Abstract
The aim of this study was to investigate if non-coplanar intensity-modulated radiation therapy (IMRT) in the post-mastectomy setting can reduce the dose to normal structures and improve target coverage. We compared this IMRT technique with a standard partial wide tangential (PWT) plan and a five-field (5F) photon-electron plan. 10 patients who underwent left-sided mastectomy were planned to 50.4 Gy using either (1) PWT to cover the internal mammary (IM) nodes and supraclavicular fields, (2) 5F comprising standard tangents, supraclavicular fields and an electron field for the IM nodes or (3) IMRT. The planning target volume (PTV) included the left chest wall, supraclavicular, axillary and IM lymph nodes. No beams were directed at the right lung, right breast or heart. Mean dose–volume histograms were constructed by combining the dose–volume histogram data from all 10 patients. The mean PTV to receive 95% of the dose (V95%) was improved with the IMRT plan to 94.2% from 91.4% (p = 0.04) with the PWT plan and from 87.7% (p = 0.012) with the 5F plan. The mean V110% of the PTV was improved to 3.6% for the IMRT plan from 16.8% (p = 0.038) for the PWT plan and from 51.8% (p = 0.001) for the 5F plan. The mean fraction volume receiving 30 Gy (v30Gy) of the heart was improved with the IMRT plan to 2.3% from 7.5% (p = 0.01) for the PWT plan and 4.9% (p = 0.02) for the 5F plan. In conclusion, non-coplanar IMRT results in improved coverage of the PTV and a lower heart dose when compared with a 5F or PWT plan.
Several prospective studies have shown the benefit of post-mastectomy radiation in reducing locoregional recurrences and increasing overall survival [1–3]. These trials included comprehensive radiation to the chest wall and regional nodes including the internal mammary, axillary and supraclavicular regions.
Comprehensive post-mastectomy radiation is technically difficult given the complexity of the target volume and its close proximity to critical structures including the heart, lung, brachial plexus and contralateral breast [4, 5]. Several studies have examined different three-dimensional (3D) radiation techniques comparing target coverage and dose to the neighbouring critical structures [6–12]. To date, there is no gold standard for the delivery of post-mastectomy radiation that adequately covers the regional nodes while avoiding the underlying critical structures. Each technique described in the literature is optimised and chosen to account for the individual patient's unique anatomy.
Recently, intensity-modulated radiation therapy (IMRT) has been evaluated in the left-sided post-mastectomy setting in technical feasibility studies [13]. These studies show that IMRT improves dose homogeneity and significantly spares the heart and left lung [13–16]. Most published studies to date, however, have utilised coplanar IMRT beams directed from all around the patient. The primary drawback of this technique is increased dose to the contralateral normal lung and breast because beams pass through these structures [13–16].
We present a novel beam arrangement for the delivery of IMRT to the regional lymphatics and chest wall in patients who have undergone a left-sided mastectomy. The beams are arranged in an ipsilateral, non-coplanar manner to effectively spare the right lung and breast from receiving any direct radiation dose. This approach is compared with a partially wide tangential (PWT) and five-field (5F) arrangement.
Methods and materials
10 consecutive patients with left-sided breast cancer who were previously treated with radiation therapy were retrospectively identified for this study. All patients had undergone a left-modified radical mastectomy without the placement of an expander or implant, an axillary dissection of levels I–II and had an intact contralateral breast.
Patients were CT simulated and positioned using a breast board (Qfix, Wyckoff, NJ) with their head turned to the right and left arm raised above their head. The sternum was kept parallel to the couch with a bridge angle of 7.4–12.5 degrees.
Treatment planning
The critical structures and the clinical target volumes (CTV) were contoured and reviewed by a radiation oncologist (MM) specialising in breast cancer. The nodal regions including the supraclavicular fossa (S/Clav), the axilla (AX) and internal mammary (IM) nodes were contoured according to previously published guidelines [13, 15, 17].
The chest wall was contoured as the region of remaining muscle and breast tissue that would normally have been encompassed in tangential portal fields with standard tangents. It extended from the mid-sternum to the mid-axillary line and from the inferior clavicular head to 2 cm below the inframammary fold.
The planning target volume (PTV) was a combination of the chest wall expanded by 0.5 cm, the IM nodes expanded by 1 cm (but 0.5 cm posteriorly to limit lung dose), and the remaining nodal volumes expanded by 1 cm. A 0.5 cm bolus was placed over the chest wall to ensure adequate coverage of the skin in the treatment field. The PTV was limited by the surrounding skin and did not extend into the bolus.
Normal critical structures that were specifically analysed included the heart, the lungs, the brachial plexus and the contralateral breast.
IMRT planning
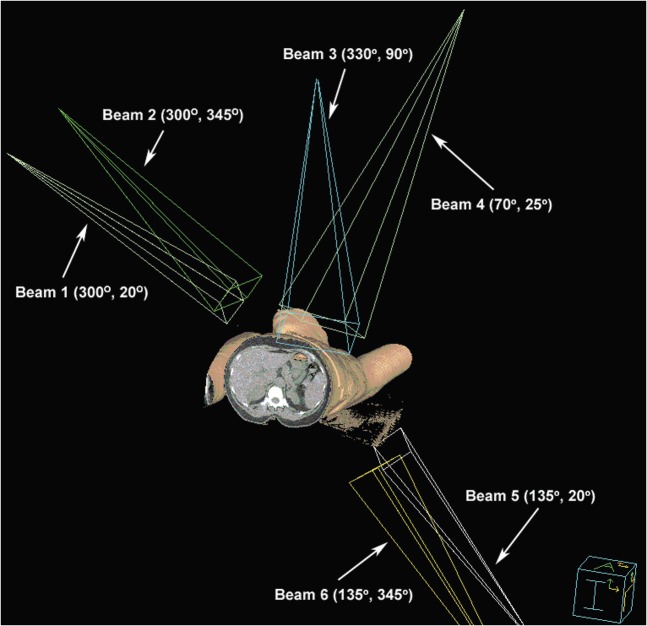
The IMRT plan used six non-coplanar beams directed around the left chest wall and all nodal regions. The beams were directed in a manner to minimise dose to the contralateral breast and right lung. A 3D representation is shown in Figure 1.
Figure 1.
Non-coplanar intensity-modulated radiation therapy beam arrangement as defined according to IEC 61217 (Gantry Angle, Table Kick) [34].
For all IMRT plans, 6 MV photons were utilised on the Pinnacle3 Planning System (version 7.6, Philips Laboratories, Milpitas, CA). Plans were designed to be delivered with a step and shoot method on a Varian 21 Linear Accelerator (Varian Medical Systems, Palo Alto, CA).
Mean dose was prescribed as 50.4 Gy in 1.8 Gy increments for the PTV. The dose–volume constraints utilised for the PTV and normal critical structures are shown in Table 1. These dose limits were based on previously published series [18–23]. The dose was normalised to ensure that 95% of the PTV received 50.4 Gy.
Table 1. Dose–volume constraints for target and critical structures.
| Structures | Volume (%) | Dose (Gy) | Weighting priority |
| Planning target volume | 95 | 50.4 | 95 |
| Internal mammary nodes | 95 | 50.4 | 95 |
| Supraclavicular nodes | 95 | 50.4 | 95 |
| Axillary levels I, II, III | 95 | 50.4 | 95 |
| Left lung | 45 | 10 | 95 |
| 20 | 20 | 95 | |
| Right lung | 3 | 5 | 92 |
| Heart | 2 | 30 | 97 |
| 10 | 10 | 97 | |
| Right breast | 1 | 2 | 95 |
Partially wide tangents
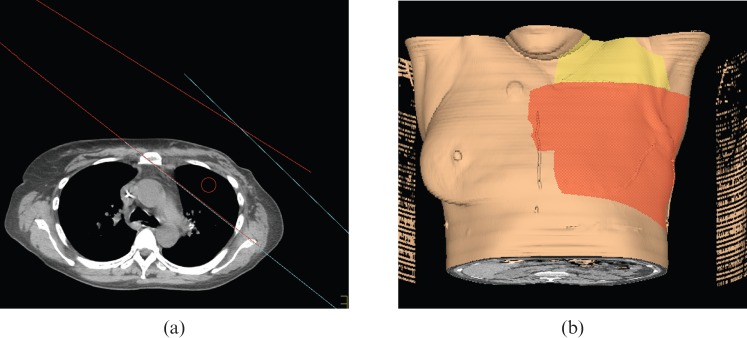
PWT fields were planned to cover the same PTV and have been described in detail elsewhere [12]. The gantry angle of the lateral beam was chosen to minimise dose to the contralateral breast. A typical beam arrangement is shown in Figure 2. The S/Clav and AX PTVs were covered using matched medial anterior-oblique/posterior-oblique fields angled approximately 10 degrees away from the spinal cord and prescribed to mid-plane. The prescription was to cover 95% of the PTV to 50.4 Gy.
Figure 2.
Depiction of partially wide tangent field arrangement. (a) Axial CT slice: red, medial tangent; blue, lateral tangent. (b) Three-dimensional depiction: red, surface path of medial and lateral tangents; yellow, surface path of anteroposterior (AP) and posteroanterior (PA) supraclavicular beam.
Five-field technique
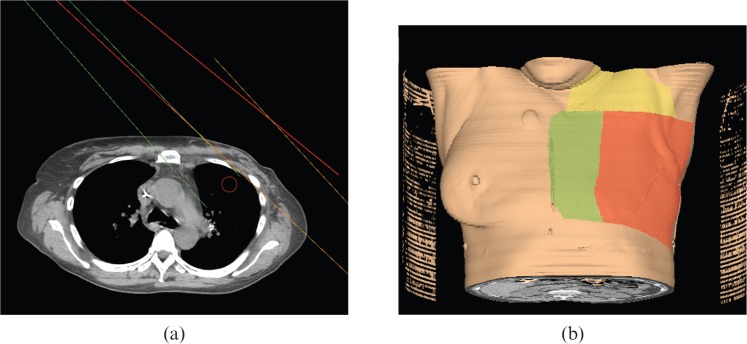
A 5F technique (two tangents, S/Clav, AX, IM nodes) was planned on all scans and optimised individually to cover the same PTV [24]. Medial and lateral tangents were used to treat the lateral chest wall. The IM nodes and medial chest wall were treated with a separate electron beam that was angled 5 degrees less to minimise overlapping field hotspots. A typical beam arrangement is shown in Figure 3. If the IM nodes were inadequately covered, a photon beam was added and angled to be matched with the medial tangent and the electron beam. The S/Clav and AX PTVs were covered with the same standard fields used in the PWT plan. The prescription was to cover 95% of the PTV to 50.4 Gy.
Figure 3.
Depiction of five-field arrangement. (a) Axial CT slice: red, medial tangent; orange, lateral tangent; green, angled IM (internal mammary) electron beam. (b) Three-dimensional depiction: red, surface path of medial and lateral tangents; yellow, surface path of anteroposterior (AP) and posteroanterior (PA) supraclavicular beam; green, surface path of angled IM electron beam.
For the PWT and 5F techniques, the plans were optimised individually for each patient and wedges as well as “field-in-field” multi-leaf collimators (MLCs) (i.e. forward planned modulation) were used to minimise hotspots where possible.
Plan assessment
Each IMRT plan was compared individually with the PWT and 5F plan. In all three plans the intended prescription was to cover 95% of the PTV to 50.4 Gy. Each plan was then analysed individually regarding its clinical acceptability. In certain cases with the 5F and PWT plans, it was decided that the regional lymphatics (IM, AX and S/Clav nodes) may receive less than 50.4 Gy in order to create an acceptable plan without inordinate hotspots. In all the plans 50.4 Gy was delivered to 95% of the left chest wall.
The coverage of the PTV, which included the left chest wall, the IM nodes, the S/Clav and AX, was calculated using the 95% isodose line as adequate coverage. Evaluation of the volume of target regions receiving 110% of the dose (V110%) was used as a parameter to judge dose heterogeneity and the prevalence of hotspots. The dose to critical structures including the right breast, the right lung, the V30Gy of the heart (i.e. the fraction volume receiving 30 Gy) and V20Gy of the left lung (i.e. the fraction volume receiving 20 Gy) was also evaluated.
Mean values of the studied parameters were calculated from the IMRT and conventional plans for all 10 patients. Comparisons were made between the IMRT plan and each of the conventional techniques using a paired two-tailed Student's t-test. A two-tailed p-value of <0.05 was considered statistically significant. Statistical analysis was performed with SPSS, version 13.0 (SPSS Inc. Chicago, IL).
Results
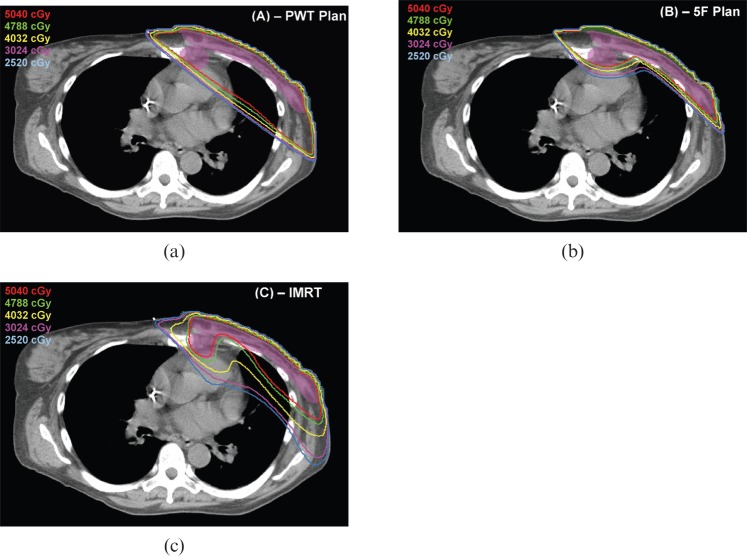
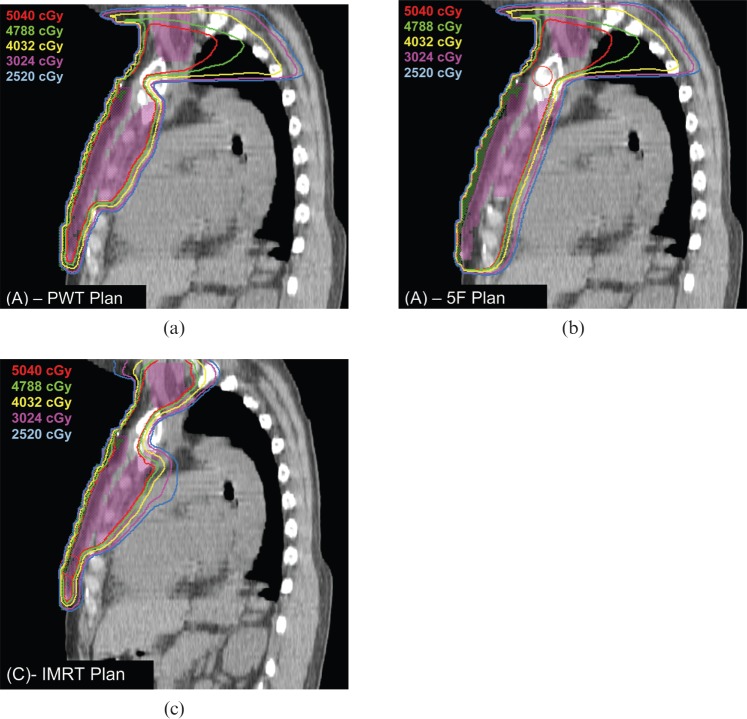
A typical patient example is shown in Figures 4 and 5 to illustrate the different isodose distributions seen in the IMRT, PWT and 5F plans.
Figure 4.
Isodose distribution of patient number 8. (a) Partially wide tangent (PWT), (b) five-field (5F) and (c) non-coplanar intensity-modulated radiation therapy (IMRT) in axial plane. Isodose lines are as follows: 100%–5040 cGy (red), 95%–4788 cGy (green), 80%–4032 cGy (yellow), 60%–3024 cGy (purple), 50%–2520 cGy (light blue). PTV shaded purple and bolus shaded green.
Figure 5.
Isodose distribution of patient number 8. (a) Partially wide tangent (PWT), (b) five-field (5F) and (c) non-coplanar intensity-modulated radiation therapy (IMRT) in sagittal plane. Isodose lines are as follows: 100%–5040 cGy (red), 95%–4788 cGy (green), 80%–4032 cGy (yellow), 60%–3024 cGy (purple), 50%–2520 cGy (light blue). PTV shaded purple and bolus shaded green.
The mean dose–volume indices from all 10 patients for the PTV and critical structures for the IMRT, PWT and 5F plans are shown in Table 2. The IMRT plan resulted in a significantly improved V95% (i.e. volume of target regions receiving 95% of the dose) of the PTV and AX levels II–III when compared with both conventional techniques. Furthermore, the IMRT plan had significantly improved dose homogeneity with reduced V110% of the PTV, S/Clav, AX level III and IM nodes. The IMRT plan has a significantly lower mean V20Gy left lung, V30Gy heart than the PWT plan. The 5F plan has a significantly lower left lung V20Gy but a higher heart V30Gy compared with the IMRT plan. Furthermore, the IMRT plan had a significantly improved dose homogeneity with reduced V110% of the PTV, S/Clav, AX level III and IM nodes. The IMRT plan had no hotspots greater than 110% in the brachial plexus, while the 5F plan had a V110% of 18.8% (p = 0.01).
Table 2. Mean dose–volume indices for non-coplanar intensity-modulated radiation therapy, partially wide tangents and a five-field plan.
| IMRT (%) | Partially wide tangents (%) | p-value (compared with IMRT plan) | Five-field (%) | p-value (compared with IMRT plan) | |
| V95% of PTV | 94.2 | 91.4 | 0.042 | 87.7 | 0.012 |
| V95% of S/C | 99.0 | 88.0 | 0.061 | 91.0 | 0.117 |
| V95% of AX level I | 99.8 | 96.9 | 0.063 | 85.4 | 0.111 |
| V95% of AX level II | 99.9 | 92.5 | 0.034 | 67.0 | 0.004 |
| V95% of AX level III | 99.8 | 85.6 | 0.002 | 65.0 | 0.015 |
| V95% of IM nodes | 98.0 | 99.6 | 0.045 | 81.8 | 0.036 |
| V110% of PTV | 3.6 | 16.8 | 0.038 | 51.8 | 0.001 |
| V110% of S/Clav | 0.5 | 24.5 | 0.041 | 33.8 | 0.003 |
| V110% of AX level I | 1.3 | 15.8 | 0.084 | 23.6 | 0.030 |
| V110% of AX level II | 0.4 | 11.6 | 0.088 | 26.8 | 0.008 |
| V110% of AX level III | 0.6 | 12.9 | 0.040 | 36.5 | 0.006 |
| V110% of IM nodes | 0.8 | 42.9 | 0.010 | 52.6 | 0.001 |
| V20Gy left lung | 29.8 | 38.4 | 0.016 | 23.1 | 0.047 |
| V30Gy heart | 2.3 | 7.5 | 0.010 | 4.9 | 0.020 |
| V110% brachial plexus | 0 | 5.7 | 0.070 | 18.8 | 0.010 |
V95%, the volume of target regions receiving 95% of the dose. V110%, the volume of target regions receiving 110% of the dose. V20Gy, the fraction volume receiving 20 Gy. V30Gy, the fraction volume receiving 30 Gy. AX, axillary; IM, internal mammary; IMRT, intensity-modulated radiation therapy; PTV, planning target volume; S/Clav, supraclavicular fossa.
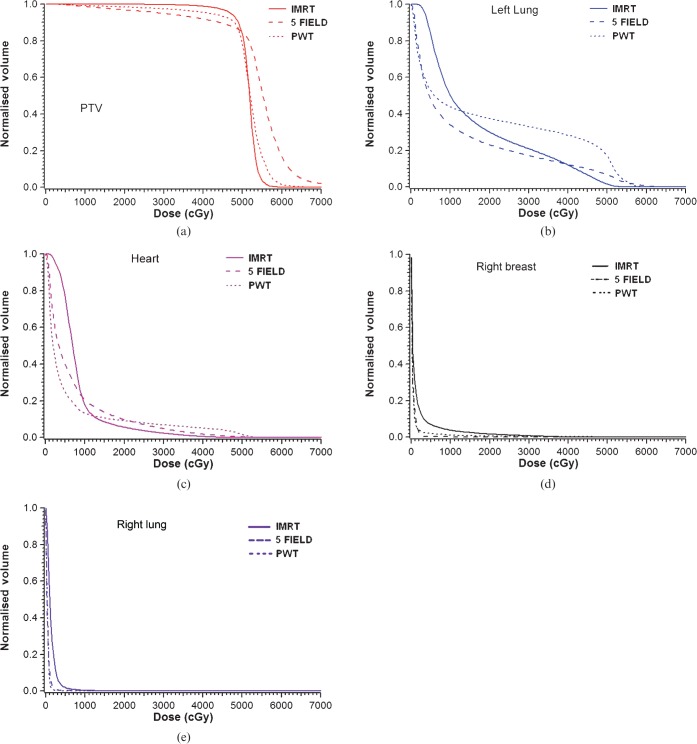
Mean dose–volume histograms were constructed by combining the dose–volume histogram data from all 10 patients. The mean dose–volume histograms for the PTV, the left lung, heart, right breast and right lung comparing the IMRT, PWT and 5F plan are shown in Figure 6. The IMRT plan substantially spared a greater portion of left lung tissue and heart when compared with the partially wide tangential field. Furthermore, the heart high-dose region was spared with the IMRT plan when compared with the 5F plan. The volume of the right lung receiving >2 Gy (V2Gy) was 0%, 0% and 2% for the PWT, 5F and IMRT plan, respectively. The volume of the right breast receiving >2 Gy was 2.8%, 2.0% and 12.8% for the PWT, 5F and IMRT plan, respectively.
Figure 6.
Combined dose–volume histograms (n = 10 plans) for partially wide tangents (PWT), five-field and non-coplanar intensity-modulated radiation therapy (IMRT) for (a) planning tumour volume (PTV), (b) left lung, (c) heart, (d) right breast and (e) right lung.
Discussion
This study is the first to demonstrate the feasibility of non-coplanar IMRT in the post-mastectomy setting for patients with left-sided breast cancer. The utilisation of non-coplanar IMRT resulted in significantly improved target coverage and dose homogeneity as compared with PWT and 5F plans.
Three studies have examined the utilisation of coplanar IMRT to deliver radiation therapy in the post-mastectomy setting [13, 15, 16]. Krueger et al [15] utilised a nine-field (9F) coplanar technique with beams circumferentially and equally spaced around the patient and showed that dose to the chest wall target was more uniform with IMRT as compared with PWTs, but the field arrangements in this plan passed through the contralateral breast and lung. Lomax et al [16] also compared a similar 9F coplanar technique with five fields using electrons and showed improved target–dose homogeneity with IMRT, but they too reported increased doses through the contralateral breast and lung. Clinically, there is significant concern of low-dose radiation to the contralateral breast in a known breast cancer patient as these doses may be carcinogenic. Recognising the inherent problems with the 9F technique (i.e. contralateral fields), Dogan et al [13] compared the 360 degree, 9F IMRT plan with an ipsilateral two-field (2F), four-field (4F) and six-field (6F) coplanar IMRT plan. The 4F and 6F plans were based on two and three pairs of tangents, respectively.
The coverage of the PTV with coplanar and our non-coplanar IMRT is improved in a similar fashion as with the conventional techniques. Given that target coverage is improved with IMRT, the issue then becomes homogeneity and avoidance of critical structures.
Avoidance of ipsilateral critical structures
Ischaemic heart disease is a long-term toxicity of breast irradiation and correlates to radiation dose [19, 25, 26]. The non-coplanar IMRT resulted in a significantly improved heart V30Gy when compared with both the PWT and 5F plans. Dogan et al [13] reported a V30Gy of 5.6% utilising nine coplanar beam configurations. Remouchamps et al [27] was able to achieve a V30Gy of 3.1% using a combination of breath hold and deep tangents with IMRT. Our mean heart V30Gy of 2.3% is comparable with these other studies.
The non-coplanar IMRT plan had a slightly higher mean V20Gy for the left lung than the 5F plan. This was owing to multiple non-coplanar lateral beams that were necessary to provide adequate target coverage, but also increased the lung dose compared with the 5F plan. Dogan et al [13] obtained a left lung V20Gy of 20–25% using 9F co-planar IMRT and near 15–25% for the 2F, 4F and 6F IMRT plan. Krueger et al [15], using a 9F co-planar beam arrangement with IMRT in the post-mastectomy setting, reported a left lung V20Gy of 25–47%. Our mean V20Gy of 29.8% is comparable with these other studies and is clinically acceptable [28, 29].
Dose to contralateral critical structures
Standard tangents can result in a scatter dose to the contralateral breast of 0.5 to 2.5 Gy [30, 31]. Although an absolute zero dose to the opposite breast is an ideal cost function in IMRT planning, it is more practical to limit the dose to be as low as possible in order to allow adequate dose to the medial aspect of the chest wall and the IM nodes. The primary drawback of coplanar IMRT techniques is that tissue not normally irradiated with conventional techniques is exposed to a low dose of irradiation. In our plan, we can lower contralateral dose significantly because our treatment beams are not directed at and do not exit through the contralateral structures. The volume of contralateral breast receiving ≥2 Gy is only 12.8% in our IMRT plan, as compared with 52.2% with the 9F coplanar technique [15]. Lomax et al [16], using the same 9F technique, report a contralateral breast volume receiving ≥5 Gy of 50% for an initial plan (IMX1). A second plan (IMX2), when optimised to decrease organ dose at the risk of tumour coverage, still gives ≥5 Gy to 10%. This non-coplanar technique delivers a lower dose to the contralateral breast compared with other previously published IMRT techniques; however, it is still slightly higher compared with the 5F and PWT plans.
For similar reasons, our technique gives negligible dose to the contralateral lung with results of V2Gy of 2%. In comparison, the V10Gy of the right lung using the 9F coplanar technique is nearly 50–60% according to Lomax et al [16] and 10% according to Krueger and colleagues [15].
Extra-normal tissue avoidance with IMRT
This IMRT technique allows for the reduction of dose to incidentally treated regions that would otherwise be included with conventional treatments. The S/Clav regions can be treated to the proper depth while minimising dose to the posterior shoulder (Figure 5c). Omission of the posterior shoulder and dose homogeneity in this area may clinically lead to a decreased incidence of fibrosis, costochondritis, frozen shoulder symptoms, brachial plexopathy, arm stiffness and lymphoedema [32, 33].
Conclusions
This non-coplanar IMRT technique shows improved coverage of the target structures and has the potential to reduce doses to the surrounding critical structures when compared with other conventional techniques such as a PWT or 5F plan. This IMRT technique compares well with previously described IMRT methods, but avoids the large and practical drawback of increased dose to normal tissues like the contralateral breast and lung, as seen with co-planar techniques.
References
- 1.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949–55 [DOI] [PubMed] [Google Scholar]
- 2.Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst 2005;97:116–26 [DOI] [PubMed] [Google Scholar]
- 3.Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641–8 [DOI] [PubMed] [Google Scholar]
- 4.Goodman RL, Grann A, Saracco P, Needham MF. The relationship between radiation fields and regional lymph nodes in carcinoma of the breast. Int J Radiat Oncol Biol Phys 2001;50:99–105 [DOI] [PubMed] [Google Scholar]
- 5.Krasin M, McCall A, King S, Olson M, Emami B. Evaluation of a standard breast tangent technique: a dose-volume analysis of tangential irradiation using three-dimensional tools. Int J Radiat Oncol Biol Phys 2000;47:327–33 [DOI] [PubMed] [Google Scholar]
- 6.Pierce LJ, Butler JB, Martel MK, Normolle DP, Koelling T, Marsh RB, et al. Postmastectomy radiotherapy of the chest wall: dosimetric comparison of common techniques. Int J Radiat Oncol Biol Phys 2002;52:1220–30 [DOI] [PubMed] [Google Scholar]
- 7.van derLaan HP, Dolsma WV, van't Veld AA, Bijl HP, Langendijk JA. Comparison of normal tissue dose with three-dimensional conformal techniques for breast cancer irradiation including the internal mammary nodes. Int J Radiat Oncol Biol Phys 2005;63:1522–30 [DOI] [PubMed] [Google Scholar]
- 8.Severin D, Connors S, Thompson H, Rathee S, Stavrev P, Hanson J. Breast radiotherapy with inclusion of internal mammary nodes: a comparison of techniques with three-dimensional planning. Int J Radiat Oncol Biol Phys 2003;55:633–44 [DOI] [PubMed] [Google Scholar]
- 9.Hehr T, Classen J, Huth M, Durst I, Christ G, Bamberg M, et al. Postmastectomy radiotherapy of the chest wall. Comparison of electron-rotation technique and common tangential photon fields. Strahlenther Onkol 2004;180:629–36 [DOI] [PubMed] [Google Scholar]
- 10.McNeely LK, Leavitt DD, Egger MJ, Stewart JR. Dose volume histogram analysis of lung radiation from chest wall treatment: comparison of electron arc and tangential photon beam techniques. Int J Radiat Oncol Biol Phys 1991;21:515–20 [DOI] [PubMed] [Google Scholar]
- 11.Pakisch B, Stucklschweiger G, Leitner H, Poier E, Poschauko J, Ottl M, et al. Evaluation of irradiation techniques in breast carcinoma using dose-volume histograms. Comparison of the heart and lung exposure. Strahlenther Onkol 1991;167:158–64 [PubMed] [Google Scholar]
- 12.Arthur DW, Arnfield MR, Warwicke LA, Morris MM, Zwicker RD. Internal mammary node coverage: an investigation of presently accepted techniques. Int J Radiat Oncol Biol Phys 2000;48:139–46 [DOI] [PubMed] [Google Scholar]
- 13.Dogan N, Cuttino L, Lloyd R, Bump EA, Arthur DW. Optimized dose coverage of regional lymph nodes in breast cancer: the role of intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2007;68:1238–50 [DOI] [PubMed] [Google Scholar]
- 14.Cavey ML, Bayouth JE, Endres EJ, Pena JM, Colman M, Hatch S. Dosimetric comparison of conventional and forward-planned intensity-modulated techniques for comprehensive locoregional irradiation of post-mastectomy left breast cancers. Med Dosim 2005;30:107–16 [DOI] [PubMed] [Google Scholar]
- 15.Krueger EA, Fraass BA, McShan DL, Marsh R, Pierce LJ. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys 2003;56:1023–37 [DOI] [PubMed] [Google Scholar]
- 16.Lomax AJ, Cella L, Weber D, Kurtz JM, Miralbell R. Potential role of intensity-modulated photons and protons in the treatment of the breast and regional nodes. Int J Radiat Oncol Biol Phys 2003;55:785–92 [DOI] [PubMed] [Google Scholar]
- 17.Madu CN, Quint DJ, Normolle DP, Marsh RB, Wang EY, Pierce LJ. Definition of the supraclavicular and infraclavicular nodes: implications for three-dimensional CT-based conformal radiation therapy. Radiology 2001;221:333–9 [DOI] [PubMed] [Google Scholar]
- 18.Gyenes G, Gagliardi G, Lax I, Fornander T, Rutqvist LE. Evaluation of irradiated heart volumes in stage I breast cancer patients treated with postoperative adjuvant radiotherapy. J Clin Oncol 1997;15:1348–53 [DOI] [PubMed] [Google Scholar]
- 19.Gyenes G. Late cardiac morbidity and mortality in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol 2007;25:2489; author reply 2489–90. [DOI] [PubMed] [Google Scholar]
- 20.Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin's disease in children and adolescents. J Clin Oncol 1993;11:1208–15 [DOI] [PubMed] [Google Scholar]
- 21.Fogliata A, Nicolini G, Alber M, Asell M, Dobler B, El-Haddad M, et al. IMRT for breast. A planning study. Radiother Oncol 2005;76:300–10 [DOI] [PubMed] [Google Scholar]
- 22.Mayo CS, Urie MM, Fitzgerald TJ. Hybrid IMRT plans — concurrently treating conventional and IMRT beams for improved breast irradiation and reduced planning time. Int J Radiat Oncol Biol Phys 2005;61:922–32 [DOI] [PubMed] [Google Scholar]
- 23.Marks LB, Yu X, Vujaskovic Z, Small W, Jr, Folz R, Anscher MS. Radiation-induced lung injury. Semin Radiat Oncol 2003;13:333–45 [DOI] [PubMed] [Google Scholar]
- 24.Hurkmans CW, Saarnak AE, Pieters BR, Borger JH, Bruinvis IA. An improved technique for breast cancer irradiation including the locoregional lymph nodes. Int J Radiat Oncol Biol Phys 2000;47:1421–9 [DOI] [PubMed] [Google Scholar]
- 25.Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s–1990s. Int J Radiat Oncol Biol Phys 2007;69:1484–95 [DOI] [PubMed] [Google Scholar]
- 26.Gagliardi G, Lax I, Ottolenghi A, Rutqvist LE. Long-term cardiac mortality after radiotherapy of breast cancer — application of the relative seriality model. Br J Radiol 1996;69:839–46 [DOI] [PubMed] [Google Scholar]
- 27.Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys 2003;55:392–406 [DOI] [PubMed] [Google Scholar]
- 28.Lind PA, Wennberg B, Gagliardi G, Rosfors S, Blom-Goldman U, Lidestahl A, et al. ROC curves and evaluation of radiation-induced pulmonary toxicity in breast cancer. Int J Radiat Oncol Biol Phys 2006;64:765–70 [DOI] [PubMed] [Google Scholar]
- 29.Pierce LJ, Butler JB, Martel MK, Normolle DP, Koelling T, Marsh RB, et al. Postmastectomy radiotherapy of the chest wall: dosimetric comparison of common techniques. Int J Radiat Oncol Biol Phys 2002;52:1220–30 [DOI] [PubMed] [Google Scholar]
- 30.Storm HH, Andersson M, Boice JD, Jr, Blettner M, Stovall M, Mouridsen HT, et al. Adjuvant radiotherapy and risk of contralateral breast cancer. J Natl Cancer Inst 1992;84:1245–50 [DOI] [PubMed] [Google Scholar]
- 31.Boice JD, Jr, Harvey EB, Blettner M, Stovall M, Flannery JT. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med 1992;326:781–5 [DOI] [PubMed] [Google Scholar]
- 32.Sugden EM, Rezvani M, Harrison JM, Hughes LK. Shoulder movement after the treatment of early stage breast cancer. Clin Oncol (R Coll Radiol) 1998;10:173–81 [DOI] [PubMed] [Google Scholar]
- 33.Rietman JS, Dijkstra PU, Hoekstra HJ, Eisma WH, Szabo BG, Groothoff JW, et al. Late morbidity after treatment of breast cancer in relation to daily activities and quality of life: a systematic review. Eur J Surg Oncol 2003;29:229–38 [DOI] [PubMed] [Google Scholar]
- 34.Bureau Central de la Commission Electrotechnique International IEC 6217: Radiotherapy equipment-coordinates, movements and Scales. Edition 1. Switzerland. 1996 [Google Scholar]