Abstract
To evaluate the clinical significance of incidental focal prostate fluorodeoxyglucose (FDG) uptake, we reviewed 18-F-FDG positron emission tomography (PET)/CT scans from 2003 to 2007 and selected cases with focal FDG uptake in prostate. Cases of known prostate cancer were excluded. The maximum standardised uptake value (SUVmax), site (central or peripheral) and pattern (discrete or ill-defined) of FDG uptake, calcification (present or absent) and prostate volume (<30 or ≥30 cc) were recorded. The PET/CT findings were correlated with serum prostate-specific antigen (PSA) levels, imaging studies, clinical follow-up and biopsy. Of a total of 5119 cases, 63 (1.2%) demonstrated focal FDG uptake in prostate. Eight cases were lost to follow-up. Among the 55 cases with follow-up, malignancy was confirmed by biopsy in 3 (5.4%). The three malignant cases had SUVmax values of 3.3, 3.6 and 2.3, and all were noted in the peripheral portion of prostate; two of these cases had a discrete FDG uptake pattern, none had calcification corresponding to the FDG uptake area and one had a prostatic volume greater than 30 cc. The mean SUVmax of 52 benign cases was 3.2 ± 1.7 and focal FDG uptake was noted in the peripheral portion in 34 (65%), 20 (38%) cases showed a discrete FDG uptake pattern, 35 (67%) were accompanied by calcification and 32 (62%) had a prostatic volume greater than 30 cc. The majority of cases demonstrating focal FDG uptake in prostate were benign and no PET/CT finding could reliably differentiate benign from malignant lesions; however, when discrete focal FDG uptake without coincidental calcification is seen, particularly in the peripheral zone of the prostate, further clinical evaluation is recommended.
18-Fluoro-2-deoxyglucose positron emission tomography (18-F-FDG PET) scanning has been used worldwide for staging and restaging of various malignancies, such as head and neck, breast, lung, oesophageal, colorectal and gynaecological cancers, lymphoma and melanoma [1–3]. In addition, reports have demonstrated a potential role of PET in cancer screening in asymptomatic participants. A PET scan can evaluate the whole body and allows for the early detection of hypermetabolic pre-malignant or malignant lesions [4, 5].
When PET images are obtained for cancer evaluation or preventative health check-up, incidental focal FDG uptake is sometimes noted in variable sites. Physiological uptake, benign lesions (such as inflammation) or unexpected cancers can be the cause [6]. According to previous PET studies with large numbers of cases, second primary cancers were detected in 1.2–4.8% of patients with known cancer. The second tumours were found in variable sites such as thyroid, lung, colon, oesophagus, breast and parotid gland, among others [7–9]. Patients with squamous cell carcinoma of the head and neck have a high risk of a second primary cancer of the lung or oesophagus. In head and neck cancer patients, the detection rate of a second primary cancer by PET scan is reported to be as high as 18% [8]. In PET studies of healthy volunteers, Kojima et al [10] reported incidental cancer detection rates of 0.7%, sensitivity of 70.6% and specificity of 94.0%. Of 2487 male patients, 2 showed abnormal FDG uptake in the prostate; histological examination confirmed these to be malignant lesions. In another study [11], incidental prostate cancer was rarely reported in cancer screening FDG PET studies of healthy men. Of 1629 men, 2 cases had incidental focal uptake in the prostate that was later confirmed as cancer.
From our experience, incidental focal FDG uptake in the prostate gland is encountered from time to time when reading PET/CT scans. To our knowledge, there are inadequate data on how to interpret such focal FDG uptake of prostate glands. The first aim of this paper was to examine the frequency of incidental focal FDG uptake in prostate on PET/CT scans performed for the evaluation of known cancer or preventative health check-up. The second aim was to determine the clinical significance of such FDG uptake in prostate. Lastly, we wanted to examine and compare the PET/CT features of prostate between benign and malignant cases.
Methods and materials
Patients
A total of 5119 PET/CT scans of male patients performed for cancer evaluation or health check-up from November 2003 to October 2007 at our hospital were retrospectively reviewed. Among the 5119 PET/CT scans, 63 cases demonstrated incidental focal FDG uptake of the prostate gland. Cases where PET/CT scan was performed for staging or restaging of prostate cancer were excluded. Also, cases without further study were excluded. Thus, a total of 55 cases demonstrating incidental focal FDG uptake of prostate with follow-up were included in this study.
The ethics committee of our institution does not require patient consent for retrospective review of imaging studies.
The 18-F-FDG PET/CT scan
All patients fasted for at least 6 h before the PET/CT study. 18-F-FDG was injected intravenously (370–555 MBq) and scanning began 60 min later. None of the patients had blood glucose levels >130 mg dL–1 before the injection. No iv contrast agent was administered. Studies were acquired on combined PET/CT in-line systems, either Biograph Duo or Biograph Truepoint (Siemens Medical Solutions Knoxville, TN). The acquisition time was 2–3 min per bed position. All patients were in a supine position with their arms raised. CT began at the orbitomeatal line and progressed to the upper thigh (130 kVp, 80 mA and 5 mm slice thickness; 120 kVp, 50 mA and 5 mm slice thickness). PET followed immediately over the same body region. The CT data were used for attenuation correction and images were reconstructed using a standard ordered-subset expectation maximisation (OSEM) algorithm. The axial spatial resolution was 6.5 mm or 4.5 mm at the centre of the field of view.
Interpretation
All PET/CT images were reviewed at a workstation with fusion software (Syngo; Siemens) that provided multiplanar reformatted images and displayed PET images after attenuation correction, CT images and PET/CT fusion images. The images were closely searched for focal uptake in prostate by two physicians who were board certified in both nuclear medicine and radiology.
Incidental focal FDG uptake was defined as discrete FDG activity higher than the surrounding prostate gland on visual analysis. Images showing elongated FDG activity on coronal and sagittal views or FDG activity located in the centre of the prostate gland, are highly likely to result from prostatic urethral uptake and were excluded. The maximum standardised uptake value (SUVmax) in prostate was obtained from transaxial views.
Cases were grouped according to several variables: the site of FDG uptake (central or peripheral); the pattern of FDG uptake (discrete or ill-defined) on axial view; calcification (present or absent); and prostate volume (<30 cc or ≥30 cc). The volume of the prostate gland was calculated from ultrasound or CT images. Long-axis diameters of the prostate were obtained from axial, sagittal and coronal views. The volume of prostate was then estimated using the prostate volume ellipsoid formula: width (cm) × length (cm) × height (cm) × 0.52.
Method of diagnosis
PET/CT findings were correlated with results of biopsy, serum prostate-specific antigen (PSA) levels, imaging studies and urological examination. Patients with abnormally increased PSA levels (normal range 0–4 ng mL–1) or no PSA level checked within 3 months after the PET/CT scan had either imaging studies with a minimum 1 year follow-up or urological examination. Biopsy was performed in suspicious cases by the urologist. A total of 8 cases were confirmed by biopsy, 43 patients had their PSA level checked soon after the PET/CT scan, imaging studies such as transrectal ultrasound (n = 10), CT (n = 9), magnetic resonance (n = 2) or follow-up PET/CT (n = 7) were obtained in 28 patients and 30 patients were examined by a urologist.
Results
Among 5119 PET/CT images, 63 cases (1.2%) demonstrated incidental focal FDG uptake of the prostate. Eight cases were excluded owing to loss at follow-up. Of a total of 55 cases (mean age 57±11 years; age range 31–83 years) included in this study, 23 PET/CT scans were performed for preventative health check-up. The remaining 32 cases were performed for evaluation of known cancer (Table 1). Among the 55 cases, 3 (5.4%) were confirmed to be malignant and 52 were benign.
Table 1. Indications for performing positron emission tomography (PET)/CT scan.
| Indication for PET/CT | Initial staging (n) | Restaging (n) | Total (n) |
| Head and neck cancer | 3 | 4 | 7 |
| Lung cancer | 4a | 4 | 8 |
| Stomach cancer | 3a | 1 | 4 |
| Colorectal cancer | 4 | 5 | 9 |
| Hepatocellular carcinoma | 1 | 0 | 1 |
| Sarcoma | 0 | 2 | 2 |
| Lymphoma | 1 | 0 | 1 |
| Health check-up | 0 | 0 | 23 |
aOne lung and one stomach cancer patient had a history of another primary cancer, hepatocellular carcinoma.
Malignant lesions
Among the 55 cases, 3 (5.4%) cases were confirmed to be adenocarcinoma by needle biopsy (Figure 1). FDG PET/CT features of the three cancers are described in Table 2. In two cases there was focal calcification in the prostate, but the calcification did not correspond to the focal FDG uptake area.
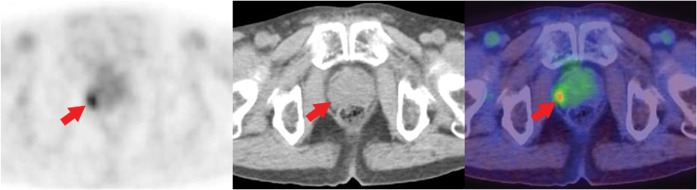
Figure 1.
Positron emission tomography (PET)/CT scan of a 74-year-old man performed for follow-up of a known malignant sarcoma axial PET image, axial CT image, axial PET/CT fusion image. A discrete focal fluorodeoxyglucose (FDG) uptake with a maximum standardised uptake value (SUVmax) of 3.3 (arrow) was noted in the right marginal side of the prostate. Adenocarcinoma was confirmed by biopsy.
Table 2. Cases confirmed as malignancy (n = 3).
| Patient 1 | Patient 2 | Patient 3 | |
| Age (years) | 74 | 73 | 78 |
| Indication of PET/CT scan | Restaging, malignant sarcoma in the nasal cavity | Initial staging, hypopharyngeal cancer | Initial staging, lung cancer |
| Gleason score | 6 (3 + 3) | 8 (3 + 5) | 8 (4 + 4) |
| PSA level (ng mL–1) | 2.82 | 16.28 | >100 |
| SUVmax of prostate lesion | 3.3 | 3.6 | 2.3 |
| Site of tumour | Periphery | Periphery | Periphery |
| FDG uptake pattern | Discrete | Discrete | Ill-defined |
| Accompanying calcification | Absent | Present | Present |
| Prostate volume (cc) | 23.4 | 20.5 | 37.0 |
FDG, fluorodeoxyglucose; PET, positron emission tomography; PSA, prostate-specific antigen; SUVmax, maximum standardised uptake value.
Benign lesions
Of the 55 cases, 52 (94.6%) were benign. Four patients had increased PSA levels, but malignancy was excluded by further imaging studies and biopsy (Figure 2). In this study, 36 patients with PSA levels within the normal range and no abnormality on imaging or physical examination were considered benign. In 12 cases, PSA levels were not checked soon after the PET/CT exam, but no abnormal finding was noted in further imaging studies or on examination by the urologist and these were considered benign cases.
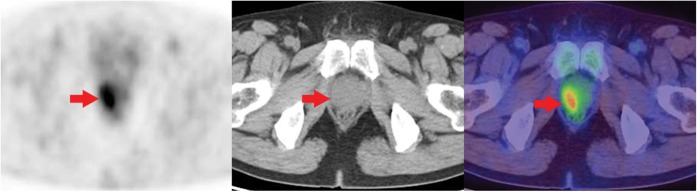
Figure 2.
Positron emission tomography (PET)/CT scan of a 54-year-old man performed for restaging of known colon cancer. Axial PET image, axial CT image, axial PET/CT fusion image. There was no evidence of locoregional tumour recurrence or metastasis. However, a focal fluorodeoxyglucose (FDG) uptake with a maximum standardised uptake value (SUVmax) of 3.9 (arrow) was noted in the right lateral aspect of the prostate gland. The serum prostate-specific antigen level was increased to 14.06 ng mL–1. Needle biopsy confirmed nodular hyperplasia with focal chronic inflammation.
The mean SUVmax of the 52 benign lesions was 3.2±1.7 (range 1.3–13.2). The features of the focal FDG uptake are shown in Table 3. In 34 cases (65%) calcification was present in the prostate gland and 26 cases showed calcification corresponding to the area of FDG uptake. The mean volume of the prostate gland was 37.7±17.1 cc (range 16.0–81.9 cc).
Table 3. Cases confirmed as benign lesions (n = 52).
| Category | Number of cases |
| Site of FDG uptake | |
| Central | 17 (33%) |
| Peripheral | 35 (67%) |
| FDG uptake pattern | |
| Discrete | 20 (38%) |
| Ill-defined | 32 (62%) |
| Accompanying calcification | |
| Present | 34 (65%) |
| Absent | 18 (35%) |
| Prostate volume (cc) | |
| <30 | 20 (38%) |
| ≥30 | 32 (62%) |
| Total | 52 (100%) |
FDG, fluorodeoxyglucose.
Discussion
Prostate cancer is the second most common cancer among men. Of all the cancers diagnosed in 2002 worldwide, more than one in ten were located in the prostate [12]. Transrectal ultrasound, CT, MRI or bone scintigraphy are the generally recommended imaging studies in the staging of prostate cancer [13, 14]. However, 18-F-FDG PET has a limited role in the diagnosis or staging of urological malignancies including prostate cancer, bladder cancer and renal cell carcinoma. The detection of prostate cancer using FDG PET scanning is limited by urinary excretion of the radiotracer and the low metabolic activity of prostate cancer [15–18]. In a study by Liu et al [19], FDG PET was reported as having a sensitivity of 4.0% for tissue-confirmed prostate cancer.
Incidental focal FDG uptake in the prostate is a finding encountered every now and then and can be caused by both benign and malignant conditions. Among malignancies, adenocarcinoma is the most common histological type of prostate cancer and can appear as incidental focal FDG uptake; however, other histological types can also be the cause. Rarely, neuroendocrine tumours with different biological behaviour from prostatic adenocarcinoma have been reported. Compared with the prostatic adenocarcinoma, neuroendocrine tumours show more intense FDG uptake [20]. Ho et al [21] reported high-grade urothelial carcinoma in prostate; a PET scan of this case also showed high FDG uptake with an SUVmax of 9.7 in the tumour located in the prostate. Benign conditions of prostate can also show increased FDG uptake. Focal or diffuse FDG uptake is reported in prostatitis [8, 22] or benign prostatic hyperplasia (BPH) [23–25].
Prostate cancer is confirmed by histological examination of a sample obtained by needle biopsy. PSA and digital rectal examination are useful screening tests in clinical practice [15]. In our study, the three incidental prostate cancer cases out of 55 (5.4%) were all confirmed by biopsy; two cases had high PSA levels of 16 ng mL–1 and >100 ng mL–1. Oyama et al [26] reported that the degree of FDG uptake of primary prostate tumour was higher on PET/CT scan in patients with higher PSA levels than in those with lower PSA levels. But be warned, PSA levels can increase in benign conditions such as BPH [27]. In this study, the PSA level was checked in 40 cases and concluded as benign: the mean value of PSA was 1.73±2.57 ng mL–1 (range 0.09–14.06 ng mL–1).
It is well established that prostate cancer commonly occurs in the peripheral zone of the gland [28]. In our study, all three malignant cases had focal FDG uptake in the peripheral portion of the prostate gland. Of the three malignant cases, one with advanced stage cancer showed heterogeneous FDG uptake in the peripheral portion and exhibited a definite outward bulging contour of the prostate gland in corresponding CT images. The other two malignant cases showed focal FDG uptake in the periphery portion abutting the margin of the prostate gland. Among the 52 benign cases, there was focal FDG uptake in the peripheral portion in approximately two-thirds (67%). Strictly speaking, on a closer review of the PET/CT images, only 10 cases (19%) had focal FDG uptake in the peripheral portion adjacent to the margin of the gland. The remaining 25 cases were also localised in the peripheral zone of the gland, but uptake did not abut the gland margin. When focal FDG uptake is noted in the peripheral portion of the prostate gland, marginal location appears to be more suggestive of malignancy.
Of our three malignant cases, two showed a discrete pattern of FDG uptake. The remaining case had an ill-defined uptake pattern on PET, but showed a definite bulging contour on accompanying CT. Large-sized tumours can alter the contour of the prostate gland and the CT portion of the PET/CT scan might be helpful in raising the detection rate of cancer [14].
Prostatic calcification is a common finding in older men and is encountered as calculus or intraluminal calcifications within atypical small glandular proliferations [29]. According to one previous study, prostatic calcifications were noted in 47.2% of men under 50 years old and in 86% of men over 50 years old [30]. Shoskes et al [31] reported that prostatic calcification is associated with chronic prostatitis or chronic pelvic pain syndrome; however, prostatic calcification is not meaningful in asymptomatic healthy men. Our three prostate cancer patients were more than 70 years old. Two patients had prostatic calcifications, but calcification foci did not coincide with the focal FDG uptake area. Of 34 benign cases with prostatic calcifications, the focal FDG uptake area was coincident with the calcification foci in about three-quarters; that is, focal FDG uptake lesions with coincident calcification were all confirmed as benign lesions.
Prostatic enlargement or BPH is common in older men and, although the definition varies slightly, a prostate volume approximately greater than or equal to 30 cc is considered enlargement [32]. BPH is characterised by nodular overgrowth of the epithelium and fibromuscular tissue within the transition zone and periurethral area [33]. According to previous studies, BPH and prostate cancer cannot be reliably differentiated by FDG PET [23, 34]. Our study included several cases with focal FDG uptake in the prostate gland that were later diagnosed as BPH on the basis of imaging or biopsy. Of the BPH cases, a case with SUVmax as high as 13.2 was included.
Of the eight excluded cases, six were patients from outside the hospital referred only for the PET/CT scan. In the other two cases, therapeutic intervention for severe underlying diseases preceded any evaluation of the prostate lesion and follow-up was delayed. Prostate cancer is a very common malignancy in men, but most patients with prostate cancer are diagnosed in the early stages and have a good prognosis [35]. The preferred management of localised prostate cancer is not firmly established and many studies are currently under way to compare the effectiveness and potential dangers of active intervention vs watchful waiting [36]. When incidental focal prostate uptake is detected in PET/CT of cancer patients, further evaluation and treatment choice will be based on the severity of the underlying cancer, general condition, comorbidities, age and patient preference [37].
An important limitation of our study is selection bias. Not all of the cases had histological confirmation. PSA level, imaging study and clinical follow-up were used as a tool for making the diagnosis in a large number of cases. But the most accurate tool for diagnosing prostate cancer is biopsy. Also, evaluation was not performed in all cases with incidental focal FDG uptake on PET/CT. Eight cases missing follow-up were excluded. This limitation originated from the retrospective study design. For this reason, the accurate incidence or positive predictive value of incidental prostate cancer could not be obtained and might be higher than our results indicate.
Conclusion
Focal FDG uptake of the prostate gland was incidentally noted in 1.2% of PET/CT scans performed for cancer staging or preventative health check-up in male patients. Of the reported cases of incidental focal FDG uptake in prostate, 5.4% were confirmed as malignant. All malignant lesions were noted particularly in the peripheral zone abutting the gland margin and did not have calcification in the area corresponding to FDG uptake. Most of the cases with incidental focal FDG uptake in the prostate were found to be benign lesions. Only 20% of benign lesions were noted in the peripheral zone abutting the gland margin. Screening for malignancy using a threshold SUVmax is difficult. Nevertheless, further evaluation would be prudent for discrete focal FDG uptake without coincidental calcification in the peripheral zone of prostate in elderly male patients, especially when the FDG uptake is abutting the gland margin.
References
- 1.von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology 2006;238:405–22 [DOI] [PubMed] [Google Scholar]
- 2.Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess 2007;11:iii–iv, xi–267 [DOI] [PubMed] [Google Scholar]
- 3.Yap JT, Carney JP, Hall NC, Townsend DW. Image-guided cancer therapy using PET/CT. Cancer J 2004;10:221–33 [DOI] [PubMed] [Google Scholar]
- 4.Lee JW, Kang KW, Paeng JC, Lee SM, Jang SJ, Chung JK, et al. Cancer screening using (18)F-FDG PET/CT in Korean asymptomatic volunteers: a preliminary report. Ann Nucl Med 2009;23:685–91 [DOI] [PubMed] [Google Scholar]
- 5.Minamimoto R, Senda M, Uno K, Jinnouchi S, Iinuma T, Ito K, et al. Performance profile of FDG-PET and PET/CT for cancer screening on the basis of a Japanese Nationwide Survey. Ann Nucl Med 2007;21:481–98 [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Koch S. Positron emission tomography/computed tomography potential pitfalls and artifacts. Curr Probl Diagn Radiol 2009;38:156–69 [DOI] [PubMed] [Google Scholar]
- 7.Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med 2005;46:752–7 [PubMed] [Google Scholar]
- 8.Even-Sapir E, Lerman H, Gutman M, Lievshitz G, Zuriel L, Polliack A, et al. The presentation of malignant tumours and pre-malignant lesions incidentally found on PET-CT. Eur J Nucl Med Mol Imaging 2006;33:541–52 [DOI] [PubMed] [Google Scholar]
- 9.Choi JY, Lee KS, Kwon OJ, Shim YM, Baek CH, Park K, et al. Improved detection of second primary cancer using integrated [18F] fluorodeoxyglucose positron emission tomography and computed tomography for initial tumor staging. J Clin Oncol 2005;23:7654–9 [DOI] [PubMed] [Google Scholar]
- 10.Kojima S, Zhou B, Teramukai S, Hara A, Kosaka N, Matsuo Y, et al. Cancer screening of healthy volunteers using whole-body 18F-FDG-PET scans: the Nishidai clinic study. Eur J Cancer 2007;43:1842–8 [DOI] [PubMed] [Google Scholar]
- 11.Terauchi T, Murano T, Daisaki H, Kanou D, Shoda H, Kakinuma R, et al. Evaluation of whole-body cancer screening using 18F-2-deoxy-2-fluoro-d-glucose positron emission tomography: a preliminary report. Ann Nucl Med 2008;22:379–85 [DOI] [PubMed] [Google Scholar]
- 12.Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res 2009;53:171–84 [DOI] [PubMed] [Google Scholar]
- 13.Fuchsjager M, Shukla-Dave A, Akin O, Barentsz J, Hricak H. Prostate cancer imaging. Acta Radiol 2008;49:107–20 [DOI] [PubMed] [Google Scholar]
- 14.Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology 2007;243:28–53 [DOI] [PubMed] [Google Scholar]
- 15.Takahashi N, Inoue T, Lee J, Yamaguchi T, Shizukuishi K. The roles of PET and PET/CT in the diagnosis and management of prostate cancer. Oncology 2007;72:226–33 [DOI] [PubMed] [Google Scholar]
- 16.Bouchelouche K, Oehr P. Positron emission tomography and positron emission tomography/computerized tomography of urological malignancies: an update review. J Urol 2008;179:34–45 [DOI] [PubMed] [Google Scholar]
- 17.Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol 2007;51:1511–20; discussion 1520–1 [DOI] [PubMed] [Google Scholar]
- 18.von Mallek D, Backhaus B, Muller SC, Matthies A, Palmedo H, Jaeger U, et al. Technical limits of PET/CT with 18FDG in prostate cancer. Aktuelle Urol 2006;37:218–21 [DOI] [PubMed] [Google Scholar]
- 19.Liu IJ, Zafar MB, Lai YH, Segall GM, Terris MK. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology 2001;57:108–11 [DOI] [PubMed] [Google Scholar]
- 20.Liu Y. FDG PET-CT demonstration of metastatic neuroendocrine tumor of prostate. World J Surg Oncol 2008;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho L, Quan V, Henderson R, Seto J. High-grade urothelial carcinoma of the prostate on FDG PET-CT. Clin Nucl Med 2007;32:746–7 [DOI] [PubMed] [Google Scholar]
- 22.Kao PF, Chou YH, Lai CW. Diffuse FDG uptake in acute prostatitis. Clin Nucl Med 2008;33:308–10 [DOI] [PubMed] [Google Scholar]
- 23.Effert PJ, Bares R, Handt S, Wolff JM, Bull U, Jakse G. Metabolic imaging of untreated prostate cancer by positron emission tomography with 18fluorine-labeled deoxyglucose. J Urol 1996;155:994–8 [PubMed] [Google Scholar]
- 24.Hoh CK, Seltzer MA, Franklin J, deKernion JB, Phelps ME, Belldegrun A. Positron emission tomography in urological oncology. J Urol 1998;159:347–56 [DOI] [PubMed] [Google Scholar]
- 25.Lawrentschuk N, Davis ID, Bolton DM, Scott AM. Positron emission tomography and molecular imaging of the prostate: an update. BJU Int 2006;97:923–31 [DOI] [PubMed] [Google Scholar]
- 26.Oyama N, Akino H, Kanamaru H, Okada K. Fluorodeoxyglucose positron emission tomography in diagnosis of untreated prostate cancer. Nippon Rinsho 1998;56:2052–5 [PubMed] [Google Scholar]
- 27.Reynolds MA, Kastury K, Groskopf J, Schalken JA, Rittenhouse H. Molecular markers for prostate cancer. Cancer Lett 2007;249:5–13 [DOI] [PubMed] [Google Scholar]
- 28.McNeal JE. The zonal anatomy of the prostate. Prostate 1981;2:35–49 [DOI] [PubMed] [Google Scholar]
- 29.Muezzinoglu B, Gurbuz Y. Stromal microcalcification in prostate. Malays J Pathol 2001;23:31–3 [PubMed] [Google Scholar]
- 30.Bock E, Calugi V, Stolfi V, Rossi P, D’Ascenzo R, Solivetti FM. Calcifications of the prostate: a transrectal echographic study. Radiol Med 1989;77:501–3 [PubMed] [Google Scholar]
- 31.Shoskes DA, Lee CT, Murphy D, Kefer J, Wood HM. Incidence and significance of prostatic stones in men with chronic prostatitis/chronic pelvic pain syndrome. Urology 2007;70:235–8 [DOI] [PubMed] [Google Scholar]
- 32.Roehrborn CG, Marks LS, Fenter T, Freedman S, Tuttle J, Gittleman M, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology 2004;63:709–15 [DOI] [PubMed] [Google Scholar]
- 33.Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol 2005;40:121–8 [DOI] [PubMed] [Google Scholar]
- 34.Hofer C, Laubenbacher C, Block T, Breul J, Hartung R, Schwaiger M. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur Urol 1999;36:31–5 [DOI] [PubMed] [Google Scholar]
- 35.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol;28:126–31 [DOI] [PubMed] [Google Scholar]
- 36.Wilt TJ, Brawer MK, Barry MJ, Jones KM, Kwon Y, Gingrich JR, et al. The Prostate cancer Intervention Versus Observation Trial:VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials 2009;30:81–7 [DOI] [PubMed] [Google Scholar]
- 37.Jereczek-Fossa BA, Curigliano G, Orecchia R. Systemic therapies for non-metastatic prostate cancer: review of the literature. Onkologie 2009;32:359–63 [DOI] [PubMed] [Google Scholar]