-off with distance, measurements were acquired with no attenuating material and source collimation only. The detector was placed on a moveable platform and readings taken at 1 m, 2 m and 3 m from the effective source position at the centre of the applicator sphere. The ambient dose equivalent rate at 1 m from the source was measured using a 451P-DE-SI ion chamber survey meter (Fluke Biomedical, Everett, WA). Readings were corrected for the under-response of this meter at low energies (Fluke Biomedical (operator’s manual 2005)). The HVL for this applicator size was measured previously as 1.1 mm Al [17], so an effective energy of 22 keV was used to determine the relative response [18].
Initial environmental survey
Before the system was used clinically, a prospective survey of expected dose rates was performed in the designated theatre (Figure 3). These were used to verify the suitability of the selected theatre and inform the prior risk assessment. Measurements were taken using a Victoreen 450P-DE-SI survey meter (now replaced with the Fluke 451P meter) at various positions. Readings were initially acquired using the unshielded source (probe with smallest applicator as above). Further readings were then acquired with the probe and applicator inserted into wax bolus, and with the probe and applicator in the bolus with a single layer of the tungsten–rubber sheeting on top. These later set-ups were used to simulate the actual treatment conditions described above. All readings were corrected for under-response of the meter at low energies. An effective energy of 30 keV was used to account for beam hardening in the shielding materials, doors and walls of the theatre.
Figure 3.

Diagram of theatre layout. Numerals refer to measurement locations in Tables 1 and 2. Areas with solid shading are designated controlled and those with hatching are designated supervised. Solid lines within the theatre room show the approximate positions of the two screens.
Radiation protection measures during treatment
Only the two radiotherapy operators and an anaesthetist remain in theatre during the treatment. They are each given an EPD MK2 (Thermo Scientific, Waltham, MA) electronic personal dosimeter to monitor whole body (Hp(10)) and skin (Hp(0.07)) doses. All other staff leave the theatre, including the anaesthetic and scrub rooms. A further small lead screen is placed over an air vent at the back of the theatre. This was also manufactured in-house with lead of a similar thickness to the main screen. Access hatches are emptied and locked. Controlled area signs are displayed and doors are locked to prevent accidental entry. The radiotherapy operators complete a checklist to ensure all radiation safety measures have been followed before initiating the exposure. During the irradiation, an environmental survey is performed by a third radiotherapy staff member at key locations using the 451P meter. These are listed in Table 2 and correspond to the numbered positions in Figure 3. In each case the meter was positioned just behind the respective surface and the maximum representative reading recorded. All readings were corrected for under-response of the meter, using an effective energy of 30 keV as above to account for beam hardening in the patient and shielding.
Table 2. Environmental dose survey measurements for 40 patients at key positions (1–6) around theatre shown in Figure 3, and occasional representative readings at other positions. Accuracy of values is ±20% and zero refers to readings below the background of approximately 0.1 μSv h−1.
| Location | Mean dose rate (μSv h−1) (range) |
| 1. Behind screen | 1.6 (0.4–6.4) |
| 2. Anaesthetic room door | |
| (behind screen) | 1.0 (0–5.6) |
| (not behind screen) | (0–64) |
| 3. External door | |
| (waist height, behind screen) | 1.2 (0–6.0) |
| (head height, behind screen) | 2.8 (0.2–12) |
| (waist height, not behind screen) | (0.2–20) |
| (head height, not behind screen) | (0.2–32) |
| 4. External anaesthetic room door | 0.2 (0–1.2) |
| 5. External scrub room door | 0.2 (0–0.8) |
| 6. Sluice room hatch | 1.0 (0–5.8) |
| 7. Rear waste hatch | (0–0.2) |
| Air vent (service corridor side) | 0 |
| Floor below (offices) | 0 |
| In front of screen | 240 |
| 1 m from source | 580 |
| Behind lead aprons | (0.6–2.4) |
Further one-off measurements acquired during clinical procedures are also listed in Table 2. These were taken to confirm the adequacy of the radiation protection measures implemented. They include readings taken behind lead aprons with nominal lead equivalence of 0.25 mm and 0.35 mm (Wardray Premise), positioned at the same typical distance from the source as the screen.
Results
Attenuation and inverse square dependence
Measured values of transmission through the shielding materials are shown in Table 3 for three of the four set-ups described above. The addition of detector collimation reduced the transmission values for lead sheet and bricks from a mean of 3.6 × 10−3 in the collimated beam set-up to 2.3 × 10−3. No difference was observed for values with the other materials within measurement uncertainties. The quoted accuracy of absorbed dose rate readings was 10%. The standard deviation of repeat readings was up to 7% and variation through different parts of the bricks was 2%, so these were not significant compared with the intrinsic accuracy. Measured transmission for the uncollimated source and large lead screen was 5 × 10−3. The unshielded absorbed dose rate at 1 m from the source was 11.6 mGy h−1. Readings at 2 m and 3 m from the source showed very good agreement with an inverse square dependence on distance (square of the correlation coefficient (R2) 1.000). The ambient dose equivalent rate at 1 m was 10.3 mSv h−1. This agrees with the value measured with the Unfors detector within measurement uncertainties.
Table 3. Transmission factors for a range of shielding materials. Reference data are for 50 kVp beams, taken from [16].
| Material | Thickness (mm) | Transmission |
|||
| Measured |
Reference | ||||
| Collimated beam | Uncollimated beam | Covered beama | |||
| Tungsten–rubber sheets | 0.05b | 5.4 × 10−2 | 7.7 × 10−2 | 4.8 × 10−2 | 2.2 × 10−1 |
| 0.1b | 1.5 × 10−2 | 3.8 × 10−2 | 8.7 × 10−3 | 6.8 × 10−2 | |
| Lead | 0.5 | 3.8 × 10−3 | 1.9 × 10−2 | 1.8 × 10−4 | 2 × 10−4 |
| 1.0 | 3.6 × 10−3 | 1.6 × 10−2 | <10−4 | 2 × 10−6 | |
| Plasterboard | 10 | 2.2 × 10−1 | 2.3 × 10−1 | 2.1 × 10−1 | 3.5 × 10−1 |
| Dry brick | 100 | 3.4 × 10−3 | 1.5 × 10−2 | <10−4 | 5 × 10−6c |
aAttenuating material covering the collimated source aperture.
bLead equivalent (50 keV).
cUsing reference density of 1650 kg m−3.
Initial environmental survey
Dose rates measured in the initial assessment of the designated theatre are shown in Table 1, with positions as shown in Figure 3. On the floor above, a mezzanine level used for maintenance access only when the theatre is not in use, the maximum reading was 12 μSv h−1. Behind the walls of the theatre (service corridor, main corridor and office on the opposite side), the readings were 0.2 μSv h−1 or less, similar to background levels. Theatre walls are constructed from panels of Lytag, a lightweight aggregate (12 cm thick, gross density 700–800 kg m−3; Lytag, Escrick, UK).
Table 1. Prospective dose survey results, part of the risk assessment for use of the designated theatre. Accuracy of values is ±20%. All values are for the unshielded source, with numerals corresponding to locations shown in Figure 3.
| Location | Mean dose rate (μSv h−1) |
| 1. Behind screen | 34 |
| 2. Anaesthetic room door | 640 |
| 3. External door (behind screen) | 50 |
| 4. External anaesthetic room door | 3.0 |
| 5. External scrub room door | 1.4 |
| 6. Sluice room hatch | 6.0 |
| 7. Rear waste hatch | 9.0 |
| Air vent (service corridor side) | 320 |
Values with the source inserted into wax bolus were reduced by a factor of approximately 10 compared with the initial set-up. Behind the screen the reading was 4 μSv h−1. With the addition of tungsten–rubber sheeting, most of the values were further reduced by a small amount. Behind and to the side of the screen the readings were 4 μSv h−1 and 220 μSv h−1, respectively.
Treatment surveys and staff doses
Dose rates measured during 2 years of patient treatments are shown in Table 2, with positions as shown in Figure 3. Depending on the relative position of the main lead screen, higher readings were sometimes recorded for the side of the main doors closer to the sluice room. Conversely, on four occasions when the screen was positioned to fully cover the main doors, a small region of higher dose rate was measured on the side of the internal anaesthetic room door closer to the scrub room. Variation in dose rate close to the edges and base of the screen was within measurement uncertainties. Readings taken with both thicknesses of lead apron were the same within measurement uncertainties, with the dose rate reduced to a factor of (2 ± 1)× 10−2. For the main screen, this value was 0.7 × 10−2. On the floor below, separated by at least 10 cm of concrete, there was no discernable reading above background.
Staff whole body doses measured with EPDs were not more than 1 μSv for any procedure, and 2 μSv for any individual over a year. Skin doses were up to 3 μSv for a single procedure, and 3 μSv over a year. Approximately 20 procedures were performed per year by 2 of 7 radiotherapy operators in rotation.
Discussion
The geometry of the Intrabeam source is different from typical diagnostic and therapeutic X-ray tubes operating at kilovoltage energies. X-rays are produced without collimation in an isotropic distribution, so the measurement of transmission factors requires the beam to be restricted to primary radiation travelling through the material, rather than scattered around it. In the covered beam set-up, which most closely approximates this condition, measured values for 0.5 mm lead sheet and plasterboard agreed within a factor of 2 with reference data for a diagnostic X-ray unit [16]. This represents good agreement given the differences between the Intrabeam unit with an HVL of 1.1 mm Al [17] and a typical diagnostic X-ray tube at 50 kVp with 2.5 mm Al filtration. Agreement between measured values for the tungsten–rubber sheets and reference data based on the nominal lead thickness is less close. This is because the nominal thickness of these sheets is calculated using a worst case 50 keV monoenergetic beam (Carl Zeiss Surgical (personal communication, 2010)), rather than the actual 50 kVp spectrum for this unit. A similar level of agreement to the other materials would correspond to using the reference data with an effective lead thickness of 0.1 mm per sheet. Therefore, reference transmission data for all materials considered in this study can be applied to this unit, in spite of differences in filtration and geometry, provided an effective lead thickness of 0.1 mm is used for the tungsten–rubber sheets.
In the clinical treatment environment this covered beam (enclosed) geometry is applicable to the walls, floor and ceiling of the theatre, since the radiation cannot scatter around the edges of these surfaces. Transmission through the Lytag walls was approximately 1 × 10−3. The reference data for concrete, with density scaled to match that of Lytag, gave a value of 1.5 × 10−3, which is in good agreement as above.
For alternative geometries where attenuating materials have a finite extent, measured dose rates can be dominated by incident radiation scattered around the material. Therefore, dose rate readings in the collimated beam geometry were larger than the covered beam geometry for all materials. In the uncollimated beam geometry, readings were larger again. The underlying proportion of this “scattered around” radiation can be determined by considering an attenuator with very low transmission, such as 1 mm lead sheet. Dose rate values with 0.5 mm lead sheet and bricks were similar and show that this scattered component was 0.4% of the unattenuated primary beam in the collimated beam geometry and 1.7% when the beam was uncollimated. The screen used for shielding of operators during treatment is much larger than the lead sheets considered above, so the scattered component will be reduced. Measurements using this screen in an uncollimated geometry, which is most representative of treatment conditions, gave a value of 0.5% of primary. During actual patient treatments the mean reading behind the screen was 0.7% of the value in front, which is in good agreement with the simulated conditions. Therefore, with the large screen, dose rates in clinical use are reduced to less than 1% of the unshielded value.
The initial environmental survey demonstrated that the addition of the patient (bolus) and surface shielding (sheets) reduces the external dose rates. However, the instantaneous dose rates (IDRs) were still greater than 7.5 μSv h−1 in all three rooms. If there were any gaps in the shielding the IDRs could be even greater than this. National guidelines [19] suggest areas with IDRs greater than 2000 μSv h−1 and 7.5 μSv h−1 be designated as controlled and supervised, respectively. Similar conditions for the time-averaged dose rate (TADR) (over a typical 8 h day) are 7.5 μSv h−1 and 2.5 μSv h−1, respectively. Time averaging over a maximum of two procedures per day, owing to applicator sterilisation time and other resource considerations, reduced the dose rate in the anaesthetic and scrub rooms to supervised area levels. However, since locks were only available on doors into the main corridor, and in order to clearly demarcate the theatre unit as housing a radiation procedure, all three rooms were designated as controlled areas (grey shading on Figure 3). Similarly, the access hatches were designated as supervised areas to limit their use during the procedure. Positioning the screen to cover the anaesthetic room can lead to hotspots at the external door, so a small area extending into the main corridor was designated with signage as supervised to avoid loitering. To avoid controls on the semipublic access service corridor adjoining the back of the theatre, a second smaller screen was designed to cover the air vent on the inside. No additional measures were required for adjacent floors or offices.
These measures keep doses to other staff and members of the public as low as reasonably practicable (ALARP) [19]. Effective dose rates measured during patient treatments confirmed the sufficiency of the protection measures. Variation between procedures was fairly low, so the frequency of dose surveys can be reduced once operators are familiar with the technique. However, personal monitoring with EPDs or otherwise should continue for each procedure. Measured staff doses were all negligible and do not require dose sharing within the team of trained operators. A single staff member, receiving on average 1.6 μSv h−1 behind the screen for 0.5 h per treatment, could perform over 1000 procedures per year before they would approach the dose limit for a member of the public. Therefore, no restriction on workload is required and the risk to pregnant staff is very low. However, to keep doses ALARP investigation levels were set at 15 μSv per session. A suitable annual level can be set based on the expected workload. Stacey et al [20] also found no recordable dose to any member of staff over 10 years of treatments. With a pragmatic estimate of 100 procedures per year, the maximum transmission in the walls to keep doses to other staff within annual dose constraints is approximately 1 × 10−2. Using the reference data, this corresponds to 70 mm plasterboard, 30 mm bricks or 25 mm concrete. The removal of other staff during irradiation means they do not need explicit training in radiation safety, although they should be aware of the basic nature of the equipment and access restrictions.
Measured environmental dose rates compare well with values found by other authors. Parry et al [11] measured an unshielded dose rate in the region of 10 mSv h−1 at 1 m from the source, during a simulated treatment survey with this unit. This was reduced to 40 μSv h−1 with the use of a lead–glass screen (2 mm lead equivalent), which was deemed adequate. These values are in good agreement with the 10.3 mSv h−1 (at 1 m) and 34 μSv h−1 (behind the screen) measured in our centre. Park et al [6] reported an exposure during treatment of the order of 12–15 mR h−1 at 2 m from the source, or 0.4–0.5 mGy h−1 at 1 m using the inverse square dependence on distance verified above. This is again in good agreement with the value measured in our centre of 0.6 mSv h−1 at 1 m. Differences between ambient dose equivalent and air kerma are close to unity for an effective energy of 30 keV [21]. Parry et al [11] found a single thickness of shielding sheets placed on the patient surface reduced the dose rate by a factor of approximately 10 (transmission of 10−1). A second sheet reduced the dose rate by a further factor of 3 (total transmission 3 × 10−2). Vaidya et al [22] quoted 95% shielding (transmission of 5 × 10−2) for the sheets. These values are in good agreement with those listed in Table 3 for the uncollimated and covered beam geometries, respectively.
The two screens are overengineered, in terms of lead thickness, since the same dose rate was found behind a 0.25 mm lead equivalent apron. This is because readings are dominated by scatter around the screen. Using the reference data, the thickness of lead needed to reduce transmission to 1% is 0.2 mm. Therefore, even a very thin layer of lead will reduce the transmitted beam to levels below the scattered component. The current breast trial protocol (Targit protocol v.4.0 (personal communication 2007)) recommends those remaining in the room wear a lead apron or stand behind a shielded screen. Aprons offer sufficient protection from in front and behind, but they may be cumbersome to wear and do not cover every radiosensitive organ. Screens should have a window to monitor the patient, so may be more expensive unless they can be recycled from other units, as in our centre.
Similar shielding provision is recommended with a comparable X-ray device. Axxent (Xoft, Fremont, CA) is a miniature 50 kVp X-ray source delivered using a water-filled balloon catheter. When used for partial breast irradiation, flexible lead equivalent sheets are placed over the treatment area and the remaining staff wear lead aprons or stand behind a mobile shield [23]. The methods used in this study could also be applied to this unit.
Conclusions
The radiation protection requirements of an intraoperative 50 kVp X-ray unit have been investigated under laboratory conditions, theatre simulation and during actual treatments. High dose rates close to the unshielded source require control measures to restrict access during irradiation. Shielding on the patient surface, combined with a mobile shield for staff remaining to monitor the treatment, is sufficient to reduce occupational doses to a negligible level. Even thin lead shields offer this protection, since transmission through lead is low at this energy and so the doses are dominated by scatter around the shielding rather than transmission through it. Reference transmission data for 50 kVp X-rays combined with results in this study can be used to estimate shielding requirements in other centres.
Acknowledgments
The authors would like to thank Jane Edwards for assistance with attenuation measurements and Laura Gandon for assistance with the prospective dose rate survey. We are also grateful to Katharine Piggot and all the radiotherapy and surgical staff involved in the clinical treatments.
References
- 1.Williams JR, Thwaites DI. Radiotherapy physics in practice. Oxford: Oxford University Press, 2000 [Google Scholar]
- 2.Daves JL, Mills MD. Shielding assessment of a mobile electron accelerator for intraoperative radiotherapy. J Appl Clin Med Phys 2001;2:165–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beddar AS, Biggs PJ, Chang S, Ezzell GA, Faddegon BA, Hensley FW, et al. Intraoperative radiation therapy using mobile electron linear accelerators: Report of AAPM Radiation Therapy Committee Task Group No. 72. Med Phys 2006;33:1476–89 [DOI] [PubMed] [Google Scholar]
- 4.Soriani A, Felici G, Fantini M, Paolucci M, Borla O, Evangelisti G, et al. Radiation protection measurements around a 12 MeV mobile dedicated IORT accelerator. Med Phys 2010;37:995–1003 [DOI] [PubMed] [Google Scholar]
- 5.Vaidya JS, Baum M, Tobias JS, Morgan S, D'Souza D. The novel technique of delivering targeted intraoperative radiotherapy (Targit) for early breast cancer. Eur J Surg Oncol 2002;28:447–54 [DOI] [PubMed] [Google Scholar]
- 6.Park CC, Yom SS, Podgorsak MB, Harris E, Price RA, Bevan A, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) emerging technology committee report on electronic brachytherapy. Int J Radiat Oncol Biol Phys 2010;76:963–72 [DOI] [PubMed] [Google Scholar]
- 7.Offerson BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol 2009;90:1–13 [DOI] [PubMed] [Google Scholar]
- 8.Stewart AJ, Khan AJ, Devlin PM. Partial breast irradiation: a review of techniques and indications. Br J Radiol 2010;83:369–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaidya JS, Tobias JS, Baum M, Houghton J. Protocol 99PRT/47 targeted intraoperative radiotherapy (Targit) for breast cancer. Lancet. 1999 Available from: http://www.thelancet.com/protocol-reviews/99PRT-47. [Google Scholar]
- 10.Mannino M, Yarnold J. Accelerated partial breast irradiation trials: diversity in rationale and design. Radiother Oncol 2009;91:16–22 [DOI] [PubMed] [Google Scholar]
- 11.Parry J, Sutton D, Mackay C, O'Neill J, Eljamei S, Thompson A, et al. Radiation protection aspects of setting up an intraoperative radiotherapy facility. In: Proceedings of the 8th Biennial ESTRO Meeting on Physics and Radiation Technology for Clinical Radiotherapy; 2005 September 26–29; Lisbon, Portugal. Radiother Oncol 2005;76:S202–3 [Google Scholar]
- 12.Tobias JS, Vaidya JS, Keshtgar M, Douek M, Metaxas M, Stacey C, et al. Breast-conserving surgery with intra-operative radiotherapy: the right approach for the 21st century? Clin Oncol (R Coll Radiol) 2006;18:220–8 [DOI] [PubMed] [Google Scholar]
- 13.Armoogum K, Ackland C, Gardner J. Implementation and experiences of an intraoperative radiotherapy service. J Radiother Pract 2006;5:203–10 [Google Scholar]
- 14.Vaidya JS. A novel approach for local treatment of breast cancer. PhD thesis, University College London, 2001. [Google Scholar]
- 15.Ionising Radiations Regulations 1999 Statutory instrument 1999 no. 3232. London: HMSO, 1999 [Google Scholar]
- 16.Sutton DG, Williams JR. Radiation shielding for diagnostic x-rays. London: British Institute of Radiology, 2000 [Google Scholar]
- 17.Eaton DJ, Duck S. Dosimetry measurements with an intra-operative x-ray device. Phys Med Biol 2010;55:N359–69 [DOI] [PubMed] [Google Scholar]
- 18.Hubbell JH, Seltzer SM. Tables of x-ray mass attenuation coefficients and mass energy-absorption coefficients (NISTIR 5632). Gaithersburg, MD: National Institute of Standards and Technology, 1995 [Google Scholar]
- 19.Institute of Physics and Engineering in Medicine Medical and dental guidance notes. York: IPEM, 2002 [Google Scholar]
- 20.Stacey C, Metatxas M, Morgan S, D'Souza D. The role of the radiotherapy physicist in intraoperative partial breast irradiation using a low energy x-ray source, based on 10 years clinical experience. In: Proceedings of the 50th AAPM Annual Meeting; 2008 July 27–31; Houston, TX. Med Phys 2008;35:2866 [Google Scholar]
- 21.Smith H. Conversion coefficients for use in radiological protection against external radiation. ICRP Publication 74. Ottawa, Canada: International Commission on Radiological Protection, 1996 [Google Scholar]
- 22.Vaidya JS, Tobias JS, Baum M, Wenz F, Kraus-Tiefenbacker U, D'Souza D, et al. TARGeted Intraoperative radioTherapy (TARGIT): an innovative approach to partial-breast irradiation. Semin Radiat Oncol 2005;15:84–91 [DOI] [PubMed] [Google Scholar]
- 23.Dickler A, Ivanov O, Francescatti D. Intraoperative radiation therapy in the treatment of early-stage breast cancer utilizing Xoft Axxent electronic brachytherapy. World J Surg Oncol 2009;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]