Abstract
Erythema occurs in 80–90% of women treated for breast cancer with radiation therapy. There is currently no standard treatment for radiation-induced skin reactions. This study investigates the clinical efficacy of Mepilex Lite dressings in reducing radiation-induced erythema in women with breast cancer. A total of 28 patients were recruited; of these, 24 participants presented with 34 erythematous areas of skin for analysis. When erythema was visible, each affected skin area was randomly divided into two similar halves: one half was treated using Mepilex Lite dressings, the other half with standard aqueous cream. Skin reactions were assessed by the Radiation-Induced Skin Reaction Assessment Scale. We also evaluated any potential dose build-up by the dressings using a white water phantom, the dose distribution over the breast via thermoluminescent dosimeters (TLDs) and the surface skin temperature with an infrared thermographic scanner. Mepilex Lite dressings significantly reduced the severity of radiation-induced erythema compared with standard aqueous cream (p <0.001), did not affect surface skin temperature and caused only a small (0.5 mm) dose build-up. TLD measurements showed that the inframammary fold was exposed to significantly higher doses of radiation than any other breast region (p <0.0001). Mepilex dressings reduce radiation-induced erythema.
Breast cancer is the most common malignancy for women in New Zealand. Most of these women will receive radiation therapy treatment, and skin reactions will occur in 80–90% of patients by treatment completion [1]. To date, there is no standard treatment for radiation-induced skin reactions and practice tends to be based on historical and anecdotal evidence [1–3]. A promising new range of Swedish silicon-foam skin dressings, Mepilex Lite (MV Bamford and Company Ltd, Lower Hutt, New Zealand and Mölnlycke Health Care Gothenburg, Sweden) is currently used in New Zealand for the treatment of burns and slow-healing wounds. This absorbent, self-adhesive dressing consists of a thin, flexible sheet of absorbent hydrophilic polyurethane foam bonded to a water vapour-permeable polyurethane film backing layer. The contact surface of the dressing is coated with a soft silicone adhesive layer without any added chemicals. It adheres to healthy skin, thus retaining the dressing in position but without causing trauma on removal, and provides a moist wound-healing environment. The material does not add or react to chemicals in or on the skin, does not stick to wounds and can be left on the skin for several days [1, 4].
Preliminary case studies conducted in our department showed that Mepilex Lite dressings reduced the extent of all radiation-induced skin reactions. These results are consistent with previous case studies carried out in Scotland and Stockholm [1, 5]. The current study is the first clinical study that compares the clinical efficacy of Mepilex Lite dressings on the severity of radiation-induced erythema with a standard aqueous cream using the Radiation-Induced Skin Reaction Assessment Scale (RISRAS) (Table 1) [5, 6]. Because anecdotal evidence suggests that the dressings may have a cooling effect, we also determined their effect on surface skin temperature as well as the extent of dose build-up caused by the dressings if they were left on the patient during treatment.
Table 1. Radiation-Induced Skin Reaction Assessment Scale (RISRAS).
| RISRAS (total scores between 0 and 36)a | ||||||||
| Researcher component (total scores between 0 and 24) | ||||||||
| Erythema (E) | 0 Normal skin | 1.0 Dusky pink | 2.0 Dull red | 3.0 Brilliant red | 4.0 Deep red-purple | |||
| Dry desquamation (DD) | 0 Normal skin | 1.0 (<25%)b | 2.0 (25–50%) | 3.0 (50–75%) | 4.0 (>75%) | |||
| Moist desquamation (MD) | 0 Normal skin | 1.5 (<25%) | 3.0 (25–50%) | 4.5 (50–75%) | 6.0 (>75%) | |||
| Necrosis (N) | 0 Normal skin | 2.5 (<25%) | 5.0 (25–50%) | 7.5 (50–75%) | 10.0 (>75%) | |||
| Patient component (total scores between 0 and 12) | ||||||||
| Symptoms | Not at all | A little | Quite a bit | Very much | ||||
| Do you have any tenderness, discomfort or pain of your skin in the treatment area? | 0 | 1 | 2 | 3 | ||||
| Does your skin in the treatment area itch? | 0 | 1 | 2 | 3 | ||||
| Do you have a burning sensation of your skin in the treatment area? | 0 | 1 | 2 | 3 | ||||
| To what extent have your skin reactions and your symptoms affected your day-to-day activities? | 0 | 1 | 2 | 3 | ||||
aIndividual scores for each item are added up to give a total score for the researcher and patient components of the scale. Adding the researcher and patient component scores together gives the total combined RISRAS score.
bPercentage of surface area of affected skin.
Methods and materials
This systematic inpatient controlled clinical trial investigated the effect of Mepilex Lite dressings on the extent of radiation-induced erythema in 24 breast cancer patients. Women were enrolled into the trial after giving written informed consent before the start of radiation therapy treatment. A total of 34 areas of erythema were randomly divided into 2 equal halves. One half was covered with Mepilex Lite dressings and the other half was treated with standard aqueous cream. The extent of erythema was measured using the RISRAS scale. The trial was approved by the New Zealand Lower South Island Regional Ethics Committee in May 2008 (protocol number LRS/08/05/016) and is registered in the Australia New Zealand Clinical Trials Registry (ACTRN12608000180314).
Trial outcomes
We determined the effect of Mepilex Lite dressings on (1) dose build-up, (2) severity of radiation-induced skin reactions and (3) surface skin temperature. In addition, we determined the dose distribution over the breast.
Trial endpoint
Skin measurements stopped as soon as the skin developed dry desquamation. Patient data up to the point of dry desquamation were incorporated in the final analysis. Areas of dry desquamation were covered in Mepilex Lite dressings as per department protocol, regardless of whether the area had been dressed in Mepilex Lite or treated with control cream before.
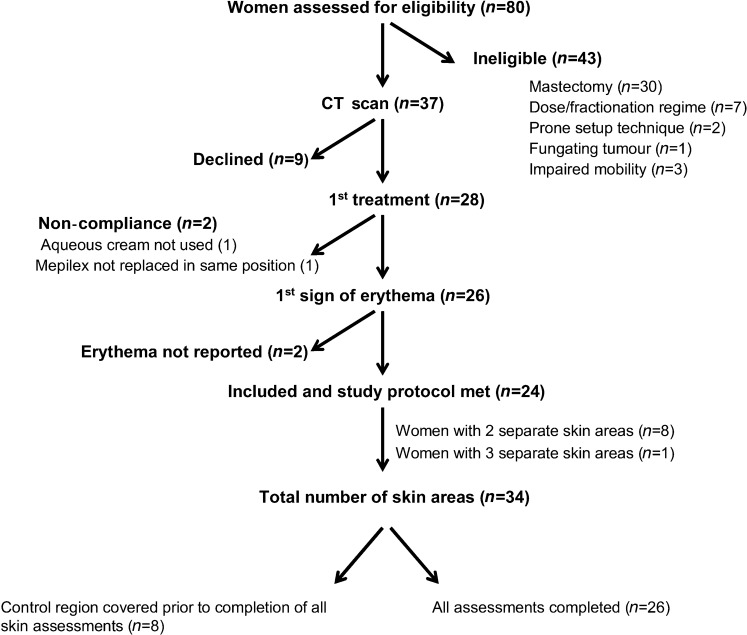
Patient recruitment and participation flow
All women receiving radiation therapy treatment for breast cancer at Dunedin Hospital, who had not had a radical mastectomy or previous irradiation of the breast, were screened for recruitment from Dunedin Oncology’s database, MOSAIQ. Recruitment occurred between June 2008 and May 2009, the flow of patients is shown in the consort diagram in Figure 1. Specific exclusion criteria were radical mastectomy, fractionation regimes other than 50 Gy in 25 fractions, prone treatment position, impaired mobility and fungating tumour. In total, 28 patients were recruited, with 24 women yielding data for analysis. Of these 24 women, 8 presented with 2 separate areas of skin and 1 presented with 3 separate areas for analysis. Areas of skin from the same woman were in different areas of the breast, each receiving a different radiation dose. A total of 34 areas from 24 women were analysed for this study, with a surface area ranging from 50 to 150 cm2.
Figure 1.
Consort diagram showing the flow of participants through the trial. Out of the initial 28 women enrolled in the trial, 24 women yielded 34 areas of skin for the final analysis.
Patient demographics
The average age of the women in this study was 58 (range 43–79) years. Five women were considered as smokers at the time of the study (smoked within last 18 months) and a further seven women had previously smoked. One woman was Maori/Lebanese and all others were identified as Caucasian (Pakeha or European Pakeha). The correlation between skin type, age, body mass index (BMI), previous sun exposure, smoking and concomitant chemotherapy is not explored in this paper.
Radiation therapy treatment
All trial participants received 50 Gy in 25 fractions; in addition, 4 women received boosts to their axilla/upper outer breast quadrant and one woman received a boost to the superior anterior breast/upper inner breast quadrant. All women were planned using a Philips BigBore CT (Phillips Healthcare Andover, MA) and Xio 3D conformal planning system (Elekta, Stockholm, Sweden). All breast tissue and the chest wall were included in the treatment region with heterogeneities taken into consideration. None of the women required bolus. Chemotherapy was given prior to radiation treatment for six women. Adriamycin and cyclophosphamide (AC) taxol was given to five and fluorouracil, epirubicin and cyclophosphamide (FEC) regime to one participant.
Application of dressings and aqueous cream
Patients doubled as their own controls to eliminate confounding patient and treatment-related factors. Intervention started from the moment the skin showed erythema, as noticed by the treating radiation therapist, which was generally about 10–14 days after the first fraction. Mepilex Lite dressings were positioned by the research radiation therapist on half of the area where erythema was present; the other half continued to be treated with aqueous cream (control). The location of the dressing was indicated by a semi-permanent marker pen so that patients could accurately reposition the dressings after showering. Erythematous skin areas were designated either number 1 (for superior or medial as appropriate) or 2 (for inferior or lateral as appropriate). Allocation of dressings and controls was random, based on order of entry into the trial (first person: one dressing, two controls; second person: one control, two dressings).
In 8 of the 24 patients, the skin treated with aqueous cream developed dry desquamation. No further skin assessments were carried out, effectively stopping the trial prematurely for these eight patients. However, patient data up to the point of dry desquamation were incorporated in the final analysis. Any areas with dry desquamation were covered in Mepilex Lite dressings as per department protocol.
Mepilex Lite dressings were provided by MV Bamford and Company Ltd and Mölnlycke Health Care; aqueous cream was obtained from AFT pharmaceuticals (Auckland, NZ) and contained 9 g of emulsifying wax, 10 g of white soft paraffin, 6 g of liquid paraffin, 1 g of phenoxyethanol in boiled and cooled purified water to 100 g.
Measurements
Dose build-up
Tissue maximum ratio (TMR) measurements were taken at various depths when a Mepilex Lite dressing was applied to the surface, thus calculating its bolus effect. A PTW RW3 Slab phantom was assembled to measure TMRs. Two slabs bored to house an Advanced Markus chamber (PTW, Sydney, Austrilia) (TW34045) were set with the entry window of the chamber at the isocentre of the beam. A source-to-axis distance (SAD) of 100 cm was maintained throughout all measurements. A 10 cm thickness of RW3 was placed below the chamber holder as backscatter material. RW3 slabs were added on top of the chamber in 1 mm increments up to 2 cm and then in 2 mm increments to a total of 4 cm. Measurements were taken with the Advanced Markus chamber in combination with PTW Unidos E electrometer (PTW) for both 6X and 18X photon beams at each depth. A 10 × 10 cm field was centred on the chamber, and three readings were averaged for each energy level at each depth. Readings were made with and without the Mepilex Lite dressing on the surface of the phantom over the chamber. The maximum value was determined for each energy level and used as the divisor to determine the TMR at any given depth.
Severity of skin reactions
The modified RISRAS was used to assess the extent of radiation-induced erythema [5]. This scale consists of a researcher component, filled in by the research radiation therapist, who scores the visible extent of the skin reactions, and a patient component, filled in by the patient, who scores the level of pain, itchiness, burning and effect on day-to-day life (Table 1). Because the trial end point was dry desquamation, we used only the extent of erythema (from 0 to 4) in addition to the patient component (from 0 to 12). Our scoring system allowed us to discriminate between small differences in skin reactions (total scores between 0 and 16). Neither the research radiation therapist, who scored all the patients, nor the patients themselves were blinded to the knowledge of which skin area was covered in dressings or treated with cream.
Timing and analysis of RISRAS measurements
RISRAS scores were determined three times a week (on Mondays, Wednesdays and Fridays) from the moment erythema was visible until completion of radiation treatment. Two more assessments were carried out, the first 1 week after completion of treatment and the second during the final check-up (usually 4 weeks after completion of treatment). All RISRAS scores for each area were added up and divided by the number of assessments, yielding an average RISRAS score for that area. The average increase in RISRAS score was determined by subtracting the score on day 1 of the assessment when the dressing was first applied.
Statistical analysis
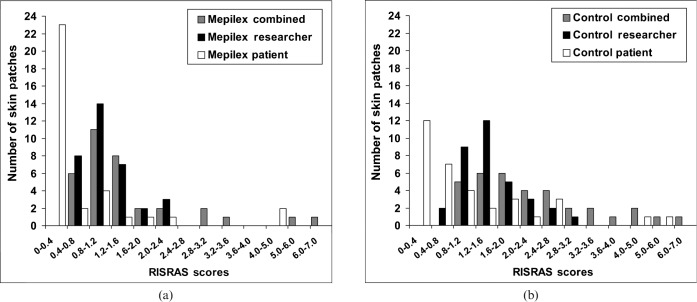
Both the combined and researcher scores were approximately normally distributed (Figure 2). For these scores, the means for the Mepilex Lite and control conditions were compared using ANOVA design of repeated measures of paired patches of erythema, with some patients having multiple patches (SAS Proc Mixed 9.1.3; SAS Institute, Cary, NC). As the patient scores were not normally distributed (Figure 2), the difference between Mepilex and control patches was performed using the non-parametric Wilcoxon signed-rank test (SPSS v 15, Chicago, IL). This involved simplifying the data set so that there was one pair of patches per patient. The selection method for this was to take the most conservative pair (null difference between Mepilex Lite and control, or control better than Mepilex Lite). This means that any potential bias will “underestimate” the reliability of differences between Mepilex and the control. In most instances, the difference between Mepilex and control conditions was similar across patches in those patients who had more than one patch.
Figure 2.
Distribution of Radiation-Induced Skin Reaction Assessment Scale (RISRAS) scores for Mepilex (a) and controls (b). RISRAS scores of skin areas were grouped into categories and displayed as number of skin areas per category, showing an overall normal distribution for the combined and researcher scores but not for the patient scores.
Surface skin temperature
Surface skin temperature was measured using an infrared thermographic scanner, Exergen Dermatemp Model DT-1001RS (Critical Assist, Auckland, New Zealand), to detect subtle skin temperature variations caused by underlying perfusion variations. Temperatures were taken at several points across the control and Mepilex areas, and this was performed twice at each point to calculate an average. Skin temperature was measured for the first 10 patients 3 times a week during every skin assessment.
Dose distribution across the breast
TLDs were used to calculate the actual dose received by the skin in different areas of the breast. These measurements were used to compare the dose that had been received with the dose that the Dunedin Oncology Department’s Xio planning system had intended giving. They were also used as a reference point for the level of skin reaction experienced by the patient. During the first week of treatment, TLDs were placed in various locations on the breast during patient positioning and set-up for treatment, as shown in Figure 3. A centre TLD (surface pseudo-iso) was positioned at the lateral location of the treatment isocentre after the lateral shift had been made. The superior, inferior, medial and lateral TLDs were positioned 2 cm from the treatment field borders having first taken into account the extent of shielding. The lateral, superior corner TLD was positioned on the lateral field edge, 2 cm from the superior border when the gantry was at 0°. The medial, superior TLD was placed 2 cm from the superior and medial border when the gantry was at its medial tangential angle. In practice, placement of TLDs was unique for each patient because of differences in breast size, treatment plan, use of shielding, beam energy and beam angles.
Figure 3.
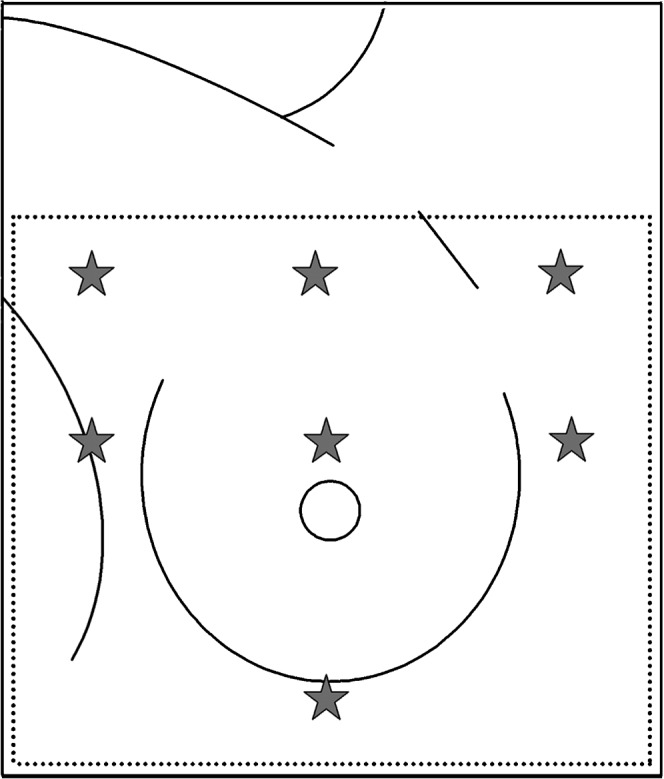
Position of thermoluminescent dosimeters (TLDs) over the breast.
Results
Mepilex Lite dressings have a very small bolus effect
According to information from Mölnlycke Health Care, radiation therapy treatment can be given through Mepilex Lite dressings without significantly affecting the radiation dose received (dose build-up or bolus effect). In order to verify this, we conducted a series of measurements in a white water phantom to determine the effect of Mepilex Lite dressings on the actual dose received. The difference in dose between Mepilex and control under 6 MV and 18 MV beams was shown to decrease with depth, demonstrating a 0.5 mm difference in dose build-up (Figure 4).
Figure 4.
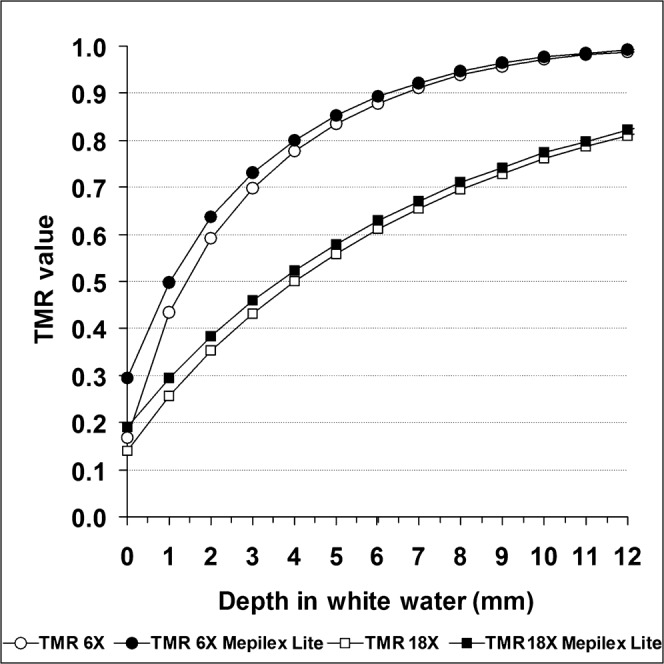
White water phantom studies. The lower two lines represent the tissue maximum ratio for a 6 MV beam at various depths in a water phantom, with and without the application of a Mepilex Lite dressing to the surface. The upper two lines are the equivalent for an 18 MV beam.
Mepilex Lite dressings decrease the extent of radiation-induced erythema
The average increase in combined, researcher and patient RISRAS scores for the 34 areas of skin analysed clearly shows that the dressings significantly decreased the extent of the radiation-induced erythema (p<0.001; Figure 5 and Table 2). In support of this, the majority (17 out of 24) of patients preferred the dressings over the cream and commented that the dressings were soothing, providing pain relief and relief from friction.
Figure 5.
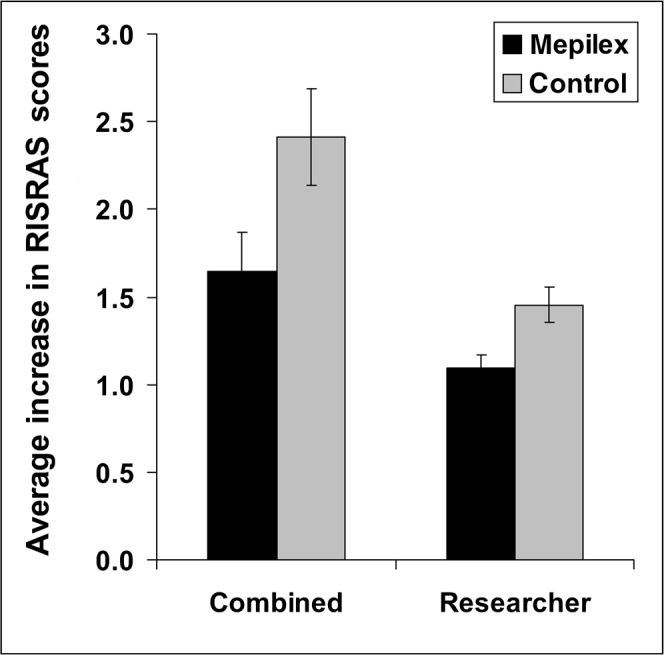
Radiation-Induced Skin Reaction Assessment Scale (RISRAS) scores broken down in separate components. Mean ± SEM of 34 skin patches. Statistical significance of difference between dressings and cream was <0.001 using an analysis of variance of repeated measurements design. Black bars, Mepilex Lite; grey bars, control.
Table 2. Statistical analysis of Radiation-Induced Skin Reaction Assessment Scale (RISRAS) scores.
| Combined scores | Researcher scores | Patient scores | |
| Test used | ANOVAa | ANOVA | Wilcoxon |
| Results | F(1,23) = 32.46 | F(1,23) = 34.01 | |
| Mean (95% CI) | Mean (95% CI) | Median (25% and 75%) | |
| Mepilex Lite | 1.645 (1.205–2.085) | 1.093 (0.9444–1.242) | 0 (0 and 0.3225) |
| Control | 2.411 (1.867–2.955) | 1.455 (1.252–1.658) | 0.305 (0 and 1) |
| p-Values | <0.001 | <0.001 | <0.001 |
CI, confidence interval.
aANOVA of repeated measures for paired patches and repeated patches of patients with more than one patch.
Mepilex Lite dressings do not affect surface skin temperature
Anecdotal evidence from patients in Dunedin and from the study done by Hatcher and Main (2004) [7] suggests that the Mepilex Lite dressings may have a “cooling” effect on the skin. Therefore, the effect of Mepilex on skin temperature was assessed using a hand-held DermaTemp infrared thermographic scanner in the first 10 patients. The average skin temperature in the study regions varied among the participants, ranging from 31 to 33.5°C (Figure 6). However, the average difference between the surface skin temperature of the dressed and undressed areas was statistically insignificant in these 10 patients (p = 0.77, unpaired two-tailed Student’s t-test). Therefore, we did not pursue skin temperature measurements in the remaining patients.
Figure 6.
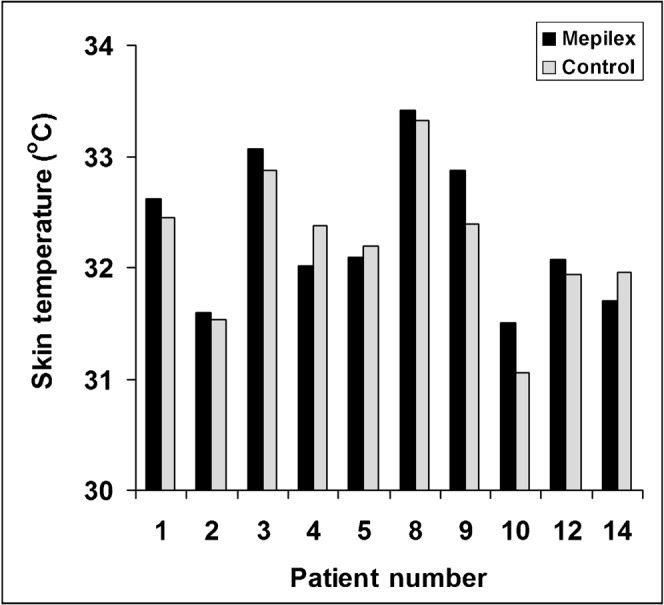
The effect of Mepilex Lite dressings on surface skin temperature of individual patients. Black bars, Mepilex Lite; grey bars, control areas.
Breast regions at risk from radiation-induced side effects
To determine the actual dose received by the skin in different areas of the breast, TLDs were positioned at various locations on the breast (Figure 3). Figure 7 displays the average dose per fraction, at different points on the breast, for all of the 24 study participants. The inframammary fold received the highest dose regardless of breast size, which was highly statistically significant (p<0.00001, unpaired two-tailed Student’s t-test). In support of this result, we found that 16 of the 24 patients (67%) developed moist desquamation in their inframammary fold. Because the skin in this area deteriorated rapidly and Mepilex Lite dressings were applied to the skin in the inframammary fold early on in the proceedings, they did not yield RISRAS scores and therefore were not part of the final analysis. The same was true for the axilla in 10 of the 24 patients (42%). With respect to the 34 skin areas that were analysed for this trial, the majority were located in the superior and central regions of the breast, showing that erythema is more often seen in regions that receive a higher radiation dose.
Figure 7.
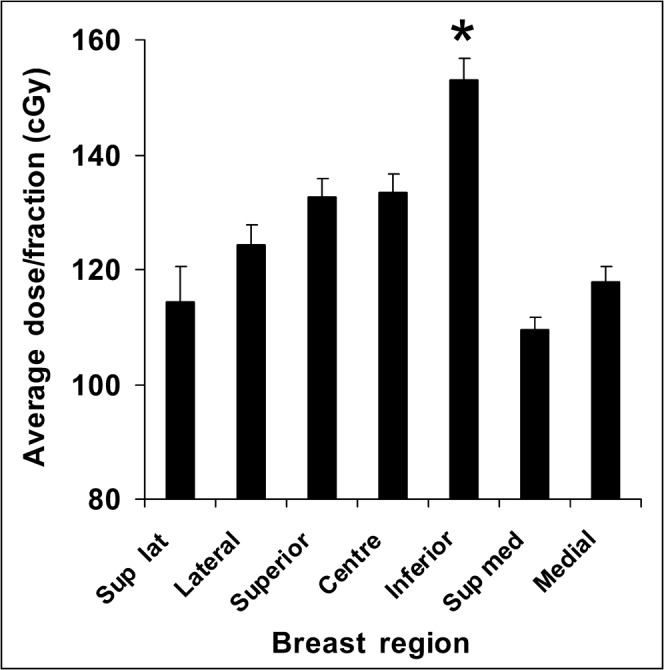
Dose distribution over the breast. Average dose to different regions of the breast in 24 women. *p<0.00001 between the inferior region and all the other regions using a paired two-tailed Student’s t-test.
Although moist desquamation was not part of our trial outcomes, it is of interest to note here that the skin of 8 of the 34 skin areas treated with aqueous cream broke down to reach dry desquamation (24%), compared with 5 of the 34 skin areas dressed with Mepilex Lite (15%). Dry desquamation led to moist desquamation in all instances, suggesting that the dressings may decrease the number of skin areas reaching moist desquamation.
Discussion
This study is the first clinical trial that has demonstrated that Mepilex Lite dressings are superior to the control in decreasing the severity of radiation-induced erythema in breast cancer patients treated with radiation therapy. Not only does this increase patient comfort and quality of life, it also reduces the chances of developing long-term consequences of acute skin inflammation such as scarring. Mepilex Lite dressings did not affect surface skin temperature and did not cause a significant dose build-up.
It is generally accepted that maintaining a moist wound environment will facilitate healing. Although there are many creams and gels on the market that attract and trap moisture at the skin surface, there is a lack of clinical evidence to support the use of topical agents [1, 3, 8]. One of the most popular skin remedies, aloe vera, is commonly used by the lay public for the treatment of various ailments, including skin burns [9]. However, a recent systematic review [10] of five randomised clinical trials together with a previous systematic review [11] showed that there is no clinical evidence that topical aloe vera prevents or decreases radiation-induced skin reactions. We used aqueous cream as a control in this study because it is a moisturising cream used in our department as standard practice of care for erythema. Aqueous cream has been shown not to affect the onset and severity of radiation-induced skin reactions [8]. The majority of our patients preferred the dressings over the cream and thought they increased comfort levels, decreased the amount of pain experienced and allowed patients to wear normal clothing. These sentiments were shared by patients from studies by Wells and MacBride [1] and Adamietz et al [4]. MacBride et al [5] found that the dressings decreased the extent of radiation-induced skin reactions in patients with breast and head and neck cancers. Although these authors documented that the dressings were generally well tolerated, two of their patients withdrew from the study owing to itching experienced after application of the dressings, which disappeared after these were removed. In the current study, none of the 24 participants experienced an adverse reaction to Mepilex Lite dressings.
Skin assessments
There are several skin toxicity scoring systems that are commonly used to assess the severity of skin reactions, notably those recommended by the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) [12]. However, there are few scales that determine the effect of skin reactions on patient comfort levels [13, 14]. The RISRAS, developed by Noble-Adams [14] and later modified by MacBride et al [5], includes a participant component, which scores symptoms such as pain, itchiness and burning, in addition to the researcher component, which scores the visual extent of the skin reaction. As the experience of each participant is unique, this scale adds valuable depth to the data, especially as the degree of erythema does not always reflect the level of discomfort experienced by the patient.
The difference between RISRAS scores for skin areas covered in dressings and those treated with cream was statistically significant for combined RISRAS scores as well as for the researcher and patient components. Although digital photographs were taken during each skin assessment, correlations between photographic and RISRAS skin assessments will be covered in another paper. These photos did show, however, that the skin reactions in both control and treated areas were fairly uniform.
Dose build-up
TLD measurements determined that the dose build-up caused by Mepilex Lite dressings is similar to that of CMS Medtec’s Unifix Thermoplastic masks, used on a daily basis for immobilisation of head and neck patients. In support of these findings, Thilmann et al [15] reported the surface percentage depth dose (PDD) with Mepilex to be 32%, which was similar to our measurements (30%). These results show radiation therapy treatment can be safely given through the dressings, allowing them to stay on the skin during treatment. However, in our study, some patients had their dressings positioned on top of reference marks and therefore these needed to be removed. For the sake of consistency, we removed all dressings prior to treatment.
Dose distribution over the breast
The TLD results clearly demonstrate that the inframammary fold receives a significantly higher dose than all other breast regions. This may be attributed to the inherent shape of a breast. Although breasts can vary in shape and size, there is always a greater separation in tissue across the breast than that found in the inframammary fold. Thus, there is less tissue for the dose to be distributed through, causing areas of high dose. This, added to the risk of skin damage caused by sweat and friction, points to the inframammary fold as being at high risk of developing severe radiation-induced skin reactions, especially in large-breasted women.
Temperature measurements showed that Mepilex Lite dressings did not affect surface skin temperature; therefore, a different explanation must be found for their effect. The dressings were originally designed for burns: they are inert and adhere to healthy skin but do not stick to wounds. Therefore, they maintain a moist environment and minimise trauma to the wound when the dressings are removed, allowing for undisturbed wound healing, especially as the dressings can stay in place for several days. We concur with MacBride et al [5] and Adamietz et al [4] that Mepilex Lite dressings protect irradiated skin from friction from clothes or adjacent tissue, lessening the severity of radiation-induced skin reactions. Irradiation of the skin causes sublethal damage to the stem cells that make up the basal layer of the skin. Any further trauma, be it mechanical (friction) or chemical (perspiration trapped in skin folds), is likely to cause more physical damage to the fragile damaged skin and to interfere with proliferation of basal stem cells, slowing down the healing process. This would explain why Mepilex Lite dressings appear to be more effective on skin that displays more severe reactions. In support of a clinical use for a protective layer of any kind, Graham et al [16] demonstrated that No Sting Barrier Film from Cavilon (3M, St Paul, MN) reduced the incidence of moist desquamation compared with the control (33% and 46% respectively) in 61 post-mastectomy patients treated with radiation therapy. In this study, No Sting was used prophylacticy on either the lateral or medial aspect of the chest wall, and the other side was treated with sorbolene (10% glycerine in aqueous cream). The authors attribute their results to the reduction in friction because, like Mepilex Lite dressings, the film is only applied every couple of days and forms a seal around the wound, limiting mechanical trauma and sealing in moisture [16].
Limitations of the study
This small randomised controlled clinical trial was unblinded, and therefore some researcher and response bias cannot be excluded. As this was a single-centre study with small patient numbers, one researcher scored the extent of visible erythema of all patients, removing interscorer variability. Randomisation of skin areas to treatment by dressings or the control was based on date of entry into the trial; further trials would benefit from computer-generated randomisation numbers. Radiation dose is the most significant contributor to the severity of skin reactions. It is therefore important to determine the actual dose received by the Mepilex and control areas. We tried to address this in this trial by measuring the dose delivered to seven predetermined points on the breast. Even though the Mepilex and control skin areas were all in either the same breast region or adjacent regions receiving similar radiation doses, further trials would benefit from measuring the dose distribution over the actual skin areas that are scored for toxicities.
The end point of this trial was dry desquamation, and RISRAS measurements were stopped when the end point was reached, possibly obscuring the effects of Mepilex Lite dressings over the full scale of skin reactions. We intend to address the limitations of this trial in a further clinical study investigating the effect of these dressings on radiation-induced moist desquamation.
In summary, this study has shown that Mepilex Lite dressings decrease the extent of radiation-induced erythema in women treated with radiation therapy to the breast.
Acknowledgments
The authors wish to acknowledge Danny Warren for the preparation of the TLD measurements and James Stanley and Gordon Purdie for statistical support. This research was supported by scholarships from the Healthcare Otago Charitable Trust, the Auckland Division of the Cancer Society of New Zealand and the Department of Radiation Therapy Targeted Research Fund. The dressings were supplied free of charge by WM Bamford & Company Ltd and Mölnlycke Health Care.
Footnotes
There is no known conflict of interest on behalf of the study researchers, the University of Otago or the Oncology department of Dunedin hospital.
References
- 1.Wells M, MacBride S. Supportive care in radiotherapy-radiation skin reactions. 2003;8:135–59 [Google Scholar]
- 2.Bolderston A, Lloyd NS, Wong RK, Holden L, Robb-Blenderman L. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer 2006;14:802–17 [DOI] [PubMed] [Google Scholar]
- 3.D’haese S, Bate T, Claes S, et al. Management of skin reactions during radiotherapy: a study of nursing practice. Eur J Cancer Care 2005;14:28–42 [DOI] [PubMed] [Google Scholar]
- 4.Adamietz I, Mose S, Harberl A. Effect of self-adhesive, silicone-coated polyamide net dressing on irradiated human skin. Oncol Invest 1995;2:277–85 [Google Scholar]
- 5.MacBride SK, Wells ME, Hornsby C, Sharp L, Finnilla K, Downie L. A case study to evaluate a new soft silicone dressing, Mepilex Lite, for patients with radiation skin reactions. Cancer Nurs 2008;31:E8–14 [DOI] [PubMed] [Google Scholar]
- 6.Noble-Adams R. Radiation-induced reactions: development of a measurement tool. Br J Nurs 1999a;8:1208–11 [DOI] [PubMed] [Google Scholar]
- 7.Hatcher A, Main N. Dressing the discomfort: managing radiation therapy induced dermatitis. Ostany wound management 2004;50:11–12 [PubMed] [Google Scholar]
- 8.Wells M, MacMillan M, Raab G, MacBride S, Bell N, Mackinnon K, et al. Does aqueous or sucralfate cream affect the severity of erythematous radiation skin reactions? A randomised controlled trial. Radiother Oncol 2004;73:153–62 [DOI] [PubMed] [Google Scholar]
- 9.Davis RH, Leitner MG, Russo JM, Byrne ME. Wound healing. Oral and topical activity of Aloe vera. J Am Podiatr Med Assoc 1989;79:559–62 [DOI] [PubMed] [Google Scholar]
- 10.Richardson J, Smith JE, McIntyre M, Thomas R, Pilkington K. Aloe vera for preventing radiation-induced skin reactions; a systematic literature review. Clin Oncol R 2005;17:478–84 [DOI] [PubMed] [Google Scholar]
- 11.Vogler BK, Erns E. Aloe vera: a systematic review of its clinical effectiveness. Br J Gen Prac 1999;49:823–8 [PMC free article] [PubMed] [Google Scholar]
- 12.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer. Int J Radiat Oncol Biol Phys 1995;31:1341–6 [DOI] [PubMed] [Google Scholar]
- 13.Berthelet E, Truong PT, Musso KRN, Grant V, Kwan W, Moravan V, et al. Preliminary Reliability and Validity Testing of a New Skin Toxicity Assessment Tool (STAT) in Breast Cancer Patients Undergoing Radiotherapy. Am J Clin Oncol 2004;27:626–31 [DOI] [PubMed] [Google Scholar]
- 14.Noble-Adams R. Radiation-induced skin reactions 3: Evaluating the RISRAS. Br J Nurs 1999b;8:1305–12 [DOI] [PubMed] [Google Scholar]
- 15.Thilmann C, Adamietz IA, Ramm U, Mose S, Saran F, Bottcher HD, et al. Radiation load on the skin using a silicone-coated polyamide wound dressing during photon and electron radiotherapy. Strahlenther Onkol 1996;172:270–4 [PubMed] [Google Scholar]
- 16.Graham P, Browne L, Capp A, Fox C, Graham J, Hollis J, et al. Randomized, paired comparison of No-Sting Barrier Film versus sorbolene cream (10% glycerine) skin care during postmastectomy irradiation. Int J Radiat Oncol Biol Phys 2004;58:241–6 [DOI] [PubMed] [Google Scholar]