Abstract
We describe the application of a novel analysis method that provides detailed maps of changes in cartilage thickness measured from MRI scans for individuals and cohorts of patients together with regional measures.
A cohort of osteoarthritis patients was imaged using a 1.0 T MR scanner over a 36-month period. Hyaline cartilage was manually segmented from a three-dimensional (3D) spoiled gradient-echo sequence with fat suppression. Representative outlines of the bone surfaces of the distal femur and proximal tibia were automatically generated from T2 weighted images using statistical models of the shape and appearance of the bones. Cartilage thickness was measured from a dense set of points representing the bony surface. The models of the bones provided a common frame of reference, relative to which change maps were generated and aggregated across the cohort and anatomically corresponding subregions of the joint to be identified.
In the reproducibility arm involving six patients, the thickness of cartilage had coefficients of variation of 2.66% within the tibiofemoral joint and 2.94% within the medial femoral condyle region. In the 9 patients (6 female, 3 male) who completed the 36-month study, the most striking observation was that lack of change in global measures of cartilage thickness concealed substantial focal changes. Specifically, the cartilage thickness within the tibiofemoral joint decreased by 0.85% per annum (95% CI −2.13% to 0.45%) with the medial femoral condyle as the region with the most significant change, decreasing by 2.43% per annum (uncorrected 95% CI −4.31% to 0.51%).
Cartilage loss is a cardinal feature of osteoarthritis (OA), and MRI permits direct visualisation of articular hyaline cartilage. Assessments of cartilage morphology from knee MRI are emerging as promising measures for monitoring OA disease progression [1]. This is of particular interest in drug development, where precise cartilage morphology biomarkers may allow the efficacy of disease-modifying drugs to be assessed in shorter and smaller clinical trials than when using only traditional clinical or imaging biomarkers such as symptoms or radiographic joint space narrowing. However, this potential has yet to be fully realised. One reason for this may be that methods of image analysis and cartilage quantification have not been sufficiently precise to detect the small changes in cartilage thickness seen as the disease progresses. Alternatively, it may be that OA represents a heterogeneous disease entity, and that improved methods of patient characterisation are needed to select subjects for clinical trials.
MR biomarkers of cartilage morphology were introduced in the early 1990s, a biomarker in this context, being “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention” [2]. Early work used three-dimensional (3D) fat-suppressed spoiled gradient-echo sequences followed by manual segmentation of cartilage in each slice and reported global measures such as compartmental volume [3] or compartmental mean thickness [4]. Although some investigators reported relatively rapid loss of tibiofemoral cartilage in OA, 4–8% per year [1], other OA studies showed little or no change. Indeed, we previously described [5] a cohort of 16 knee OA patients, followed for up to 3 years, with a mean annual loss of only 0.5%. It is unclear whether the studies reporting rapid loss have truly identified a “fast progressor” population or whether apparent lack of progression reflects inadequacies in analysis. It may be that global measures are too non-specific, and, in order to detect the heterogeneous nature of cartilage loss during OA disease progression, a refined analysis is needed to enable measurements of focal anatomical regions within the joint [6, 7].
We have previously described how regional measures of cartilage thickness provide focal information [8] and how further refinements allow measures to be restricted to regions such as the central, more load-bearing regions of the joint where others have shown that homogeneous changes may be expected [9, 10]. Such quantitative analysis of hyaline cartilage requires the precise location of the cartilage to be identified from the MR images. The current gold standard is to use manual or semi-automatic segmentation of the cartilage boundary in each image slice [11, 12]. For focal measurements of cartilage in population studies, measures must be made within anatomically equivalent regions across all patients. Other researchers extend the cartilage segmentation task to include identification of the anterior and posterior aspects of the femoral condyles [11], and this per image, slice by slice delineation of the regions both is subjective and requires expert interpretation, adding to the already high costs of manual segmentation. Others automate the task of identifying anatomical regions in each image by fitting the cartilage surfaces to a common co-ordinate frame [13] or dividing the cartilage based on the geometry of its outer edges [14]. However, although these global co-ordinate frame registration methods account for gross shape and scale variations, they do not account for local shape variation that affects the precise location of region boundaries in individuals. This is important as cartilage coverage varies between patients and also changes over time, especially in diseased patients. Therefore, regions defined according to the outer edges of the cartilage will not be consistent across patients or between time-points.
In this paper we apply a method that uses statistical models of the bones' appearance and shape to construct detailed maps showing changes in cartilage thickness over time for individuals and across subjects for a small cohort of OA patients studied previously [5]. The statistical models are also used to define anatomically consistent regions of interest across all images, within which changes in mean cartilage thickness are analysed. We measure the reproducibility of our approach and assess whether this more sensitive measure of cartilage morphology and/or regional measures alter our original finding of no change in global measures of compartmental cartilage volume.
Methods and materials
Overview
We present the re-analysis of a small MRI study of heterogeneous OA patients using a new statistical shape-modelling methodology. Briefly, at each visit, patients' knees were imaged using MR protocols designed to highlight the hyaline cartilage and endosteal bone surface. The location of the cartilage was traced manually in each image slice. The bone surfaces were identified automatically using active appearance models (AAMs), described below, which provide a model bone reference surface in each image that is underpinned by a dense set of points that are anatomically equivalent. The cartilage thickness was measured above each point of the bone reference surface and illustrated in colour-coded maps on the bone shapes. Since the reference surfaces correspond across images, maps from the same patient at different visits could be compared to illustrate changes in cartilage thickness over time. These individual change maps were also combined to illustrate the change in cartilage thickness across the study cohort. The reference surface was also used to automatically define anatomically consistent regions of interest across all images. Mean change in cartilage thickness within the regions of interest was computed from the cartilage thickness maps and analysed across the study cohort to determine the average change over time and the statistical significance of these changes. The study included a reproducibility arm in which the coefficients of variation of regional mean cartilage thickness measures were measured.
Patient recruitment and image acquisition
The original study recruited 16 patient volunteers (10 male, 6 female) with established OA [5]. The inclusion criteria were current use-related pain in index knee; age >40 years; and radiographic evidence of OA. Patients with severe joint damage (Kellgren and Lawrence grading scale (KL) grade 4 on radiographic evidence) were excluded from the study.
Patients were imaged at four time-points: baseline; either 2, 4 or 6 months; 12 months; and 36 months. During the 12-month visit, a subset of the patients was imaged twice, with repositioning in the scanner, to enable the reproducibility of the method to be analysed. Knees were positioned centrally in the coil with a minimum of rotation in order to maintain comfort in accordance with the institution's usual radiographic practice, but no special immobilisation devices were used. The range of the time between the first and last scan was 35–39 months. 11 of the original 16 patient recruits remained in the study for the 36-month duration, of whom 6 had 2 images taken during the 12-month time-point. Images were acquired on a Siemens (Erlangen, Germany) 1.0 T “Impact” clinical scanner using a Siemens circularly polarised extremity coil. A 3D spoiled gradient-echo sequence with fat suppression was used for visualisation of hyaline cartilage (repetition time/echo time (TR/TE) = 50/11 ms; 40° flip-angle; 64 sagittal image slices 1.54 mm thick; 140 mm field of view displayed on 256 × 256 matrix providing in-plane resolution of 0.55 mm; acquisition took 10 min and 16 s). A T2 weighted image was acquired to visualise the endosteal surface of the bone (TE = 90 ms; 23 sagittal image slices 3 mm thick, with 4 mm slice separation; 140 mm field of view on 256 × 256 matrix providing in-plane resolution of 0.55 mm; acquisition took 5 min and 11 s).
Cartilage segmentation
The segmentation protocol was devised in collaboration between a musculoskeletal radiologist (CEH) and experienced segmentation supervisor (MB). Manual slice-by-slice segmentation of cartilage in the gradient-echo sequence was performed by three trained segmenters following a period of training and assessment using test images. Two of the segmenters were undergraduate medical students and the third was an undergraduate physicist. Segmenters were certified when they were able to repeatedly segment the femoral, medial tibial, lateral tibial and patella cartilage compartments with an intra-observer coefficient of variation of less than 3% using blinded sets of images. Cartilage segmentation involved manual, subvoxel delineation of the hyaline cartilage boundary of femoral, medial tibial and lateral tibial cartilage in each image slice using EndPoint software (Imorphics, Manchester, UK). Each patient's data were blinded to time and segmented by a single segmenter. The segmenters collaborated with each other and consistently consulted the experienced segmenter and musculoskeletal radiologist to resolve segmentation issues. The experienced segmenter performed a central review of the final segmentations, with the musculoskeletal radiologist as consultant. During these revisions, missing slices in the segmentations were identified and added, and amendments were performed to ensure consistency in the decisions taken on the presence or lack of cartilage between all images for each subject.
Bone reference surface
In order to compare thickness measures between time-points and combine measures across patients, a common, anatomically consistent frame of reference was defined in all MR images. The bone was chosen as a reference surface because it is more consistent and stable than the cartilage, whose edge, relative to the bone anatomy, is more likely to vary between patients and time-points. AAMs of the distal femur and proximal tibia were used to identify automatically the corresponding endosteal bone surface in each of the T2 weighted images [15].
AAMs capture the shape and greyscale appearance of the same anatomical object in a set of images. The models were built from a set of similar images and manual segmentations from a previous study of 19 healthy female volunteers [16] using the minimum description length (MDL) method to define automatically a dense set of corresponding points on each example surface [17]. Although the MDL does not explicitly optimise the anatomical correspondence of the model points, evaluation of competing methods has shown it to be among the best methods for automatic identification of correspondences [18], and the anatomical correspondence of these models has been assessed previously and shown to have mean positional errors of approximately 1 mm [16]. Each model consists of a mean shape and appearance of the bone and a mathematical description of the variation of each training example in relation to the mean [15]. Models also have the ability to interpolate between training set examples to find instances of valid new shape and appearance, thus performing automatic segmentation of the object in a previously unseen image. The result of applying the AAMs to each T2 weighted image is, therefore, automatic segmentation of the distal femur and proximal tibia together with a dense set of 16 386 corresponding points on each bone surface in each image. These correspondences provide a common frame of reference between all training and automatically segmented examples.
Cartilage thickness maps
A detailed map of cartilage thickness for each patient visit was produced by measuring the thickness of the cartilage, rendered from the manual segmentations in the gradient-echo sequence, at the set of corresponding points defined on the bone reference surface in the corresponding T2 weighted image. To enable 3D measurements of cartilage thickness, closed triangulated surface representations of the cartilage surface were formed from the parallel manual slice segmentations [17]. At this stage, the volume of cartilage contained within the 3D surface was calculated to enable direct comparison with the previously published results on the same image data [5]. In order to correct for movement artefacts between the acquisitions of the gradient-echo and T2 weighted sequences, a rigid registration of the bone reference surface relative to the inner surface of the cartilage was performed.
The cartilage thickness map was constructed by defining the vector, which was perpendicular to the bone surface in 3D at each measurement point and finding its point of intersection, if any, with the inner and outer cartilage surfaces. The cartilage thickness for that measurement point was then recorded as the distance between these two points of intersection. Measurement points for which no cartilage was detected above the bone surface were labelled as having zero cartilage thickness. The result is a cartilage thickness measurement associated with each measurement point, which was displayed as a colour-coded map of cartilage thickness on each reference bone surface.
Individual and population maps
The correspondence of the cartilage thickness maps between time-points and across patients enables the reproducibility of thickness measurements and the change in cartilage thickness over time both within individual patients and averaged across the study cohort to be analysed. To examine per individual cartilage morphology, the cartilage thickness maps formed from the baseline images were subtracted from the thickness map at 36 months and illustrated on the reference bone surface of the individual at baseline. The correspondence across patients enabled population cartilage thickness change maps to be constructed by point-wise averaging of the individual change maps. These population change maps were displayed on the mean bone shape. To examine reproducibility of the thickness measurements, the coefficient of variation was calculated at each measurement point for each patient from the test–retest images acquired at the 12-month time-point. Aggregate measures of reproducibility were computed as the per point root mean square coefficient of variation across all patients and displayed on the mean bone shape.
Regional analysis
Cartilage change maps are informative but lack statistical power because significance testing of these multiple-point data requires correction for multiple comparisons. To quantify changes within anatomical regions of the joint, and determine if the changes were statistically significant across a population, average changes in cartilage thickness within regions of interest were calculated.
The bone reference shapes were utilised to define anatomically consistent regions in each patient image for all time-points. Regions of interest were defined on the mean bone reference shape as follows:
The articulating surfaces were divided into regions, based on their functionality, as defined by a consensus workshop [19].
The tibia was trivially divided to its medial and lateral aspects.
The articulating surface of the femur was divided by drawing a straight line from the inferior viewpoint, perpendicular to the bone medial and tangential to the posterior aspect of the trochlear groove; this line divides the articulating surface of the femur into the femoral trochlear, mostly in contact with the patella, and the medial and lateral femoral condyles.
A posterior boundary to the femoral condyles was also defined by drawing a straight line from the posterior viewpoint, which removes the regions only in contact with their respective trochlear articulating surfaces when the knee is fully flexed.
A trimming boundary was drawn inside the perimeter of each cartilage compartment to limit regions to the central portion of the articulating surfaces, excluding the cartilage peripheries, which we consider more prone to segmentation and, hence, measurement errors. The trimming boundary was drawn to tightly encompass the region of bone that exhibited 90% or greater cartilage coverage across the cohort of 19 healthy female volunteers in the previously mentioned study [5].
All regional boundaries were defined along the connected edges of the model correspondences, which enabled them to be propagated to any of the individual bone reference shapes while maintaining their anatomical fidelity.
Cartilage was quantified within each region for each patient and at each time-point. For each region the mean cartilage thickness was computed as the mean of the point-wise measures weighted according to the surrounding area of each point on the image bone reference surface; thus, measurement points which are more sparsely separated were given greater weighting to account for uneven distribution of points and local shape variation between subjects' knees.
Statistical analysis
Reproducibility was calculated for the regional mean thickness measures from the images of the patients imaged twice during the 12 month time-point and was expressed as a coefficient of variation. The annualised percentage change in regional cartilage thickness measures across the cohort was assessed using paired t-tests on the logarithm scale, as described by Bland and Altman [20]. The individual regional mean cartilage thicknesses were logarithm transformed (natural logarithms), and a paired t-test was conducted on the logarithm-transformed baseline and 3 year time-point pairs. Since the difference between the logarithms of two numbers is the logarithm of their ratio, this is equivalent to analysing the proportion of the baseline cartilage remaining at the 3 year visit. The resulting mean logarithm difference and 95% confidence intervals (CIs) were annualised to account for the nominal 3 year follow-up by dividing by 3, transformed back to the natural scale by taking anti-logarithms, and expressed as percentage change by subtracting 1 and multiplying by 100%.
The primary cartilage measure was agreed a priori as the overall mean cartilage thickness within the central tibiofemoral regions, trimmed to remove the edges, with follow-up analysis of the change within the four component subregions corrected for multiple comparisons by the Bonferroni correction. (See Figure 2 for illustration of regions and definition of the nomenclature.) For comparison with the original analysis of Gandy et al [5], the total cartilage volume across all compartments of the joint was also assessed. Per patient progression was examined by plotting the regional cartilage thickness against time for each patient time-point.
Figure 2.

Regions of interest on femur and tibia mean reference bone shapes. Anatomical regions illustrated by grey shading labelled using the nomenclature of Eckstein et al [19]: n, nuclear (trimmed); c, central (excluding posterior aspects of the femur); MF, medial femur; LF, lateral femur; MT, medial tibia; LT, lateral tibia.
Results
9 of the 11 patients imaged at both baseline and 37±2 months had data suitable for re-analysis (6 female, 3 male, baseline KL grades 1–3). Six of those (three female, three male) had test–retest images for the 12 month time-point. Analysis of total knee cartilage volume (patellar, femoral and tibial) revealed a non-significant decrease of 0.54% per year for the femur and tibia compartments in the nine subjects analysed (95% CI for annualised change: −1.99% to 0.93%, decreases being negative). This is consistent with the 0.53% annual loss in cartilage volume reported by Gandy et al [5] in the original analysis of this study.
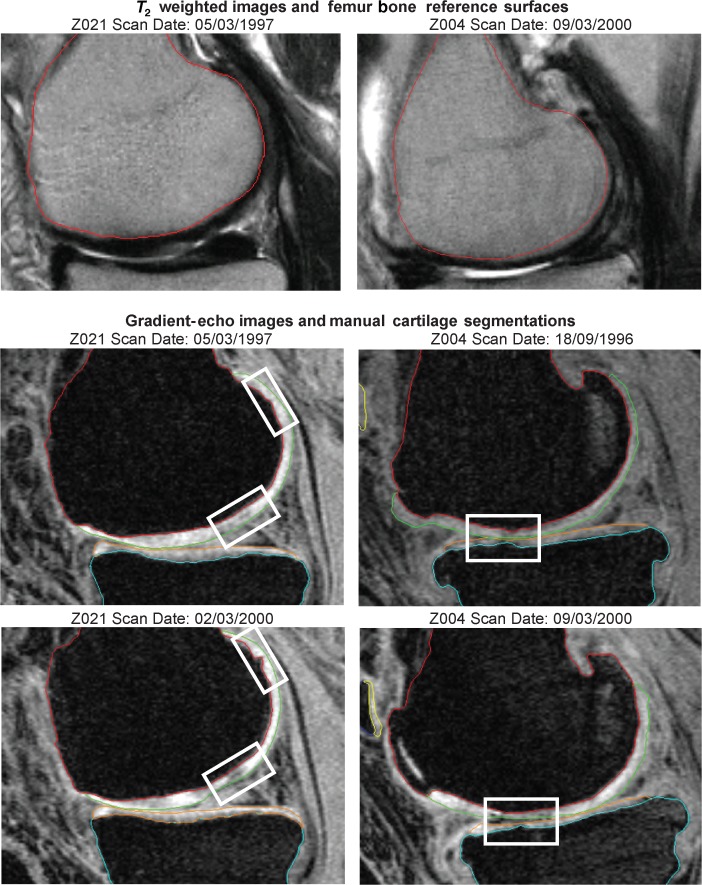
Figure 1 shows selected corresponding slices from images of two subjects at baseline and at the 36-month time-point. The result of the bone reference surface model search is shown on the T2 weighted image and the manually delineated cartilage is shown on the gradient-echo sequence. These slices illustrate distinct features of disease progression apparent in the MR images. The rigid registration of the bone surfaces to the cartilage surfaces, which corrects for movement artefacts between the acquisitions of the gradient-echo and T2 weighted sequences, had an average Euclidean translation of 1.26 mm and rotation of 8.85 degrees. Figure 2 shows the definition of the tibiofemoral joint subregions on the mean bone shapes. The results are presented for trimmed regions, which exclude the edges of cartilage coverage. Maps illustrating the standard deviation of the thickness measurement for the six patients who were imaged twice during the 12-month visit are shown in Figure 3. Reproducibility is less towards the edges of the cartilage sheet, demonstrating the uncertainty of measures of cartilage thickness in regions more prone to partial volume effects where the cartilage sheet becomes parallel to the image slices. Table 1 shows the aggregate coefficient of variation of mean thickness measurements taken for the patients who were imaged twice during the 12-month time-point visit. They demonstrate an improvement in reproducibility when the regions are trimmed to exclude the outer edges of the bone.
Figure 1.
Selected image slices from two subjects Z021 (left column) and Z004 (right column). The top row shows example slices from the T2 weighted image with the result of the femur bone model search. The bottom two rows show corresponding slices from the fat-suppressed image at baseline and 36 months with manual delineations of the femoral and tibial cartilage. The focus boxes highlight regions of osteoarthritis disease progression. Subject Z021 exhibits an increase in the size of a hole in the proximal anterior of the femoral cartilage (top box) and thinning in the central region, which was measured as a mean cartilage thickness change from 3.7 mm to 2.5 mm in the regional quantitative analysis. Subject Z004 exhibits thinning of both the femoral and tibial cartilage in the region highlighted.
Figure 3.
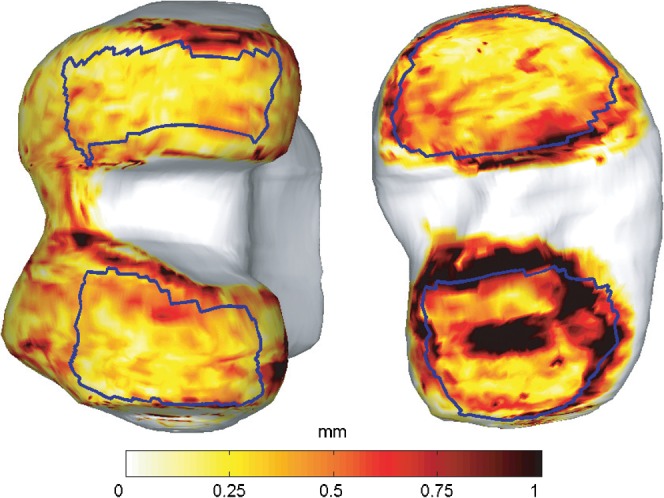
Map of the standard deviation in the difference of the point-wise thickness measurements for the six subjects who were imaged twice during the 12 month visit using a temperature colour scale to indicate the difference in cartilage thickness. Nuclear regions are shown in blue.
Table 1. Coefficients of variation for the regional mean thickness measures.
| Region | Untrimmed (%) | Trimmed (%) |
| cTF | 3.54 | 2.66 |
| MT | 5.88 | 4.63 |
| cMF | 3.28 | 2.94 |
| LT | 6.56 | 6.00 |
| cLF | 4.01 | 2.79 |
cLF, central lateral femur; cMF, central medial femur; cTF, central tibiofemoral; LT, lateral tibia; MT, medial tibia.
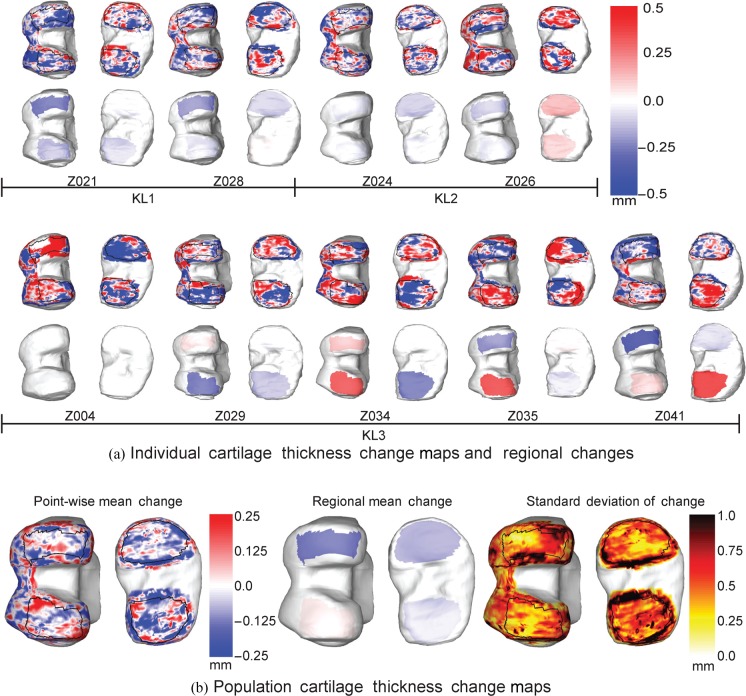
The individual change maps and subregion change maps, aligned to KL grade, are shown in Figure 4a. Blue values indicate a reduction in cartilage thickness with red regions indicating an increase in cartilage thickness. The boundaries of the subregions in the lower tier of surfaces provide evidence of the anatomical consistency of the subregions when propagated on to the individual bone surfaces. The group mean point-wise, regional change maps and standard deviation of the change maps for the nine subjects are shown in Figure 4b.
Figure 4.
(a) Individual change maps showing total point-wise (top of each pair) and regional (bottom of each pair) change between the baseline and 36-month visits for the femur (left of each pair) and the tibial plateau (right of each pair). Patients are arranged according to disease severity at baseline as assessed by KL score from radiographs. (b) Group mean point-wise, trimmed region change maps and point-wise standard deviation of change shown on the mean bone reference shape. Change, in mm, is represented using a colour map where blue is a loss and red a gain of cartilage thickness.
Figure 5 illustrates the per patient progression of mean cartilage thickness within the trimmed central medial femoral condyle region. The progression plots demonstrate the level of variability in measuring regional progression in individual patients. Change in mean cartilage thickness for the composite and individual trimmed regions of the tibiofemoral joint are plotted in Figure 6. Mean cartilage thickness across the central tibiofemoral joint, trimmed to exclude the periphery of the cartilage sheets, decreased by 0.85% per year (95% CI for annualised change −2.13% to 0.45%). The trimmed nuclear central medial femoral (ncMF) is the region with the most significant change, decreasing 2.43% per year with uncorrected 95% CI −4.31% to −0.51%, although this is not significant at the 5% level after correction for multiple comparisons across the four regions.
Figure 5.
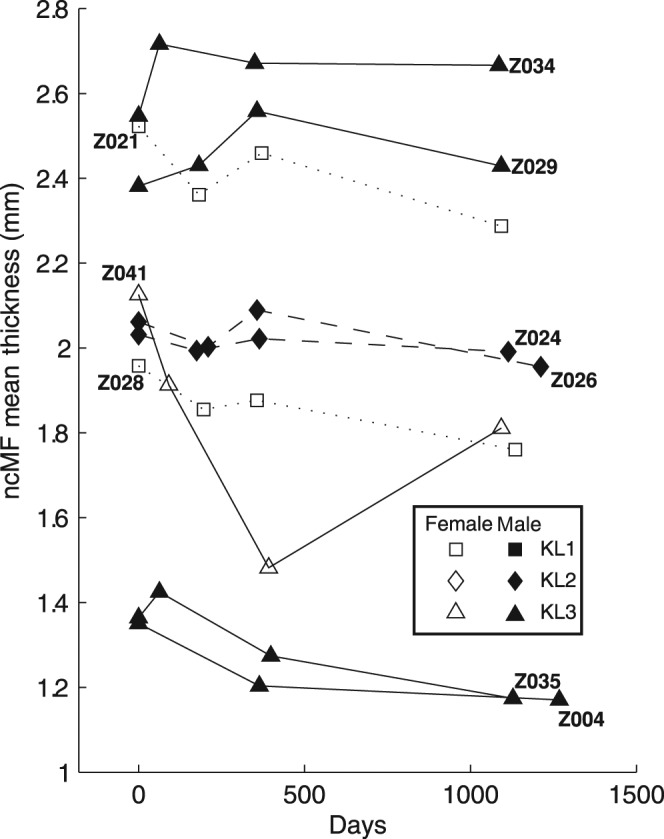
Progression of mean cartilage thickness within the trimmed nuclear, central medial femur (ncMF) compartment over time for all time-points and for all nine subjects.
Figure 6.

Annualised per cent change in mean cartilage thickness within the trimmed regions for compartments trimmed central tibiofemoral (ncTF); trimmed medial tibia (nMT); trimmed central medial femur (ncMF); trimmed lateral tibia (nLT); and trimmed central lateral femur (ncLF) over 3 years with error bars showing the 95% confidence intervals (uncorrected for multiple comparisons).
Discussion
In this paper, a new method for quantitative analysis of cartilage was applied to a study of MRI data acquired on a 1.0 T scanner for a small cohort of OA patients. An intra-observer reproducibility arm demonstrated a small coefficient of variation for regional mean thickness measurements (e.g. less than 3% in the trimmed regions), which is consistent with results from another study using the same method [8]. Interobserver reproducibility was not measured directly, although the effects of interobserver variation were minimised by ensuring that all of a subject's images were segmented by the same segmenter and through quality assurance of all segmentations by two experts.
Although our new analysis confirmed the original result for whole-knee cartilage volume: “No loss of cartilage over 3 years …” [5], the detailed focal maps and sensitive regional measures of cartilage morphology led to two striking observations. The first is that the central medial femur appeared to show fastest progression, which is consistent with previous findings [1,21]. The second and more novel is that the apparent lack of change in the global measures of volume conceals substantial focal loss of cartilage in many locations, almost balanced by equally substantial gains in cartilage thickness in other locations. This increase in thickness may be due to tissue swelling, which is known to be an early feature of cartilage degeneration in OA as a result of proteoglycan loss [22, 23]. Intriguingly, there was more loss and gain of cartilage thickness in patients with the more severe KL grades.
Even with this more precise analysis, changes in cartilage thickness are still highly variable, and more sensitive analytical measures cannot compensate entirely for patient heterogeneity with respect to annualised cartilage loss. These observations highlight still unresolved challenges in serial studies of knee OA patients, including the study of disease-modifying agents. These challenges include patient inclusion, study size and duration, and the a priori identification of the region of interest to use in the statistical analysis. Clearly, a more homogeneous population and a specific central medial femur region of interest would probably provide greater and more reproducible loss in cartilage thickness, permitting studies with fewer patients per arm and of shorter duration. Currently, we have limited knowledge on how to define this homogeneous patient group of fast progressors. One possible group is those OA knee patients who have had previous meniscal injury [24], but other groups may also exist. However, even if “fast-progressor” populations exist, it is unclear whether results from such groups would be generalisable to OA as a whole. Finally, there are limited data to suggest that the loss of cartilage thickness is of clinical relevance, with the strongest data supporting a link to time to joint replacement [25].
Acknowledgments
We wish to thank Drs Gandy, Dieppe and Watt, our original co-authors, for helpful discussion on the study. This work is supported in part by AstraZeneca and EPSRC, and the original study was supported by AstraZeneca and MRC. TGW was supported by EPSRC (DT/F003072/1).
Footnotes
CJT is currently PI on a research council grant, which involves collaboration with Imorphics Ltd., continuation of which is dependent on a significant contribution in kind from the company, and MB is an employee of the company. Imorphics Ltd. provides products and services for the quantitative analysis of medical images for clinical trials and holds the commercial exploitation rights for the work described in the paper. CJT and MB personally hold shares in Imorphics Ltd, which is a University spin-out.
References
- 1.Eckstein F, Cicuttini F, Raynauld J, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthr Cartil 2006;14(Suppl A):46–75 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95 [DOI] [PubMed] [Google Scholar]
- 3.Raynauld JP, Kauffmann C, Beaudoin G, Berthiaume MJ, deGuisei JA, Bloch DA, et al. Reliability of a quantification imaging system using magnetic resonance images to measure cartilage thickness and volume in human normal and osteoarthritic knees. Osteoarthr Cartil 2003;11:351–60 [DOI] [PubMed] [Google Scholar]
- 4.Glaser C, Draeger M, Eckstein F, Englmeier KH, Reiser M. Cartilage loss over two years in femoro-tibial osteoarthritis. Radiology 2002;225(Suppl):330 [Google Scholar]
- 5.Gandy SJ, Dieppe PA, Keen MC, Maciewicz RA, Watt I, Waterton JC. No loss of cartilage volume over three years in patients with knee osteoarthritis as assessed by magnetic resonance imaging. Osteoarthr Cartil 2002;10:929–37 [DOI] [PubMed] [Google Scholar]
- 6.Kshirsagar AA, Watson PJ, Tyler JA, Hall LD. Measurement of localized cartilage volume and thickness of human knee joints by computer analysis of three-dimensional magnetic resonance images. Invest Radiol 1998;33:289–99 [DOI] [PubMed] [Google Scholar]
- 7.Koo S, Gold GE, Andriacchi TP. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthr Cartil 2005;13:782–9 [DOI] [PubMed] [Google Scholar]
- 8.Williams TG, Holmes A, Waterton J, Maciewicz R, Nash A, Taylor C. Regional quantitative analysis of knee cartilage in a population study using MRI and model based correspondences. IEEE Int Symp on Biomedical Imaging, Arlington, VA, 2006 [Google Scholar]
- 9.Tamez-Peña JG, Barbu-McInnis M, Totterman S. Unsupervised definition of the tibia-femoral joint regions of the human knee and its applications to cartilage analysis. Proc SPIE 2006:6144 [Google Scholar]
- 10.Eckstein F, Wirth W, Lengfelder V, Stein V, Hudelmaier M, Cahue S, et al. ISMRM International Society for Magnetic Resonance in Medicine Annual Meeting; 2007. Subregional analysis of tibial cartilage changes in persons with knee osteoarthritis and malalignment. Berlin, Germany. [Google Scholar]
- 11.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state (DESS) magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the osteoarthritis initiative. Ann Rheum Dis 2005;65:433–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cicuttini F, Forbes A, Morris K, Darling S, Bailey M, Stuckey S. Gender differences in knee cartilage volume as measured by magnetic resonance imaging. Osteoarthr Cartil 1999;7:265–71 [DOI] [PubMed] [Google Scholar]
- 13.Kauffmann C, Gravel P, Godbout B, Gravel A, Beaudoin G, Raynauld J-P, et al. Computer-aided method for quantification of cartilage thickness and volume changes using MRI: Validation study using a synthetic model. IEEE Trans Biomed Eng 2003;50:978–88 [DOI] [PubMed] [Google Scholar]
- 14.Wirth E, Roth M, Kraus VB, Charles C, Hudelmaier M, Eckstein F. Regional analysis of cartilage morphology in defined anatomical subregions of femorotibial cartilages. OARSI World Congress on Osteoarthritis, 7–10 December 2006, Prague, Czech Republic. [Google Scholar]
- 15.Cootes TF, Edwards GJ, Taylor CJ. Active appearance models. IEEE Trans Patt Anal Mach Intell 2001;23:681–5 [Google Scholar]
- 16.Williams TG, Taylor CJ, Waterton JC, Holmes A. Population analysis of knee cartilage thickness maps using model based correspondences. In: ISBI 2004 IEEE Int. Symp. on Biomedical Imaging, 15–18 April, 2004. Arlington, VA: IEEE, 2004 [Google Scholar]
- 17.Williams TG, Taylor CJ, Gao Z, Waterton JC. Corresponding articular cartilage thickness measurements in the knee joint by modelling the underlying bone. Taylor C, Noble JA, IPMI 2003 Proc. 18th Int. Conf. on Information Processing in Medical Imaging, Lecture Notes in Computer Science 2732, 20–25 July 2003. Ambleside, UK: Springer, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Styner MA, Rajamani KT, Nolte L-P, Zsemlye G, Székely G, Taylor CJ, et al. Evaluation of 3D correspondence methods for model building. Information Processing in Medical Imaging Lecture Notes in Computer Science 2732 July 2003. Ambleside, UK, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B, et al. Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthr Cartil 2006;14:974–83 [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ 1996;312:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams TG, Holmes AP, Maciewicz RA, Waterton JC, Taylor CJ, Creamer P, et al. Cartilage loss in osteoarthritis detected by statistical shape analysis of magnetic resonance images. Osteoarthr Cartil 2005;13(Suppl 1):S114 [Google Scholar]
- 22.Ding C, Martel-Pelletier J, Pelletier JP, Abram F, Raynauld JP, Cicuttini F, et al. Two-year prospective longitudinal study exploring the factors associated with change in femoral cartilage volume in a cohort largely without knee radiographic osteoarthritis. Osteoarthr Cartil 2008;16:443–9 [DOI] [PubMed] [Google Scholar]
- 23.Biswal S, Hastie T, Andriacchi TP, Bergman GA, Dillingham MF, Lang P. Risk factors for progressive cartilage loss in the knee. Arthritis Rheum 2002;46:2884–92 [DOI] [PubMed] [Google Scholar]
- 24.Hunter DJ, Zhang YQ, Niu JB, Amin XTS, Clancy M, Guermazi A, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum 2006;54:795–801 [DOI] [PubMed] [Google Scholar]
- 25.Wluka AE, Ding C, Jones G, Cicuttini FM. The clinical correlates of articular cartilage defects in symptomatic knee osteoarthritis: a prospective study. Rheumatology 2005;44:1311–16 [DOI] [PubMed] [Google Scholar]