Abstract
There are several reports of adenocarcinoma developing within adenomyosis of the uterus, but imaging features of MRI, including diffusion-weighted imaging (DWI) and positron emission tomography (PET)-CT, have not been published. Herein we report a rare case of endometrioid adenocarcinoma arising from subserosal cystic adenomyosis to emphasise the unusual growth features, as well as the imaging findings of the tumour on MRI and PET-CT.
Case report
A 54-year-old post-menopausal female visited our hospital for evaluation of pelvic pain. She had no specific medical or surgical history. The serum carcinoembryonic antigen, carbohydrate antigen (CA) 125 and CA 19-9 were within normal limits.
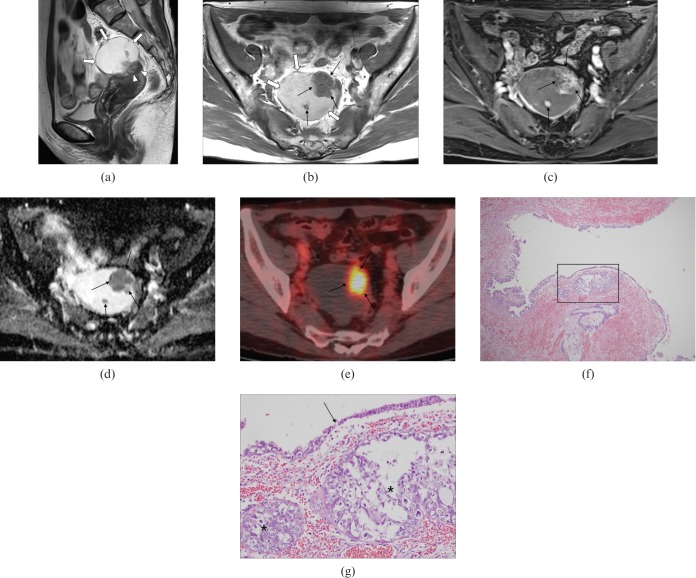
A transvaginal ultrasonogram showed a mixed cystic and solid mass adjacent to the uterus. Both ovaries were not visualised and endometrial thickness was 3 mm. Pelvic MRI was performed to evaluate the origin and additional characterisation of the tumour. Sagittal T2 weighted images demonstrated a well-circumscribed, exophytic, predominantly cystic mass with solid areas, contiguous with the anterior myometrium of the uterine fundus (Figure 1a). The cystic portion of the tumour was hyperintense on T1 weighted images (Figure 1b). The solid areas of the mass were enhanced on gadolinium (Gd)-enhanced T1 weighted images (Figure 1c) and were hypointense on the apparent diffusion coefficient (ADC) map (Figure 1d). 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT images revealed hypermetabolism with a maximal standardised uptake value of 10.3, corresponding to the solid area of the tumour (Figure 1e). The presumptive diagnosis was a uterine tumour such as a subserosal leiomyoma with secondary degeneration and possible malignant transformation. A total abdominal hysterectomy with bilateral salpingo-oophorectomy was performed.
Figure 1.
A 55-year-old woman with endometrioid adenocarcinoma arising from subserosal cystic adenomyosis. (a) Sagittal T2 weighted image demonstrates a 6×5×4.5 cm well-circumscribed, exophytic, predominantly cystic mass (arrows) contiguous with the anterior myometrium of the uterine fundus; the wall was eccentrically thickened and hypointense, reflecting myometrial hypertrophy of adenomyosis (arrowheads). (b) The cystic portion (thick arrows) shows hyperintensity on axial T1 weighted images corresponding to haemorrhagic fluid and the solid areas (thin arrows) were hypointense. (c,d) The solid areas show heterogeneous enhancement (thin arrows) on axial gadolinium-enhanced T1 weighted image (c) and hypointensity on axial apparent diffusion coefficient map (d) with increased cellularity. (e) Axial 18F-fluorodeoxyglucose positron emission tomography-CT image reveals a focal hypermetabolic area (maximal standardised uptake value of 10.3) corresponding to the solid portion of the tumour (thin arrows). (f) Photomicrograph of the surgical specimen reveals the tumour cell nests located under the cystic wall (the region in square; haematoxylin and eosin (H&E) stain, original magnification ×40). (g) The transitional zone (arrow) is seen between carcinomatous (left side) and benign (right side) epithelium with underlying tumour cell nests (asterisks). Tumour cells have prominent nucleoli with nuclear size variation and abundant cytoplasm, forming endometrioid adenocarcinoma (H&E stain, original magnification ×200).
At surgery, the exophytic and complex predominantly cystic mass contained haemorrhagic fluid and originated from the uterine fundus. Both ovaries were atrophied and intact. On microscopic examination, the mass was composed of irregularly shaped endometrial glands with surrounding stroma and dilated cysts with thinned epithelial lining cells (Figure 1f). Nodular nests of the tumour cells were located under the cystic wall and tumour cells had prominent nucleoli with nuclei of variable sizes and abundant cytoplasm. The transition from adenomyotic glandular epithelium to adenocarcinoma was also detected (Figure 1g). The endometrium was unremarkable. These features were diagnostic of endometrioid adenocarcinoma arising from cystic adenomyosis. Post-operatively, the patient received chemotherapy. No metastasis or recurrence was demonstrated on the 12 month follow-up imaging study.
Discussion
Adenomyosis is a benign entity characterised by the heterotopic growth of endometrial glands or stroma into the myometrium with adjacent smooth muscle hyperplasia [1]. Uterine adenomyosis usually manifests as diffuse disease involving the myometrium and the endometrial-myometrial junction, but it may appear as a focal lesion. The location of uterine adenomyosis is commonly intramural and less frequently subserosal [2]. Haemorrhagic and cystic changes in adenomyosis can occur, but are usually small and focal. Some cases have been reported as “cystic adenomyosis” or “adenomyotic cysts” and repeated haemorrhage during menstruation is considered a cause of extensive cyst formation [3,4].
MRI is an accurate, non-invasive modality for diagnosing adenomyosis with a high sensitivity and specificity. On MRI, adenomyosis usually appears as an ill-defined area of low signal intensity, occasionally with internal bright foci on T2 weighted images [5,6]. If the lesion has extensive cystic changes, it is homogeneously hyperintense on T1 weighted images and variable signal intensity on T2 weighted images owing to haemorrhage or proteinaceous fluid [3,7]. Cystic adenomyosis with subserosal growth is extremely rare and may be indistinguishable from uterine tumours, such as subserosal leiomyomas with cystic degeneration or even cystic ovarian tumours [6,8]. In our case, the tumour originated from the uterus on MRI, which was suggestive of a uterine tumour with subserosal growth and haemorrhagic change. Endometrioid adenocarcinoma can rarely arise from adenomyosis [1,2]. Motohara et al [9] reported the course of adenocarcinoma arising from adenomyosis on periodic MRI. They described a gradually enlarging adenomyotic lesion, which had been replaced by a new, poorly demarcated lesion with moderately high signal intensity on T2 weighted images. The malignant change within adenomyosis usually exhibits an infiltrative form and may be difficult to distinguish from typical adenomyosis without malignancy [6]. However, if the malignant change manifests as a discrete mass within adenomyosis, it can be detectable on MRI as in our case. On 18F-FDG PET, FDG generally accumulates in malignant lesions because of high glucose metabolism. Of malignant uterine tumours, cervical cancers, endometrial cancers and uterine sarcomas usually show intense FDG uptake. But FDG can also accumulate in benign uterine lesions such as leiomyomas [10]. In our case, MRI demonstated a subserosal, predominantly cystic mass with solid areas in the uterus. The cystic portion of the tumour was hyperintense on T1 weighted images, which was suggestive of haemorrhagic and/or proteinaceous fluid. These MRI features were similar to uterine tumours, such as subserosal leiomyoma with secondary degeneration. However, the solid areas of the mass were enhanced on Gd-enhanced T1 weighted images and hypointense on the ADC map, and showed a focal hypermetabolism on 18F-FDG PET-CT which was suggestive of a malignant portion.
This case is a rare example of a large subserosal cystic adenomyosis with malignant change, but its importance lies in the unusual growth features as well as the imaging findings of the tumour on MRI and PET-CT. Although this seems an exceptionally rare case, focal, unusual solid areas within an exophytic, predominantly cystic mass in the uterus should be regarded with suspicion of endometrioid adenocarcinoma arising from subserosal cystic adenomyosis.
References
- 1.Couto D, Mota F, Silva T, de Oliveira C. Adenocarcinoma arising in adenomyosis: report of an unusual case. Acta Obstet Gynecol Scand 2004;83:406–8 [DOI] [PubMed] [Google Scholar]
- 2.Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006;20:511–21 [DOI] [PubMed] [Google Scholar]
- 3.Troiano RN, Flynn SD, McCarthy S. Cystic adenomyosis of the uterus: MRI. J Magn Reson Imaging 1998;8:1198–202 [DOI] [PubMed] [Google Scholar]
- 4.Imaoka I, Kaji Y, Kobashi Y, Wada A, Honjo G, Hayashi M, et al. Cystic adenomyosis with florid glandular differentiation mimicking ovarian malignancy. Br J Radiol 2005;78:558–61 [DOI] [PubMed] [Google Scholar]
- 5.Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: MR imaging findings. Radiographics 1999;19 (Spec No):S161–70 [DOI] [PubMed] [Google Scholar]
- 6.Tamai K, Togashi K, Ito T, Morisawa N, Fujiwara T, Koyama T. MR imaging findings of adenomyosis: correlation with histopathologic features and diagnostic pitfalls. Radiographics 2005;25:21–40 [DOI] [PubMed] [Google Scholar]
- 7.Kataoka ML, Togashi K, Konishi I, Hatabu H, Morikawa K, Kojima N, et al. MRI of adenomyotic cyst of the uterus. J Comput Assist Tomogr 1998;22:555–9 [DOI] [PubMed] [Google Scholar]
- 8.La Fianza A, Abbati D, Cesari S, Morbini P. Subserous uterine adenomyosis mimicking an adnexal mass on sonography. J Clin Ultrasound 2004;32:95–7 [DOI] [PubMed] [Google Scholar]
- 9.Motohara K, Tashiro H, Ohtake H, Saito F, Ohba T, Katabuchi H. Endometrioid adenocarcinoma arising in adenomyosis: elucidation by periodic magnetic resonance imaging evaluations. Int J Clin Oncol 2008;13:266–70 [DOI] [PubMed] [Google Scholar]
- 10.Kitajima K, Murakami K, Kaji Y, Sugimura K. Spectrum of FDG PET/CT findings of uterine tumors. AJR Am J Roentgenol 2010;195:737–43 [DOI] [PubMed] [Google Scholar]